Chronic Hyperkaliemia in Chronic Kidney Disease: An Old Concern with New Answers
Abstract
:1. Introduction
2. Potassium Homeostasis
2.1. Internal Potassium Balance
2.2. External Potassium Balance
3. Regulation of Urinary Potassium Excretion
4. Molecular Mechanisms Explaining the Relationship between Potassium Intake and Blood Pressure
5. Hyperkalaemia in General Population and in Chronic Kidney Disease
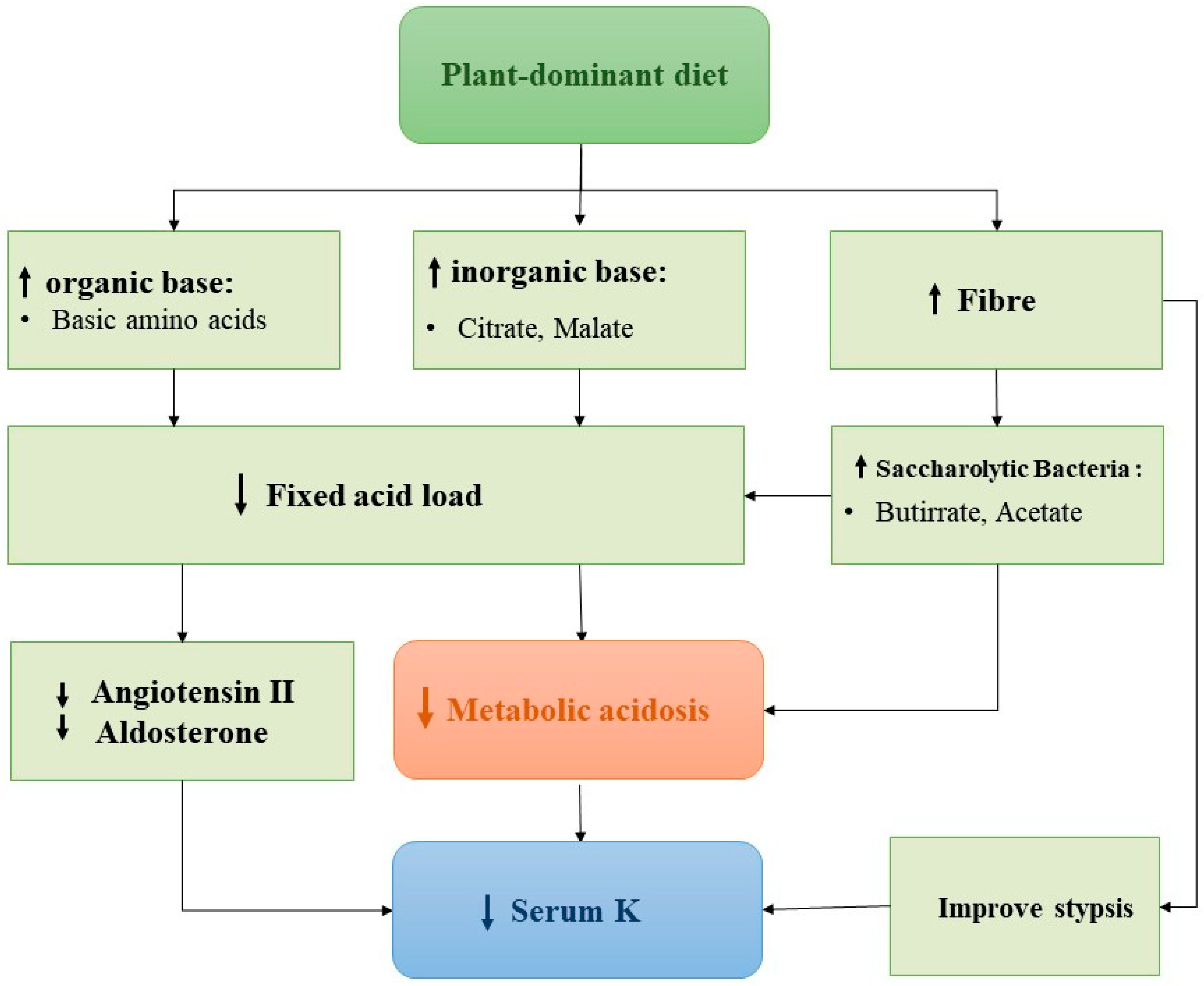
6. The Dilemma of Nutritional Approach in Chronic Kidney Disease Patients
7. Role of K-Binders on Hyperkalaemia in Chronic Kidney Disease Patients
7.1. Patiromer
7.2. Sodium Zirconium Cyclosilicate (SZC)
7.3. Strategies to Remove Potassium in Dialysis Patients
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Clase, C.M.; Carrero, J.J.; Ellison, D.H.; Grams, M.E.; Hemmelgarn, B.R.; Jardine, M.J.; Kovesdy, C.P.; Kline, G.A.; Lindner, G.; Obrador, G.T.; et al. Potassium homeostasis and management of dyskalemia in kidney diseases: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020, 97, 42–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; He, F.J.; Sun, Q.; Yuan, C.; Kieneker, L.M.; Curhan, G.C.; MacGregor, G.A.; Bakker, S.J.L.; Campbell, N.R.C.; Wang, M.; et al. 24-Hour Urinary Sodium and Potassium Excretion and Cardiovascular Risk. N. Engl. J. Med. 2022, 386, 252–263. [Google Scholar] [CrossRef]
- Neal, B.; Tian, M.; Wu, Y. Salt Substitute and Cardiovascular Events and Death. Reply. N. Engl. J. Med. 2021, 385, 2493–2494. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Matsushita, K.; Sang, Y.; Brunskill, N.J.; Carrero, J.J.; Chodick, G.; Hasegawa, T.; Heerspink, H.L.; Hirayama, A.; Landman, G.W.D.; et al. Serum potassium and adverse outcomes across the range of kidney function: A CKD Prognosis Consortium meta-analysis. Eur. Heart J. 2018, 39, 1535–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, B.F. Regulation of Potassium Homeostasis. Clin. J. Am. Soc Nephrol. 2015, 10, 1050–1060. [Google Scholar] [CrossRef] [Green Version]
- Gumz, M.L.; Rabinowitz, L.; Wingo, C.S. An Integrated View of Potassium Homeostasis. N. Engl. J. Med. 2015, 373, 1787–1788. [Google Scholar] [CrossRef] [Green Version]
- Hoorn, E.J.; Gritter, M.; Cuevas, C.A.; Fenton, R.A. Regulation of the Renal NaCl Cotransporter and Its Role in Potassium Homeostasis. Physiol. Rev. 2020, 100, 321–356. [Google Scholar] [CrossRef]
- Terker, A.S.; Zhang, C.; McCormick, J.A.; Lazelle, R.A.; Zhang, C.; Meermeier, N.P.; Siler, D.A.; Park, H.J.; Fu, Y.; Cohen, D.M.; et al. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015, 21, 39–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, N.; Shoda, W.; Wang, Y.; Mandai, S.; Furusho, T.; Takahashi, D.; Zeniya, M.; Sohara, E.; Rai, T.; Uchida, S. Role of ClC-K and barttin in low potassium-induced sodium chloride cotransporter activation and hypertension in mouse kidney. Biosci. Rep. 2018, 38, BSR20171243. [Google Scholar] [CrossRef] [Green Version]
