Genome-Wide Identification of Switchgrass Laccases Involved in Lignin Biosynthesis and Heavy-Metal Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification and Characterization of the Laccase Gene Family in Switchgrass
2.2. Phylogenetic and Gene Duplication Analysis of the Laccase Proteins
2.3. Gene Structure Analysis and Identification of Conserved Motifs as Well as Cis-Acting Elements
2.4. PvLac Expression Profiles in Various Switchgrass Tissues
2.5. Plant Materials and Treatments
2.6. RNA Extraction and qRT-PCR Analysis
2.7. Protein Extraction and Enzyme Activity Assay
2.8. Lignin Analysis
3. Results
3.1. Identification and Characterization of LACs in the Switchgrass Genome
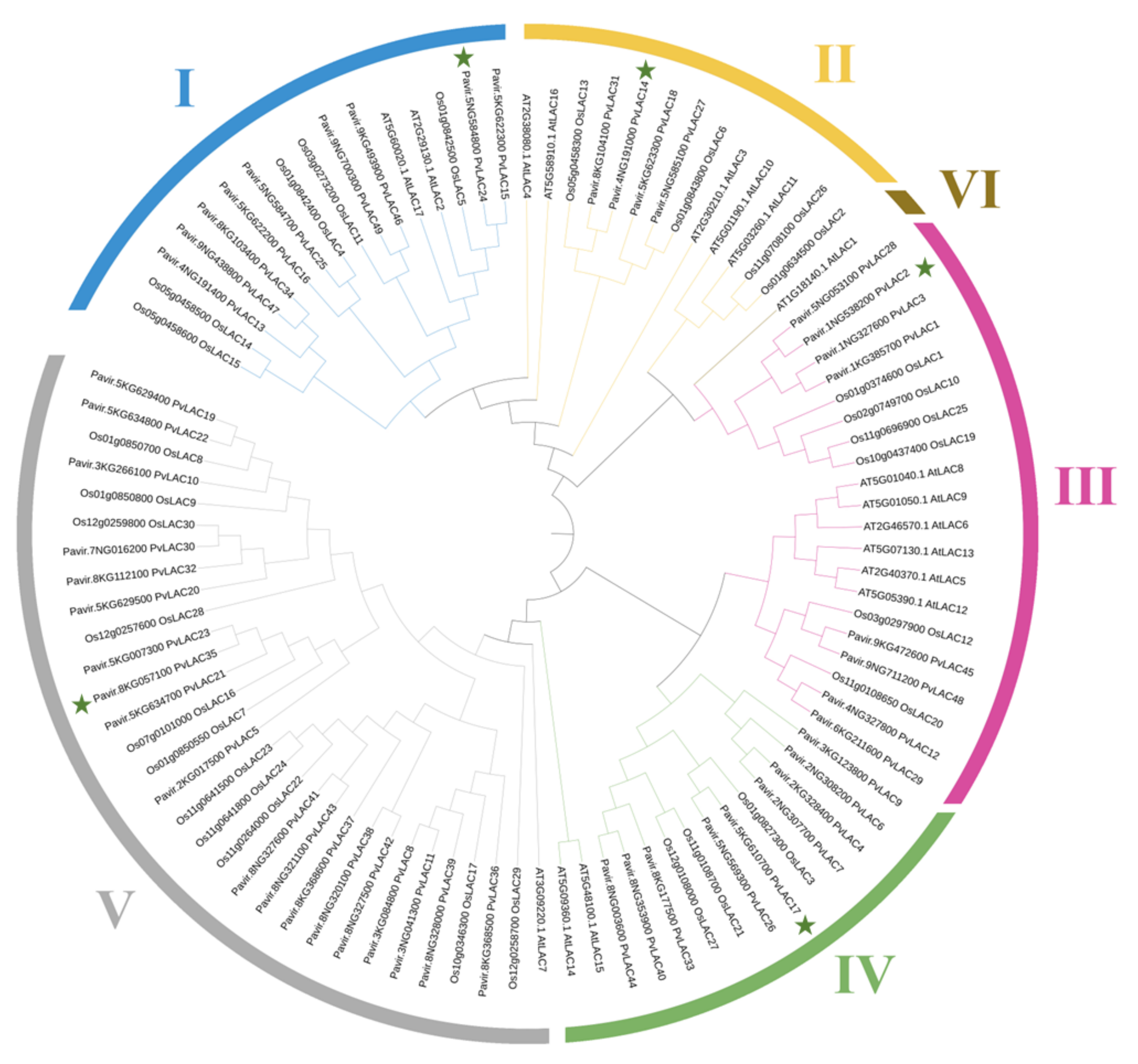
3.2. Phylogenetic Analysis of PvLac Family
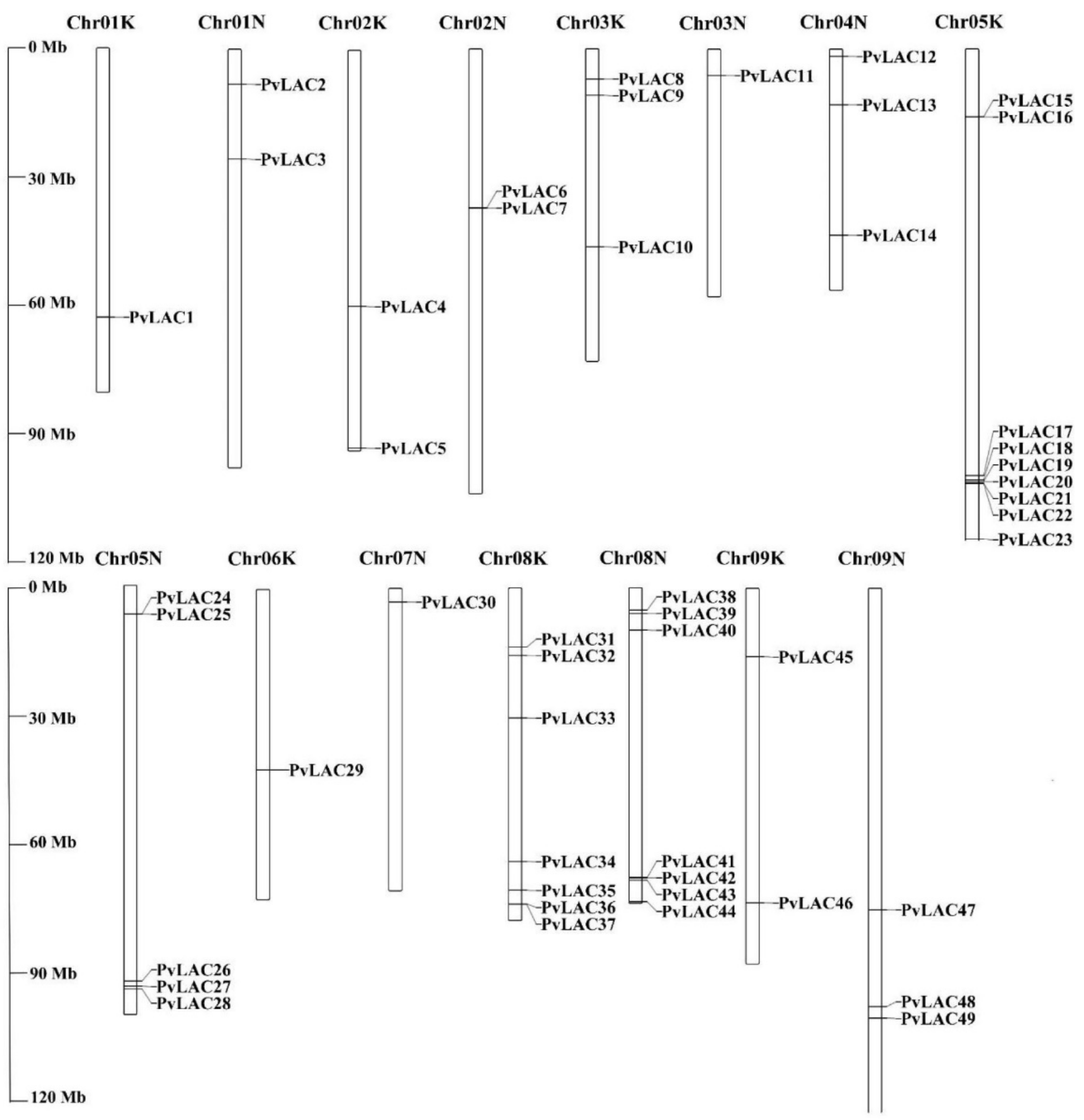
3.3. Chromosomal Locations and Duplication Events in PvLac Gene Family
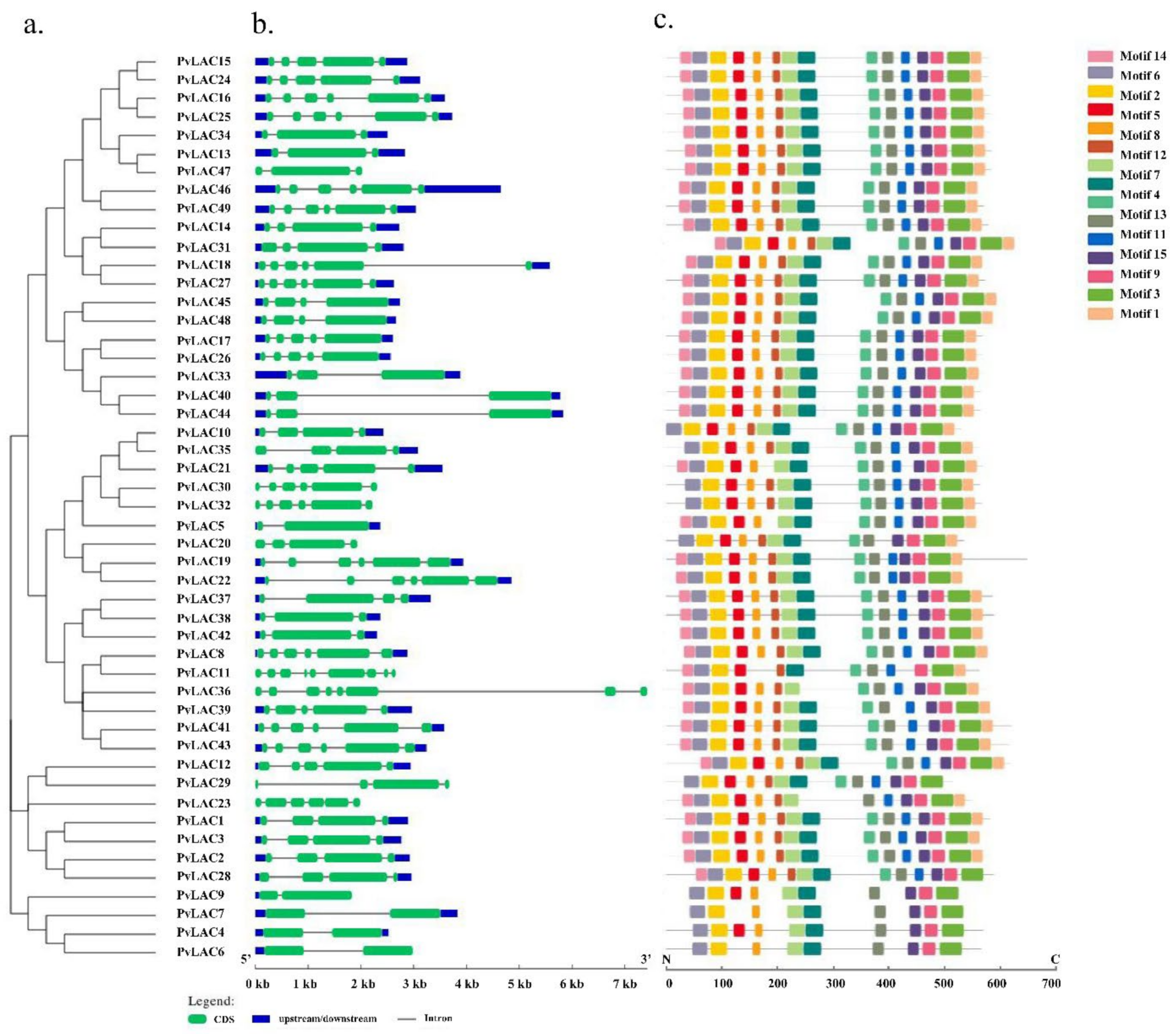
3.4. Gene Structure, miRNA Target Site Prediction and Conserved Motif Analysis of PvLac Family
3.5. Cis-Elements Analysis of PvLac Promoters
3.6. Expression Patterns of PvLac Genes in Different Tissues
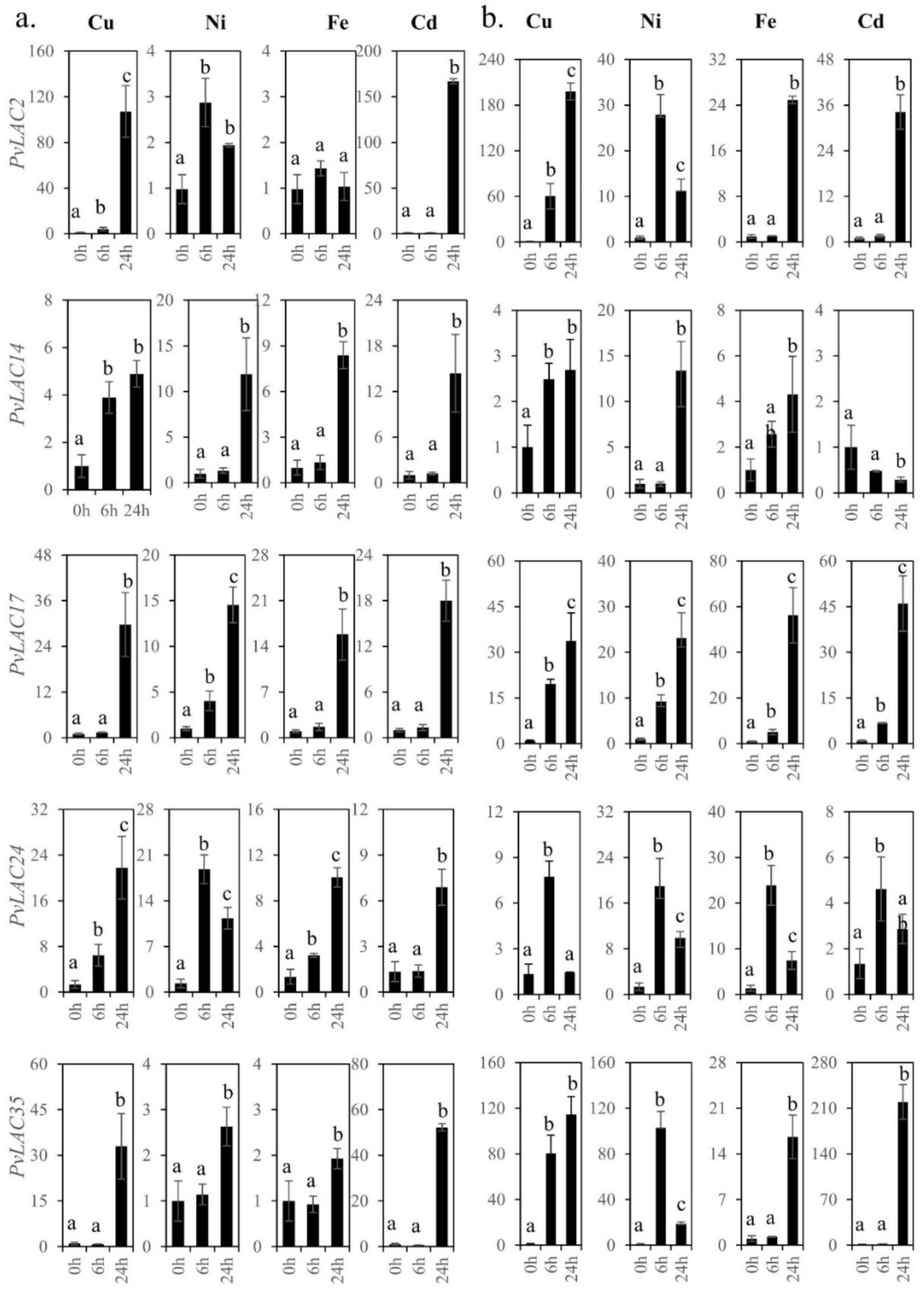
3.7. Expression Patterns of Five PvLac Genes in Response to Various Heavy-Mental Treatments
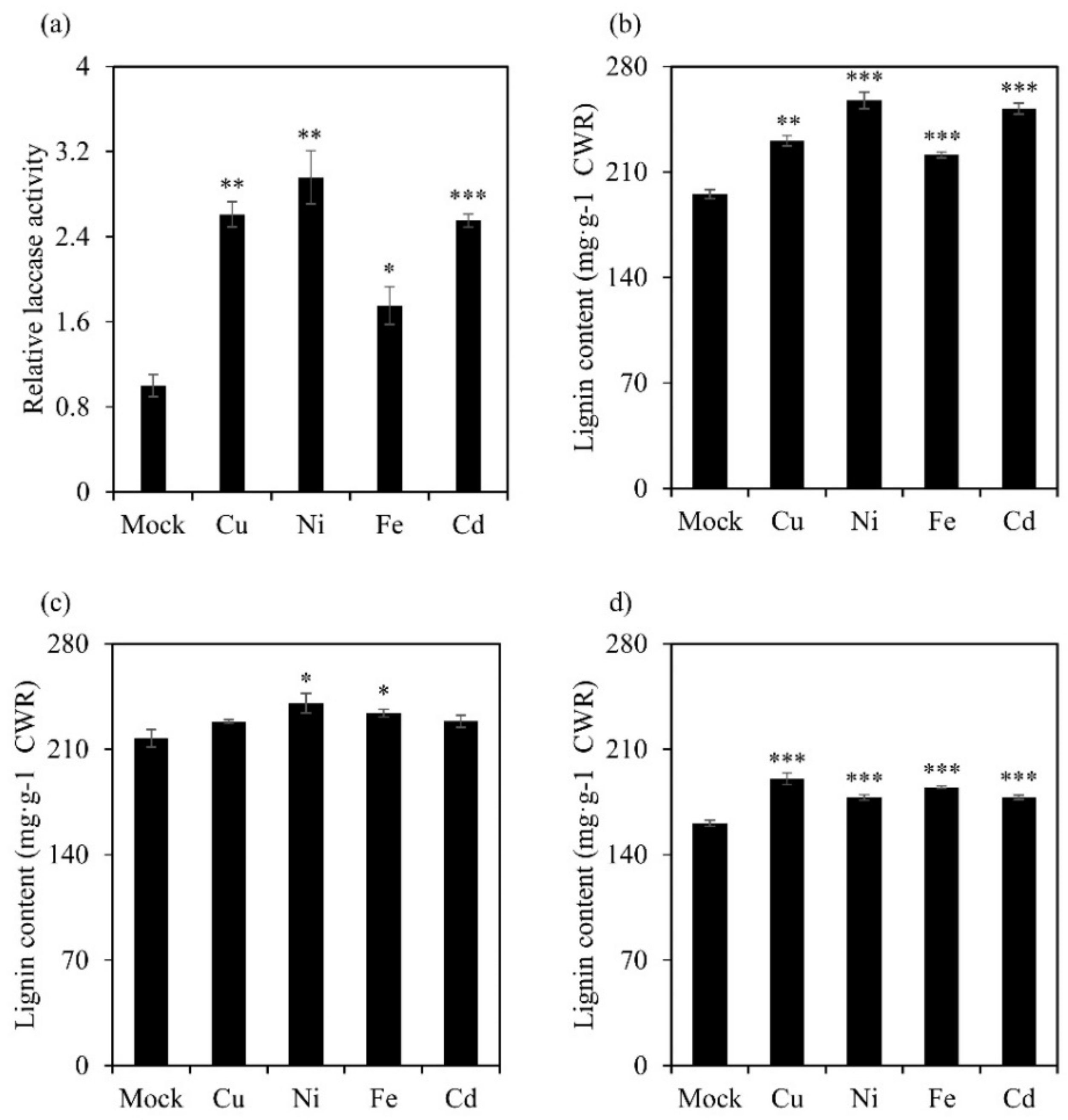
3.8. Heavy-Metal Treatment Induced Both Lignin Content and Laccase Activity in Switchgrass
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Lac | Laccases |
| COMT | Caffeic acid 3-O-methyltransferase |
| CCoAOMT | Caffeyl CoA 3-O-methyltransferase |
References
