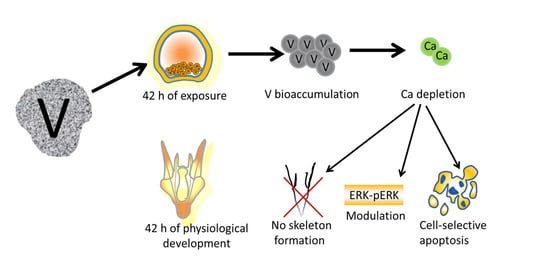
Toxicity of Vanadium during Development of Sea Urchin Embryos: Bioaccumulation, Calcium Depletion, ERK Modulation and Cell-Selective Apoptosis
Abstract
:1. Introduction
2. Results
2.1. V-Bioaccumulation Competes with Ca Uptake
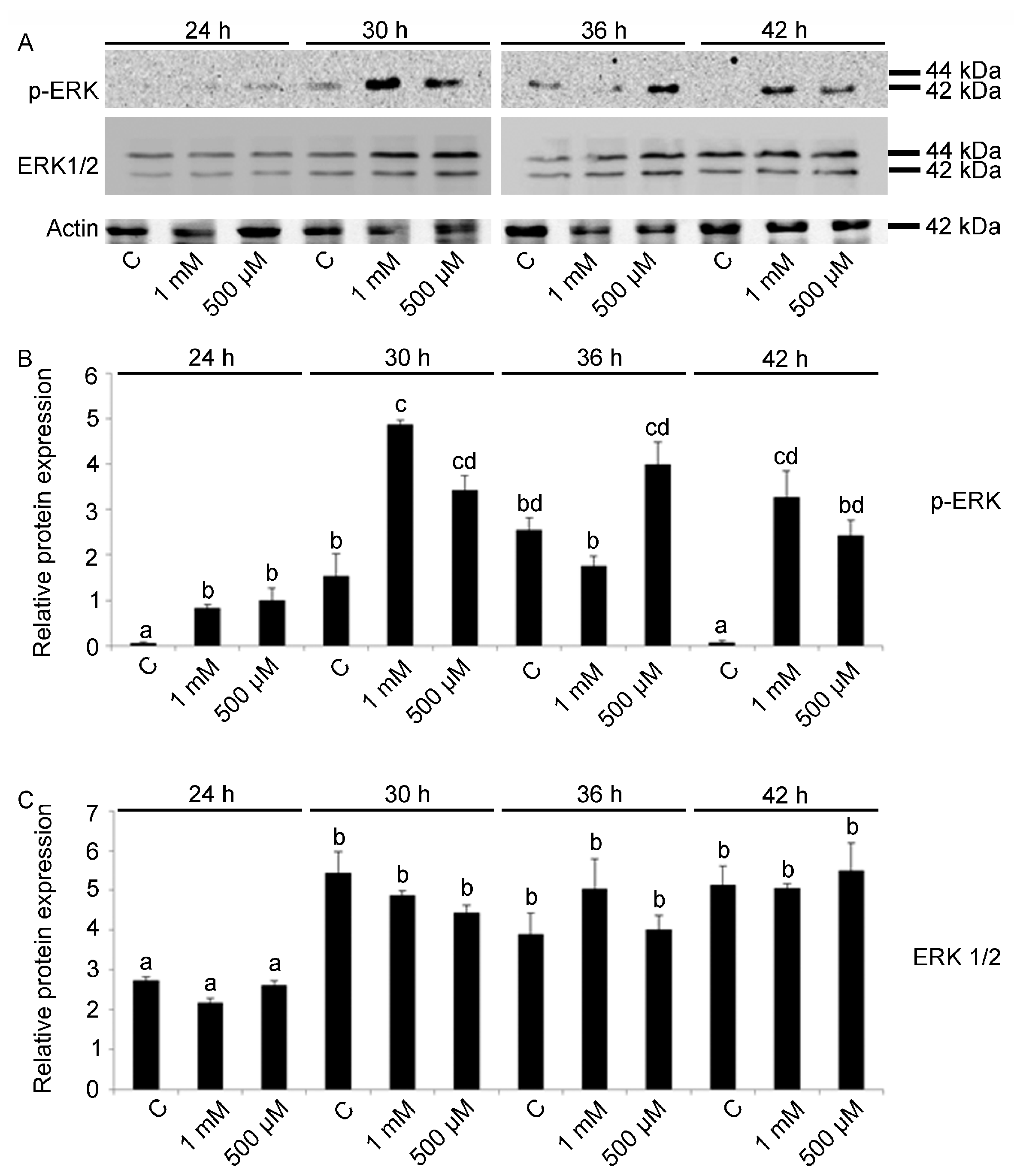
2.2. ERK Modulation on Embryos Exposed to V
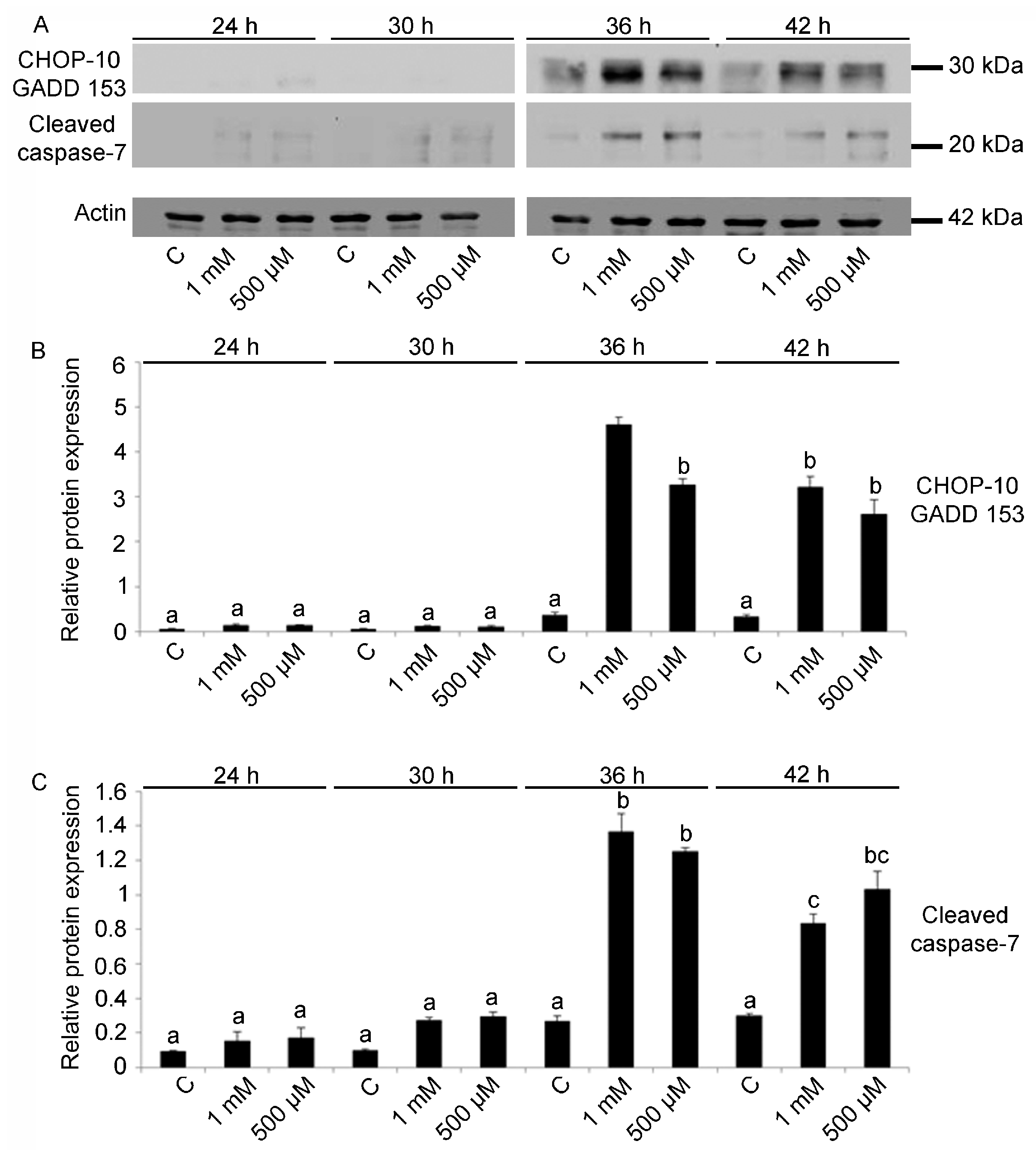
2.3. Cell Cycle Arrest and Apoptosis
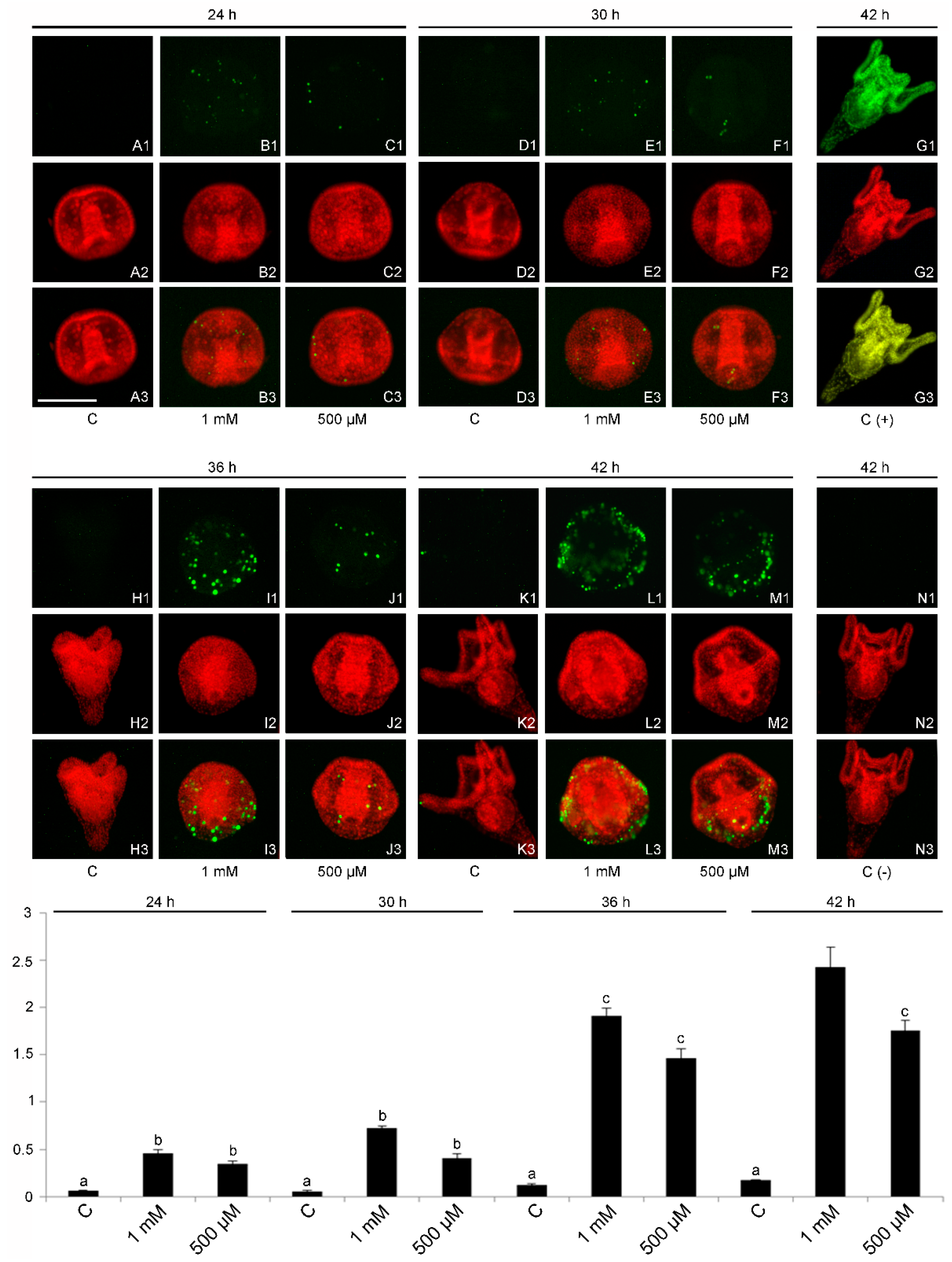
2.4. DNA Fragmentation and Evidence of a Cell-Selective Apoptosis
3. Discussion
4. Materials and Methods
4.1. Embryos Cultures and V-Exposure
4.2. V and Ca Quantitative Analysis
4.3. Electrophoretic Analysis and Immunoblotting
4.4. TUNEL Assay and Quantitative Fragmented DNA Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandiyan, J.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K.A.; Ahmed, Z.; Al-Mulahim, N.; Jagadheesan, R.; Krishnappa, K. An assessment of level of heavy metals pollution in the water, sediment and aquatic organisms: A perspective of tackling environmental threats for food security. Saudi J. Biol. Sci. 2020, 28, 1218–1225. [Google Scholar] [CrossRef]
- Jezierska, B.; Ługowska, K.; Witeska, M. The effects of heavy metals on embryonic development of fish (a review). Fish. Physiol. Biochem. 2008, 35, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, R.; Roccheri, M.C. Heavy Metals and Metalloids as Autophagy Inducing Agents: Focus on Cadmium and Arsenic. Cells 2012, 1, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarelli, R.; Roccheri, M.C. Marine Invertebrates as Bioindicators of Heavy Metal Pollution. Open J. Met. 2014, 04, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.; Chianese, T.; Chiarelli, R.; Roccheri, M.C.; Scudiero, R. Toxicological Impact of Rare Earth Elements (REEs) on the Reproduction and Development of Aquatic Organisms Using Sea Urchins as Biological Models. Int. J. Mol. Sci. 2022, 23, 2876. [Google Scholar] [CrossRef]