- Ferdaus, M.Z.; Barber, K.W.; López-Cayuqueo, K.I.; Terker, A.S.; Argaiz, E.R.; Gassaway, B.M.; Chambrey, R.; Gamba, G.; Rinehart, J.; McCormick, J.A. SPAK and OSR1 play essential roles in potassium homeostasis through actions on the distal convoluted tubule. J. Physiol. 2016, 594, 4945–4966. [Google Scholar] [CrossRef] [Green Version]
- Layton, A.T.; Edwards, A.; Vallon, V. Renal potassium handling in rats with subtotal nephrectomy: Odelling and analysis. Am. J. Physiol. Renal Physiol. 2018, 314, F643–F657. [Google Scholar] [CrossRef] [Green Version]
- Veiras, L.C.; Girardi, A.C.C.; Curry, J.; Pei, L.; Ralph, D.L.; Tran, A.; Castelo-Branco, R.C.; Pastor-Soler, N.; Arranz, C.T.; Yu, A.S.L.; et al. Sexual Dimorphic Pattern of Renal Transporters and Electrolyte Homeostasis. J. Am. Soc. Nephrol. 2017, 28, 3504–3517. [Google Scholar] [CrossRef]
- Chen, Y.; Sang, Y.; Ballew, S.H.; Tin, A.; Chang, A.R.; Matsushita, K.; Coresh, J.; Kalantar-Zadeh, K.; Molnar, M.Z.; Grams, M.E. Race, Serum Potassium, and Associations with ESRD and Mortality. Am. J. Kidney Dis. 2017, 70, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.R.; Sang, Y.; Leddy, J.; Yahya, T.; Kirchner, H.L.; Inker, L.A.; Matsushita, K.; Ballew, S.H.; Coresh, J.; Grams, M.E. Antihypertensive Medications and the Prevalence of Hyperkalemia in a Large Health System. Hypertension 2016, 67, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Sriperumbuduri, S.; McArthur, E.; Hundemer, G.L.; Canney, M.; Tangri, N.; Leon, S.J.; Bota, S.; Bugeja, A.; Akbari, A.; Knoll, G.; et al. Initial and Recurrent Hyperkalemia Events in Patients with CKD in Older Adults: A Population-Based Cohort Study. Can. J. Kidney Health Dis. 2021, 8, 20543581211017408. [Google Scholar] [CrossRef] [PubMed]
- Drawz, P.E.; Babineau, D.C.; Rahman, M. Metabolic complications in elderly adults with chronic kidney disease. J. Am. Geriatr. Soc. 2012, 60, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.; Kalantar-Zadeh, K.; Lu, J.L.; Turban, S.; Anderson, J.E.; Kovesdy, C.P. Association of hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: The role of race. Nephron Clin. Pract. 2012, 120, c8–c16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarafidis, P.A.; Blacklock, R.; Wood, E.; Rumjon, A.; Simmonds, S.; Fletcher-Rogers, J.; Ariyanayagam, R.; Al-Yassin, A.; Sharpe, C.; Vinen, K. Prevalence and factors associated with hyperkalemia in predialysis patients followed in a low-clearance clinic. Clin. J. Am. Soc. Nephrol. 2012, 7, 1234–1241. [Google Scholar] [CrossRef] [Green Version]
- Nakhoul, G.N.; Huang, H.; Arrigain, S.; Jolly, S.E.; Schold, J.D.; Nally, J.V., Jr.; Navaneethan, S.D. Serum Potassium, End-Stage Renal Disease and Mortality in Chronic Kidney Disease. Am. J. Nephrol. 2015, 41, 456–463. [Google Scholar] [CrossRef]