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.; Jaiswal, N.; Singh, S.; Pandey, V.P.; Dwivedi, U.N. Molecular docking and dynamics simulation analyses unraveling the differential enzymatic catalysis by plant and fungal laccases with respect to lignin biosynthesis and degradation. J. Biomol. Struct. Dyn. 2015, 33, 1835–1849. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Swiderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkolazka, A.; Paszczynski, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Davis, E.J.; Ballif, J.; Liang, M.; Bushman, E.; Haroldsen, V.; Torabinejad, J.; Wu, Y. Mutant identification and characterization of the laccase gene family in Arabidopsis. J. Exp. Bot. 2006, 57, 2563–2569. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Wang, B.; Ding, L.; Wang, Y.; Luo, L.; Zhang, Y.; Kong, W. Genome-wide identification and characterization of laccase gene family in Citrus sinensis. Gene 2019, 689, 114–123. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Wang, X.; Shen, Z.; Zheng, L. Comprehensive Analysis of Rice Laccase Gene (OsLAC) Family and Ectopic Expression of OsLAC10 Enhances Tolerance to Copper Stress in Arabidopsis. Int. J. Mol. Sci. 2017, 18, 209. [Google Scholar] [CrossRef]
- Simoes, M.S.; Carvalho, G.G.; Ferreira, S.S.; Hernandes-Lopes, J.; de Setta, N.; Cesarino, I. Genome-wide characterization of the laccase gene family in Setaria viridis reveals members potentially involved in lignification. Planta 2020, 251, 46. [Google Scholar] [CrossRef]
- Wang, J.; Feng, J.; Jia, W.; Fan, P.; Bao, H.; Li, S.; Li, Y. Genome-Wide Identification of Sorghum bicolor Laccases Reveals Potential Targets for Lignin Modification. Front. Plant Sci. 2017, 8, 714. [Google Scholar] [CrossRef]
- Zhao, Q.; Nakashima, J.; Chen, F.; Yin, Y.; Fu, C.; Yun, J.; Shao, H.; Wang, X.; Wang, Z.Y.; Dixon, R.A. Laccase is necessary and nonredundant with peroxidase for lignin polymerization during vascular development in Arabidopsis. Plant Cell 2013, 25, 3976–3987. [Google Scholar] [CrossRef]