- WHO Regional office for Europe. Air Quality Guidelines for Europe Second Edition, Copenhagen, Denmark, Chapter 6.12 Vanadium; WHO Regional Office for Europe: Copenhagen, Denmark, 2000; pp. 1–9. [Google Scholar]
- Rana, D.; Kumar, A. Is there a Role for Sodium Orthovanadate in the Treatment of Diabetes? Curr. Diabetes Rev. 2019, 15, 284–287. [Google Scholar] [CrossRef]
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J. Trace Elements Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; McLauchlan, C.C.; Rompel, A.; Crans, D.C. Polyoxidovanadates’ interactions with proteins: An overview. Coord. Chem. Rev. 2021, 454, 214344. [Google Scholar] [CrossRef]
- Andreucci, M.; Solomon, R.; Tasanarong, A. Side Effects of Radiographic Contrast Media: Pathogenesis, Risk Factors, and Prevention. BioMed Res. Int. 2014, 2014, 741018. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.; Byrne, M.; Roccheri, M.C.; Chiarelli, R. Interactive effects of increased temperature and gadolinium pollution in Paracentrotus lividus sea urchin embryos: A climate change perspective. Aquat. Toxicol. 2021, 232, 105750. [Google Scholar] [CrossRef] [PubMed]
- Reissig, F.; Kopka, K.; Mamat, C. The impact of barium isotopes in radiopharmacy and nuclear medicine–From past to presence. Nucl. Med. Biol. 2021, 98–99, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Telgmann, L.; Sperling, M.; Karst, U. Determination of gadolinium-based MRI contrast agents in biological and environmental samples: A review. Anal. Chim. Acta 2013, 764, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.; Bonaventura, R.; Byrne, M.; Roccheri, M.; Matranga, V. Effects of exposure to gadolinium on the development of geographically and phylogenetically distant sea urchins species. Mar. Environ. Res. 2016, 128, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinsino, A.; Matranga, V.; Trinchella, F.; Roccheri, M.C. Sea urchin embryos as an in vivo model for the assessment of manganese toxicity: Developmental and stress response effects. Ecotoxicology 2009, 19, 555–562. [Google Scholar] [CrossRef]