- Luo, J.; Brunelli, S.M.; Jensen, D.E.; Yang, A. Association between Serum Potassium and Outcomes in Patients with Reduced Kidney Function. Clin. J. Am. Soc. Nephrol. 2016, 11, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Betts, K.A.; Woolley, J.M.; Mu, F.; McDonald, E.; Tang, W.; Wu, E.Q. The prevalence of hyperkalemia in the United States. Curr. Med. Res. Opin. 2018, 34, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Pitt, B.; Reaven, N.; Funk, S.; McGaughey, K.; Wilson, D.; Bushinsky, D.A. Association of Serum Potassium with All-Cause Mortality in Patients with and without Heart Failure, Chronic Kidney Disease, and/or Diabetes. Am. J. Nephrol. 2017, 46, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.; Bayés-Genís, A.; Zannad, F.; Rossignol, P.; Núñez, E.; Bodí, V.; Miñana, G.; Santas, E.; Chorro, F.J.; Mollar, A.; et al. Long-Term Potassium Monitoring and Dynamics in Heart Failure and Risk of Mortality. Circulation 2018, 137, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Minutolo, R.; Chiodini, P.; Bellizzi, V.; Nappi, F.; Russo, D.; Borrelli, S.; Garofalo, C.; Iodice, C.; De Stefano, T.; et al. Competing-Risk Analysis of Death and End Stage Kidney Disease by Hyperkalaemia Status in Non-Dialysis Chronic Kidney Disease Patients Receiving Stable Nephrology Care. J. Clin. Med. 2018, 7, 499. [Google Scholar] [CrossRef] [Green Version]
- Ramos, C.I.; Gonzalez-Ortiz, A.; Espinosa-Cuevas, A.; Avesani, C.M.; Carrero, J.J.; Cuppari, L. Does dietary potassium intake associate with hyperkalemia in patients with chronic kidney disease? Nephrol. Dial. Transplant. 2021, 36, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Saito, H.; Iwasaki, T.; Oda, A.; Watanabe, S.; Kanno, M.; Kimura, H.; Shimabukuro, M.; Asahi, K.; Watanabe, T.; et al. Association between serum potassium levels and adverse outcomes in chronic kidney disease: The Fukushima CKD cohort study. Clin. Exp. Nephrol. 2021, 25, 410–417. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, C.; Cumetti, A.; Pardini, E.; Mannucci, C.; Serio, P.; Morganti, R.; Cupisti, A. Prevalence and correlates of hyperkalemia in a renal nutrition clinic. Intern. Emerg. Med. 2021, 16, 125–132. [Google Scholar] [CrossRef]
- Panuccio, V.; Leonardis, D.; Tripepi, R.; Versace, M.C.; Torino, C.; Tripepi, G.; D’Arrigo, G.; Mallamaci, F.; Zoccali, C. Epidemiology of hyperkalemia in CKD patients under nephrological care: A longitudinal study. Intern. Emerg. Med. 2021, 16, 1803–1811. [Google Scholar] [CrossRef]
- Provenzano, M.; De Francesco, M.; Iannazzo, S.; Garofalo, C.; Andreucci, M.; Genualdo, R.; Borrelli, S.; Minutolo, R.; Conte, G.; De Nicola, L. Cost-analysis of persistent hyperkalaemia in non-dialysis chronic kidney disease patients under nephrology care in Italy. Int. J. Clin. Pract. 2020, 74, e13475. [Google Scholar] [CrossRef] [PubMed]