- He, F.; Machemer-Noonan, K.; Golfier, P.; Unda, F.; Dechert, J.; Zhang, W.; Hoffmann, N.; Samuels, L.; Mansfield, S.D.; Rausch, T.; et al. The in vivo impact of MsLAC1, a Miscanthus laccase isoform, on lignification and lignin composition contrasts with its in vitro substrate preference. BMC Plant Biol. 2019, 19, 552. [Google Scholar] [CrossRef]
- Le Bris, P.; Wang, Y.; Barbereau, C.; Antelme, S.; Cezard, L.; Legee, F.; D’Orlando, A.; Dalmais, M.; Bendahmane, A.; Schuetz, M.; et al. Inactivation of LACCASE8 and LACCASE5 genes in Brachypodium distachyon leads to severe decrease in lignin content and high increase in saccharification yield without impacting plant integrity. Biotechnol. Biofuels 2019, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Fan, C.; Li, X.; Li, Y.; Hu, J.; Li, C.; Luo, K. LACCASE14 is required for the deposition of guaiacyl lignin and affects cell wall digestibility in poplar. Biotechnol. Biofuels 2020, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Hiraide, H.; Tobimatsu, Y.; Yoshinaga, A.; Lam, P.Y.; Kobayashi, M.; Matsushita, Y.; Fukushima, K.; Takabe, K. Localised laccase activity modulates distribution of lignin polymers in gymnosperm compression wood. New Phytol. 2021, 230, 2186–2199. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhuo, C.; Xiao, X.; Wang, X.; Docampo-Palacios, M.; Chen, F.; Dixon, R.A. Substrate Specificity of LACCASE8 Facilitates Polymerization of Caffeyl Alcohol for C-Lignin Biosynthesis in the Seed Coat of Cleome hassleriana. Plant Cell 2020, 32, 3825–3845. [Google Scholar] [CrossRef]