- Matranga, V.; Pinsino, A.; Bonaventura, R.; Costa, C.; Karakostis, K.; Martino, C.; Russo, R.; Zito, F. Cellular and molecular bases of bio-mineralization in sea urchin embryos. Cah. De Biol. Mar. 2013, 54, 467–478. [Google Scholar]
- Chiarelli, R.; Agnello, M.; Bosco, L.; Roccheri, M. Sea urchin embryos exposed to cadmium as an experimental model for studying the relationship between autophagy and apoptosis. Mar. Environ. Res. 2014, 93, 47–55. [Google Scholar] [CrossRef]
- Martino, C.; Chiarelli, R.; Bosco, L.; Roccheri, M.C. Induction of skeletal abnormalities and autophagy in Paracentrotus lividus sea urchin embryos exposed to gadolinium. Mar. Environ. Res. 2017, 130, 12–20. [Google Scholar] [CrossRef]
- Martino, C.; Chiarelli, R.; Roccheri, M.C.; Matranga, V.; Byrne, M. Effects of magnesium deprivation on development and biomineralization in the sea urchin Arbacia lixula. Invertebr. Reprod. Dev. 2019, 63, 165–176. [Google Scholar] [CrossRef]
- Chiarelli, R.; Agnello, M.; Roccheri, M.C. Sea urchin embryos as a model system for studying autophagy induced by cadmium stress. Autophagy 2011, 7, 1028–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinsino, A.; Roccheri, M.C.; Costa, C.; Matranga, V. Manganese Interferes with Calcium, Perturbs ERK Signaling, and Produces Embryos with No Skeleton. Toxicol. Sci. 2011, 123, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Martino, C.; Costa, C.; Roccheri, M.C.; Koop, D.; Scudiero, R.; Byrne, M. Gadolinium perturbs expression of skeletogenic genes, calcium uptake and larval development in phylogenetically distant sea urchin species. Aquat. Toxicol. 2017, 194, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, R.; Martino, C.; Roccheri, M.C.; Cancemi, P. Toxic effects induced by vanadium on sea urchin embryos. Chemosphere 2021, 274, 129843. [Google Scholar] [CrossRef]
- Chiarelli, R.; Martino, C.; Roccheri, M.C.; Geraci, F. Vanadium Toxicity Monitored by Fertilization Outcomes and Metal Related Proteolytic Activities in Paracentrotus lividus Embryos. Toxics 2022, 10, 83. [Google Scholar] [CrossRef]