- Galloway, C.D.; Valys, A.V.; Shreibati, J.B.; Treiman, D.L.; Petterson, F.L.; Gundotra, V.P.; Albert, D.E.; Attia, Z.I.; Carter, R.E.; Asirvatham, S.J.; et al. Development and Validation of a Deep-Learning Model to Screen for Hyperkalemia from the Electrocardiogram. JAMA Cardiol. 2019, 4, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R.; Lakkis, J.I.; Jaar, B.; Rocco, M.V.; Choi, M.J.; Kramer, H.J.; Ku, E. Use of Renin-Angiotensin System Blockade in Advanced CKD: An NKF-KDOQI Controversies Report. Am. J. Kidney Dis. 2018, 72, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Shin, J.I.; Chen, T.K.; Inker, L.A.; Coresh, J.; Alexander, G.C.; Jackson, J.W.; Chang, A.R.; Grams, M.E. Association Between Renin-Angiotensin System Blockade Discontinuation and All-Cause Mortality Among Persons with Low Estimated Glomerular Filtration Rate. JAMA Intern. Med. 2020, 180, 718–726. [Google Scholar] [CrossRef]
- Santoro, A.; Perrone, V.; Giacomini, E.; Sangiorgi, D.; Alessandrini, D.; Degli Esposti, L. Association between hyperkalemia, RAASi non-adherence and outcomes in chronic kidney disease. J. Nephrol. 2022, 35, 463–472. [Google Scholar] [CrossRef]
- Leon, S.J.; Whitlock, R.; Rigatto, C.; Komenda, P.; Bohm, C.; Sucha, E.; Bota, S.E.; Tuna, M.; Collister, D.; Sood, M.; et al. Hyperkalemia-Related Discontinuation of Renin-Angiotensin-Aldosterone System Inhibitors and Clinical Outcomes in CKD: A Population-Based Cohort Study. Am. J. Kidney Dis. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar] [CrossRef] [PubMed]
- Mottl, A.K.; Alicic, R.; Argyropoulos, C.; Brosius, F.C.; Mauer, M.; Molitch, M.; Nelson, R.G.; Perreault, L.; Nicholas, S.B. KDOQI US Commentary on the KDIGO 2020 Clinical Practice Guideline for Diabetes Management in CKD. Am. J. Kidney Dis. 2022, 79, 457–479. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Buchanan, L.A.; Ji, C.; Siani, A.; Miller, M.A. Systematic review and meta-analysis of randomised controlled trials on the effects of potassium supplements on serum potassium and creatinine. BMJ Open 2016, 6, e011716. [Google Scholar] [CrossRef] [Green Version]
- Gritter, M.; Wouda, R.; Yeung, S.; Wieers, M.; Geurts, F.; de Ridder, M.; Ramakers, C.; Vogt, L.; de Borst, M.; Rotmans, J.; et al. Effects of Short-Term Potassium Chloride Supplementation in Patients with Chronic Kidney Disease. J. Am. Soc. Nephrol. 2022, ASN.2022020147. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. 2017, 377, 1765–1776. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl. S1), S1–S107. [Google Scholar] [CrossRef]
- Borrelli, S.; De Nicola, L.; Minutolo, R.; Conte, G.; Chiodini, P.; Cupisti, A.; Santoro, D.; Calabrese, V.; Giannese, D.; Garofalo, C.; et al. Current Management of Hyperkalemia in Non-Dialysis CKD: Longitudinal Study of Patients Receiving Stable Nephrology Care. Nutrients 2021, 13, 942. [Google Scholar] [CrossRef] [PubMed]