- Goyal, D.; Yadav, A.; Prasad, M.; Singh, T.B.; Shrivastav, P.; Ali, A.; Dantu, P.K.; Mishra, S. Effect of Heavy Metals on Plant Growth: An Overview. In Contaminants in Agriculture: Sources, Impacts and Management; Naeem, M., Ansari, A.A., Gill, S.S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 79–101. [Google Scholar]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Su, N.; Ling, F.; Xing, A.; Zhao, H.; Zhu, Y.; Wang, Y.; Deng, X.; Wang, C.; Xu, X.; Hu, Z.; et al. Lignin synthesis mediated by CCoAOMT enzymes is required for the tolerance against excess Cu in Oryza sativa. Environ. Exp. Bot. 2020, 175, 104059. [Google Scholar] [CrossRef]
- Kováčik, J.; Bačkor, M. Phenylalanine Ammonia-Lyase and Phenolic Compounds in Chamomile Tolerance to Cadmium and Copper Excess. Water Air Soil Pollut. 2007, 185, 185–193. [Google Scholar] [CrossRef]
- Tahara, K.; Norisada, M.; Hogetsu, T.; Kojima, K. Aluminum tolerance and aluminum-induced deposition of callose and lignin in the root tips of Melaleuca and Eucalyptus species. J. For. Res.-Jpn. 2005, 10, 325–333. [Google Scholar] [CrossRef]
- Van de Mortel, J.E.; Almar Villanueva, L.; Schat, H.; Kwekkeboom, J.; Coughlan, S.; Moerland, P.D.; Ver Loren Van Themaat, E.; Koornneef, M.; Aarts, M.G.M. Large Expression Differences in Genes for Iron and Zinc Homeostasis, Stress Response, and Lignin Biosynthesis Distinguish Roots of Arabidopsis thaliana and the Related Metal Hyperaccumulator Thlaspi caerulescens. Plant Physiol. 2006, 142, 1127–1147. [Google Scholar] [CrossRef]
- Fu, C.; Mielenz, J.R.; Xiao, X.; Ge, Y.; Hamilton, C.Y.; Rodriguez, M.; Chen, F.; Foston, M.; Ragauskas, A.; Bouton, J.; et al. Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc. Natl. Acad. Sci. USA 2011, 108, 3803–3808. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, N.; Hisano, H.; Cao, Y.; Wu, F.; Liu, W.; Bao, Y.; Wang, Z.Y.; Fu, C. Simultaneous regulation of F5H in COMT-RNAi transgenic switchgrass alters effects of COMT suppression on syringyl lignin biosynthesis. Plant Biotechnol. J. 2019, 17, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Yuan, S.; Wen, X.; Xie, Z.; Lou, L.; Hu, B.; Cai, Q.; Xu, B. Transcriptome analysis of Cd-treated switchgrass root revealed novel transcripts and the importance of HSF/HSP network in switchgrass Cd tolerance. Plant Cell Rep. 2018, 37, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Cary, T.J.; Rylott, E.L.; Zhang, L.; Routsong, R.M.; Palazzo, A.J.; Strand, S.E.; Bruce, N.C. Field trial demonstrating phytoremediation of the military explosive RDX by XplA/XplB-expressing switchgrass. Nat. Biotechnol. 2021, 39, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef]
- Moore, K.J.; Moser, L.E.; Vogel, K.P.; Waller, S.S.; Johnson, B.E.; Pedersen, J.F. Describing and quantifying growth stages of perennial forage grasses. Agron. J. 1991, 83, 1073–1077. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, L.; He, F.; Zhao, F.; Shen, Z.; Zheng, L. Transcriptional and physiological analyses identify a regulatory role for hydrogen peroxide in the lignin biosynthesis of copper-stressed rice roots. Plant Soil. 2015, 387, 323–336. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Turlapati, P.V.; Kim, K.W.; Davin, L.B.; Lewis, N.G. The laccase multigene family in Arabidopsis thaliana: Towards addressing the mystery of their gene function(s). Planta 2011, 233, 439–470. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, X.; Zhong, R.; Liu, B.; Zhang, X.; Fang, F.; Zhang, Z.; Pang, X. Laccase-Mediated Flavonoid Polymerization Leads to the Pericarp Browning of Litchi Fruit. J. Agric. Food Chem. 2021, 69, 15218–15230. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, K.; Wang, S.; Lou, Y.; Zhu, C.; Gao, Z. Genome-wide analysis of laccase genes in moso bamboo highlights PeLAC10 involved in lignin biosynthesis and in response to abiotic stresses. Plant Cell Rep. 2020, 39, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lin, J.; Dong, F.; Ma, Q.; Wu, S.; Ma, X.; Fatima, M.; Jia, H.; Ming, R. Genomic and Allelic Analyses of Laccase Genes in Sugarcane (Saccharum spontaneum L.). Trop. Plant Biol. 2019, 12, 219–229. [Google Scholar] [CrossRef]
- Lu, S.; Li, Q.; Wei, H.; Chang, M.; Tunlaya-Anukit, S.; Kim, H.; Liu, J.; Song, J.; Sun, Y.; Yuan, L.; et al. Ptr-miR397a is a negative regulator of laccase genes affecting lignin content in Populus trichocarpa. Proc. Natl. Acad. Sci. USA 2013, 110, 10848–10853. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, G.; Zheng, K.; Zhu, X.; Ma, J.; Wang, D.; Tang, K.; Feng, X.; Leng, J.; Yu, H.; et al. The Soybean Laccase Gene Family: Evolution and Possible Roles in Plant Defense and Stem Strength Selection. Genes 2019, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Caparrós-Ruiz, D.; Fornalé, S.; Civardi, L.; Puigdomènech, P.; Rigau, J. Isolation and characterisation of a family of laccases in maize. Plant Sci. 2006, 171, 217–225. [Google Scholar] [CrossRef]
- He, F. Exploring the Function of Laccases from Miscanthus Sinensis in Lignin Biosynthesis. Ph.D. Thesis, Heidelberg University, Heidelberg, Germany, 2019. [Google Scholar]
- Ranocha, P.; Chabannes, M.; Chamayou, S.; Danoun, S.; Jauneau, A.; Boudet, A.M.; Goffner, D. Laccase down-regulation causes alterations in phenolic metabolism and cell wall structure in poplar. Plant Physiol. 2002, 129, 145–155. [Google Scholar] [CrossRef]