- Nakano, E.; Okazaki, K.; Iwamatsu, T. Accumulation of radioactive calcium in larvae of the sea urchin Pseudocentrotus depressus. Biol. Bull. 1963, 125, 125–132. [Google Scholar] [CrossRef]
- Livingston, B.; Killian, C.; Wilt, F.; Cameron, A.; Landrum, M.; Ermolaeva, O.; Sapojnikov, V.; Maglott, D.; Buchanan, A.; Ettensohn, C. A genome-wide analysis of biomineralization-related proteins in the sea urchin Strongylocentrotus purpuratus. Dev. Biol. 2006, 300, 335–348. [Google Scholar] [CrossRef] [Green Version]
- Ettensohn, C.A. Lessons from a gene regulatory network: Echinoderm skeletogenesis provides insights into evolution, plasticity and morphogenesis. Development 2009, 136, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Röttinger, E.; Besnardeau, L.; Lepage, T. A Raf/MEK/ERK signaling pathway is required for development of the sea urchin embryo micromere lineage through phosphorylation of the transcription factor Ets. Development 2004, 131, 1075–1087. [Google Scholar] [CrossRef] [Green Version]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanisms of ERK-induced cell death—Apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef]
- Wang, X.; Kuroda, M.; Sok, J.; Batchvarova, N.; Kimmel, R.; Chung, P.; Zinszner, H.; Ron, D. Identification of novel stress-induced genes downstream of chop. EMBO J. 1998, 17, 3619–3630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roccheri, M.C.; Tipa, C.; Bonaventura, R.; Matranga, V. Physiological and induced apoptosis in sea urchin larvae undergoing metamorphosis. Int. J. Dev. Biol. 2002, 46, 801–806. [Google Scholar] [PubMed]
- Agnello, M.; Filosto, S.; Scudiero, R.; Rinaldi, A.M.; Roccheri, M.C. Cadmium induces an apoptotic response in sea urchin embryos. Cell Stress Chaperones 2007, 12, 44–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filosto, S.; Roccheri, M.C.; Bonaventura, R.; Matranga, V. Environmentally relevant cadmium concentrations affect development and induce apoptosis of Paracentrotus lividus larvae cultured in vitro. Cell Biol. Toxicol. 2008, 24, 603–610. [Google Scholar] [CrossRef]