- Bernier-Jean, A.; Wong, G.; Saglimbene, V.; Ruospo, M.; Palmer, S.C.; Natale, P.; Garcia-Larsen, V.; Johnson, D.W.; Tonelli, M.; Hegbrant, J.; et al. Dietary Potassium Intake and All-Cause Mortality in Adults Treated with Hemodialysis. Clin. J. Am. Soc. Nephrol. 2021, 16, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Noori, N.; Kalantar-Zadeh, K.; Kovesdy, C.P.; Murali, S.B.; Bross, R.; Nissenson, A.R.; Kopple, J.D. Dietary potassium intake and mortality in long-term hemodialysis patients. Am. J. Kidney Dis. 2010, 56, 338–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St-Jules, D.E.; Goldfarb, D.S.; Sevick, M.A. Nutrient Non-equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients? J. Renal Nutr. 2016, 26, 282–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathialahan, T.; Maclennan, K.A.; Sandle, L.N.; Verbeke, C.; Sandle, G.I. Enhanced large intestinal potassium permeability in end-stage renal disease. J. Pathol. 2005, 206, 46–51. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Mills, K.T.; Appel, L.J.; Yang, W.; Chen, J.; Lee, B.T.; Rosas, S.E.; Porter, A.; Makos, G.; Weir, M.R.; et al. Chronic Renal Insufficiency Cohort Study Investigators. Urinary Sodium and Potassium Excretion and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 1202–1212. [Google Scholar] [CrossRef]
- Kim, H.W.; Park, J.T.; Yoo, T.H.; Lee, J.; Chung, W.; Lee, K.B.; Chae, D.W.; Ahn, C.; Kang, S.W.; Choi, K.H.; et al. KNOW-CKD Study Investigators. Urinary Potassium Excretion and Progression of CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 330–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Grams, M.E.; Coresh, J.; Rebholz, C.M. Plant-Based Diets and Incident CKD and Kidney Function. Clin. J. Am. Soc. Nephrol. 2019, 14, 682–691. [Google Scholar] [CrossRef] [Green Version]
- Jhee, J.H.; Kee, Y.K.; Park, J.T.; Chang, T.I.; Kang, E.W.; Yoo, T.H.; Kang, S.W.; Han, S.H. A Diet Rich in Vegetables and Fruit and Incident CKD: A Community-Based Prospective Cohort Study. Am. J. Kidney Dis. 2019, 74, 491–500. [Google Scholar] [CrossRef]
- Kelly, J.T.; Palmer, S.C.; Wai, S.N.; Ruospo, M.; Carrero, J.J.; Campbell, K.L.; Strippoli, G.F.M. Healthy Dietary Patterns and Risk of Mortality and ESRD in CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2017, 12, 272–279. [Google Scholar] [CrossRef]
- Banerjee, T.; Carrero, J.J.; McCulloch, C.; Burrows, N.R.; Siegel, K.R.; Morgenstern, H.; Saran, R.; Powe, N.R.; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. Dietary Factors and Prevention: Risk of End-Stage Kidney Disease by Fruit and Vegetable Consumption. Am. J. Nephrol. 2021, 52, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.A.; Coresh, J.; Anderson, C.A.M.; Appel, L.J.; Grams, M.E.; Crews, D.C.; Mills, K.T.; He, J.; Scialla, J.; Rahman, M.; et al. Adherence to Healthy Dietary Patterns and Risk of CKD Progression and All-Cause Mortality: Findings from the CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2021, 77, 235–244. [Google Scholar] [CrossRef]
- Cupisti, A.; Kovesdy, C.P.; D’Alessandro, C.; Kalantar-Zadeh, K. Dietary Approach to Recurrent or Chronic Hyperkalaemia in Patients with Decreased Kidney Function. Nutrients 2018, 10, 261. [Google Scholar] [CrossRef] [Green Version]