- Liang, M.; Davis, E.; Gardner, D.; Cai, X.; Wu, Y. Involvement of AtLAC15 in lignin synthesis in seeds and in root elongation of Arabidopsis. Planta 2006, 224, 1185–1196. [Google Scholar] [CrossRef]
- Khandal, H.; Singh, A.P.; Chattopadhyay, D. The MicroRNA397b -LACCASE2 Module Regulates Root Lignification under Water and Phosphate Deficiency. Plant Physiol. 2020, 182, 1387–1403. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Shan, X. Adsorption of metal ions on lignin. J. Hazard. Mater. 2008, 151, 134–142. [Google Scholar] [CrossRef]
- Ederli, L.; Reale, L.; Ferranti, F.; Pasqualini, S. Responses induced by high concentration of cadmium in Phragmites australis roots. Physiol. Plantarum. 2004, 121, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G.; Komatsu, S. Toxicity of heavy metals and metal-containing nanoparticles on plants. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2016, 1864, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, L.; Liu, Z. Rapid effect of cadmium on lignin biosynthesis in soybean roots. Plant Sci. 2007, 172, 632–639. [Google Scholar] [CrossRef]
- Bernal, M.; Kramer, U. Involvement of Arabidopsis Multi-Copper Oxidase-Encoding LACCASE12 in Root-to-Shoot Iron Partitioning: A Novel Example of Copper-Iron Crosstalk. Front. Plant Sci. 2021, 12, 688318. [Google Scholar] [CrossRef]


| Gene Name | Gene ID | Number of Amino Acids (aa) | Molecular Weight (kDa) | Signal Peptide Cleavage Site | Signal Peptide Length | ORF Length | pI |
|---|---|---|---|---|---|---|---|
| PvLAC1 | Pavir.1KG385700 | 581 | 63.6 | VAA-TP | 24 | 1746 | 6.76 |
| PvLAC2 | Pavir.1NG538200 | 581 | 64.0 | VAA-SP | 24 | 1746 | 6.39 |