- Chiarelli, R.; Martino, C.; Agnello, M.; Bosco, L.; Roccheri, M.C. Autophagy as a defense strategy against stress: Focus on Paracentrotus lividus sea urchin embryos exposed to cadmium. Cell Stress Chaperones 2015, 21, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Agnello, M.; Filosto, S.; Scudiero, R.; Rinaldi, A.M.; Roccheri, M.C. Cadmium accumulation induces apoptosis in P. Lividus embryos. Caryologia 2006, 59, 403–408. [Google Scholar]
- Byrne, M.; Lamare, M.; Winter, D.; Dworjanyn, S.A.; Uthicke, S. The stunting effect of a high CO2 ocean on calcification and development in sea urchin larvae, a synthesis from the tropics to the poles. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120439. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Serra, M.; Consales, C.; Livigni, A.; I Arnone, M. Role of the ERK-mediated signaling pathway in mesenchyme formation and differentiation in the sea urchin embryo. Dev. Biol. 2004, 268, 384–402. [Google Scholar] [CrossRef] [Green Version]
- Agell, N.; Bachs, O.; Rocamora, N.; Villalonga, P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca2+, and Calmodulin. Cell. Signal. 2002, 14, 649–654. [Google Scholar] [CrossRef]
- Kumano, M.; Carroll, D.; Denu, J.M.; Foltz, K.R. Calcium-Mediated Inactivation of the MAP Kinase Pathway in Sea Urchin Eggs at Fertilization. Dev. Biol. 2001, 236, 244–257. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Wu, D.; Hirao, A.; Lahti, J.M.; Liu, L.; Mazza, B.; Kidd, V.J.; Mak, T.W.; Ingram, A.J. ERK Activation Mediates Cell Cycle Arrest and Apoptosis after DNA Damage Independently of p53. J. Biol. Chem. 2002, 277, 12710–12717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarelli, R.; Martino, C.; Roccheri, M.C. Cadmium stress effects indicating marine pollution in different species of sea urchin employed as environmental bioindicators. Cell Stress Chaperones 2019, 24, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, M.A.; Costa, S.; Gianguzza, M.; Roccheri, M.C.; Gianguzza, F. Effects of cadmium exposure on sea urchin