- Carrero, J.J.; González-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, V.M.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin. J. Am. Soc. Nephrol. 2013, 8, 371–381. [Google Scholar] [CrossRef] [PubMed]
- St-Jules, D.E.; Clegg, D.J.; Palmer, B.F.; Carrero, J.J. Can Novel Potassium Binders Liberate People with Chronic Kidney Disease from the Low-Potassium Diet? A Cautionary Tale. Clin. J. Am. Soc. Nephrol. 2022, 17, 467–472. [Google Scholar] [CrossRef]
- Palmer, B.F.; Carrero, J.J.; Clegg, D.J.; Colbert, G.B.; Emmett, M.; Fishbane, S.; Hain, D.J.; Lerma, E.; Onuigbo, M.; Rastogi, A.; et al. Clinical Management of Hyperkalemia. Mayo Clin. Proc. 2021, 96, 744–762. [Google Scholar] [CrossRef]
- Noel, J.A.; Bota, S.E.41; Petrcich, W.; Garg, A.X.; Carrero, J.J.; Harel, Z.; Tangri, N.; Clark, E.G.; Komenda, P.; Sood, M.M. Risk of Hospitalization for Serious Adverse Gastrointestinal Events Associated With Sodium Polystyrene Sulfonate Use in Patients of Advanced Age. JAMA Intern. Med. 2019, 179, 1025–1033. [Google Scholar] [CrossRef]
- Laureati, P.; Xu, Y.; Trevisan, M.; Schalin, L.; Mariani, I.; Bellocco, R.; Sood, M.M.; Barany, P.; Sjolander, A.; Evans, M.; et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: A nationwide study. Nephrol. Dial. Transplant. 2020, 35, 1518–1526. [Google Scholar] [CrossRef] [Green Version]
- Pitt, B.; Anker, S.D.; Bushinsky, D.A.; Kitzman, D.W.; Zannad, F.; Huang, I.Z.; PEARL-HF Investigators. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur. Heart J. 2011, 32, 820–828. [Google Scholar] [CrossRef] [Green Version]
- Weir, M.R.; Bakris, G.L.; Bushinsky, D.A.; Mayo, M.R.; Garza, D.; Stasiv, Y.; Wittes, J.; Christ-Schmidt, H.; Berman, L.; Pitt, B.; et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N. Engl. J. Med. 2015, 372, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Bakris, G.L.; Pitt, B.; Weir, M.R.; Freeman, M.W.; Mayo, M.R.; Garza, D.; Stasiv, Y.; Zawadzki, R.; Berman, L.; Bushinsky, D.A.; et al. Effect of Patiromer on Serum Potassium Level in Patients with Hyperkalemia and Diabetic Kidney Disease: The AMETHYST-DN Randomized Clinical Trial. JAMA 2015, 314, 151–161. [Google Scholar] [CrossRef]
- Bakris, G.L.; Woods, S.D.; Alvarez, P.J.; Arthur, S.P.; Kumar, R. Hyperkalemia Management in Older Adults with Diabetic Kidney Disease Receiving Renin-Angiotensin-Aldosterone System Inhibitors: A Post Hoc Analysis of the AMETHYST-DN Clinical Trial. Kidney Med. 2021, 3, 360–367.e1. [Google Scholar] [CrossRef]
- Agarwal, R.; Rossignol, P.; Romero, A.; Garza, D.; Mayo, M.R.; Warren, S.; Ma, J.; White, W.B.; Williams, B. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019, 394, 1540–1550. [Google Scholar] [CrossRef]
- Patiromer for the Management of Hyperkalemia in Subjects Receiving RAASi for HFrEF—DIAMOND. Available online: https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2022/04/02/15/56/DIAMOND (accessed on 5 May 2022).
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. FIDELIO-DKD Investigators. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. FIGARO-DKD Investigators. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Packham, D.K.; Rasmussen, H.S.; Lavin, P.T.; El-Shahawy, M.A.; Roger, S.D.; Block, G.; Qunibi, W.; Pergola, P.; Singh, B. Sodium zirconium cyclosilicate in hyperkalemia. N. Engl. J. Med. 2015, 372, 222–231. [Google Scholar] [CrossRef] [Green Version]
- Kosiborod, M.; Rasmussen, H.S.; Lavin, P.; Qunibi, W.Y.; Spinowitz, B.; Packham, D.; Roger, S.D.; Yang, A.; Lerma, E.; Singh, B. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: The HARMONIZE randomized clinical trial. JAMA 2014, 312, 2223–2233, Erratum in JAMA 2015, 313, 526. [Google Scholar] [CrossRef]
- Spinowitz, B.S.; Fishbane, S.; Pergola, P.E.; Roger, S.D.; Lerma, E.V.; Butler, J.; von Haehling, S.; Adler, S.H.; Zhao, J.; Singh, B.; et al. ZS-005 Study Investigators. Sodium Zirconium Cyclosilicate among Individuals with Hyperkalemia: A 12-Month Phase 3 Study. Clin. J. Am. Soc. Nephrol. 2019, 14, 798–809. [Google Scholar] [CrossRef] [Green Version]
- Fishbane, S.; Ford, M.; Fukagawa, M.; McCafferty, K.; Rastogi, A.; Spinowitz, B.; Staroselskiy, K.; Vishnevskiy, K.; Lisovskaja, V.; Al-Shurbaji, A.; et al. A Phase 3b, Randomized, Double-Blind, Placebo-Controlled Study of Sodium Zirconium Cyclosilicate for Reducing the Incidence of Predialysis Hyperkalemia. J. Am. Soc. Nephrol. 2019, 30, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Bilbrey, G.L.; Carter, N.W.; White, M.G.; Schilling, J.F.; Knochel, J.P. Potassium deficiency in chronic renal failure. Kidney Int. 1973, 4, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Bastl, C.; Hayslett, J.P.; Binder, H.J. Increased large intestinal secretion of potassium in renal insufficiency. Kidney Int. 1977, 12, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Basile, C.; Libutti, P.; Lisi, P.; Teutonico, A.; Vernaglione, L.; Casucci, F.; Lomonte, C. Ranking of factors determining potassium mass balance in bicarbonate haemodialysis. Nephrol. Dial. Transplant. 2015, 30, 505–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pun, P.H. Dialysate potassium concentration: Should mass balance trump electrophysiology? Semin. Dial. 2018, 31, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Pun, P.H.; Lehrich, R.W.; Honeycutt, E.F.; Herzog, C.A.; Middleton, J.P. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011, 79, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Brunelli, S.M.; Spiegel, D.M.; Du Mond, C.; Oestreicher, N.; Winkelmayer, W.C.; Kovesdy, C.P. Serum-to-dialysate potassium gradient and its association with short-term outcomes in hemodialysis patients. Nephrol. Dial. Transplant. 2018, 33, 1207–1214. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Ogata, C.; Aihara, N.; Sasaki, O.; Yoshihara, F.; Nakahama, H.; Inenaga, T.; Kimura, G.; Kawano, Y. QTc dispersion in haemodialysis patients with cardiac complications. Nephrology 2005, 10, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Galetta, F.; Caprioli, R.; Morelli, E.; Tintori, G.C.; Franzoni, F.; Lippi, A.; Meola, M.; Rindi, P.; Barsotti, G. Potassium removal increases the QTc interval dispersion during hemodialysis. Nephron 1999, 82, 122–126. [Google Scholar] [CrossRef]