development assessed by SSH and RT-qPCR: Metallothionein genes and their differential induction. Mol. Biol. Rep. 2012, 40, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Badmaev, V.; Prakash, S.; Majeed, M. Vanadium: A Review of its Potential Role in the Fight Against Diabetes. J. Altern. Complement. Med. 1999, 5, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.E.; Shoo, L.P.; Isaac, J.L.; Hoffmann, A.; Langham, G. Towards an Integrated Framework for Assessing the Vulnerability of Species to Climate Change. PLoS Biol. 2008, 6, 2621–2626. [Google Scholar] [CrossRef]
- Hamdoun, A.; Epel, D. Embryo stability and vulnerability in an always changing world. Proc. Natl. Acad. Sci. USA 2007, 104, 1745–1750. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiarelli, R.; Scudiero, R.; Memoli, V.; Roccheri, M.C.; Martino, C. Toxicity of Vanadium during Development of Sea Urchin Embryos: Bioaccumulation, Calcium Depletion, ERK Modulation and Cell-Selective Apoptosis. Int. J. Mol. Sci. 2022, 23, 6239. https://doi.org/10.3390/ijms23116239
Chiarelli R, Scudiero R, Memoli V, Roccheri MC, Martino C. Toxicity of Vanadium during Development of Sea Urchin Embryos: Bioaccumulation, Calcium Depletion, ERK Modulation and Cell-Selective Apoptosis. International Journal of Molecular Sciences. 2022; 23(11):6239. https://doi.org/10.3390/ijms23116239
Chicago/Turabian StyleChiarelli, Roberto, Rosaria Scudiero, Valeria Memoli, Maria Carmela Roccheri, and Chiara Martino. 2022. "Toxicity of Vanadium during Development of Sea Urchin Embryos: Bioaccumulation, Calcium Depletion, ERK Modulation and Cell-Selective Apoptosis" International Journal of Molecular Sciences 23, no. 11: 6239. https://doi.org/10.3390/ijms23116239
APA StyleChiarelli, R., Scudiero, R., Memoli, V., Roccheri, M. C., & Martino, C. (2022). Toxicity of Vanadium during Development of Sea Urchin Embryos: Bioaccumulation, Calcium Depletion, ERK Modulation and Cell-Selective Apoptosis. International Journal of Molecular Sciences, 23(11), 6239. https://doi.org/10.3390/ijms23116239