- Santoro, A.; Mancini, E.; London, G.; Mercadal, L.; Fessy, H.; Perrone, B.; Cagnoli, L.; Grandi, E.; Severi, S.; Cavalcanti, S. Patients with complex arrhythmias during and after haemodialysis suffer from different regimens of potassium removal. Nephrol. Dial. Transplant. 2008, 23, 1415–1421. [Google Scholar] [CrossRef] [Green Version]
- Blumberg, A.; Roser, H.W.; Zehnder, C.; Müller-Brand, J. Plasma potassium in patients with terminal renal failure during and after haemodialysis; relationship with dialytic potassium removal and total body potassium. Nephrol. Dial. Transplant. 1997, 12, 1629–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nicola, L.; Bellizzi, V.; Minutolo, R.; Cioffi, M.; Giannattasio, P.; Terracciano, V.; Iodice, C.; Uccello, F.; Memoli, B.; Di Iorio, B.R.; et al. Effect of dialysate sodium concentration on interdialytic increase of potassium. J. Am. Soc. Nephrol. 2000, 11, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Conte, G.; Dal Canton, A.; Imperatore, P.; De Nicola, L.; Gigliotti, G.; Pisanti, N.; Memoli, B.; Fuiano, G.; Esposito, C.; Andreucci, V.E. Acute increase in plasma osmolality as a cause of hyperkalemia in patients with renal failure. Kidney Int. 1990, 38, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrelli, S.; Matarazzo, I.; Lembo, E.; Peccarino, L.; Annoiato, C.; Scognamiglio, M.R.; Foderini, A.; Ruotolo, C.; Franculli, A.; Capozzi, F.; et al. Chronic Hyperkaliemia in Chronic Kidney Disease: An Old Concern with New Answers. Int. J. Mol. Sci. 2022, 23, 6378. https://doi.org/10.3390/ijms23126378
Borrelli S, Matarazzo I, Lembo E, Peccarino L, Annoiato C, Scognamiglio MR, Foderini A, Ruotolo C, Franculli A, Capozzi F, et al. Chronic Hyperkaliemia in Chronic Kidney Disease: An Old Concern with New Answers. International Journal of Molecular Sciences. 2022; 23(12):6378. https://doi.org/10.3390/ijms23126378
Chicago/Turabian StyleBorrelli, Silvio, Ida Matarazzo, Eugenio Lembo, Laura Peccarino, Claudia Annoiato, Maria Rosaria Scognamiglio, Andrea Foderini, Chiara Ruotolo, Aldo Franculli, Federica Capozzi, and et al. 2022. "Chronic Hyperkaliemia in Chronic Kidney Disease: An Old Concern with New Answers" International Journal of Molecular Sciences 23, no. 12: 6378. https://doi.org/10.3390/ijms23126378
APA StyleBorrelli, S., Matarazzo, I., Lembo, E., Peccarino, L., Annoiato, C., Scognamiglio, M. R., Foderini, A., Ruotolo, C., Franculli, A., Capozzi, F., Yavorskiy, P., Merheb, F., Provenzano, M., La Manna, G., De Nicola, L., Minutolo, R., & Garofalo, C. (2022). Chronic Hyperkaliemia in Chronic Kidney Disease: An Old Concern with New Answers. International Journal of Molecular Sciences, 23(12), 6378. https://doi.org/10.3390/ijms23126378