| PvLAC3 | Pavir.1NG327600 | 575 | 63.0 | AQA-LR | 24 | 1728 | 6.62 |
| PvLAC4 | Pavir.2KG328400 | 570 | 62.4 | ARA-AT | 25 | 1713 | 6.95 |
| PvLAC5 | Pavir.2KG017500 | 569 | 62.1 | ADA-AV | 24 | 1710 | 8.55 |
| PvLAC6 | Pavir.2NG308200 | 566 | 62.4 | ARA-AT | 24 | 1701 | 6.81 |
| PvLAC7 | Pavir.2NG307700 | 568 | 62.4 | ARA-AT | 20 | 1706 | 8.84 |
| PvLAC8 | Pavir.3KG084800 | 619 | 68.5 | TMA-LP | 25 | 1860 | 7.09 |
| PvLAC9 | Pavir.3KG123800 | 560 | 61.2 | AEA-KV | 19 | 1683 | 6.51 |
| PvLAC10 | Pavir.3KG266100 | 530 | 58.2 | / | / | 1593 | 5.19 |
| PvLAC11 | Pavir.3NG041300 | 563 | 62.1 | TMA-LP | 22 | 1692 | 6.44 |
| PvLAC12 | Pavir.4NG327800 | 619 | 67.1 | / | / | 1860 | 8.98 |
| PvLAC13 | Pavir.4NG191400 | 585 | 63.0 | AEA-ET | 32 | 1758 | 6.92 |
| PvLAC14 | Pavir.4NG191000 | 579 | 62.6 | AAA-RT | 29 | 1740 | 9.78 |
| PvLAC15 | Pavir.5KG622300 | 578 | 62.9 | AEG-AI | 24 | 1737 | 7.64 |
| PvLAC16 | Pavir.5KG622200 | 582 | 63.3 | VQG-IT | 28 | 1749 | 8.83 |
| PvLAC17 | Pavir.5KG610700 | 569 | 62.7 | AGA-EV | 22 | 1710 | 5.90 |
| PvLAC18 | Pavir.5KG623300 | 580 | 63.4 | AAG-DT | 34 | 1743 | 9.05 |
| PvLAC19 | Pavir.5KG629400 | 649 | 67.6 | ADA-AT | 16 | 1950 | 5.81 |
| PvLAC20 | Pavir.5KG629500 | 535 | 58.0 | / | / | 1608 | 5.95 |
| PvLAC21 | Pavir.5KG634700 | 570 | 60.7 | AHA-AT | 18 | 1713 | 5.82 |
| PvLAC22 | Pavir.5KG634800 | 647 | 67.2 | ADA-AT | 16 | 1944 | 6.10 |
| PvLAC23 | Pavir.5KG007300 | 549 | 60.6 | VSS-AE | 22 | 1650 | 9.63 |
| PvLAC24 | Pavir.5NG584800 | 578 | 63.0 | TEG-AI | 24 | 1737 | 7.21 |
| PvLAC25 | Pavir.5NG584700 | 584 | 63.4 | VQG-IT | 29 | 1755 | 8.73 |
| PvLAC26 | Pavir.5NG569300 | 569 | 62.8 | AGA-EV | 22 | 1710 | 5.97 |
| PvLAC27 | Pavir.5NG585100 | 573 | 62.8 | AAG-DT | 27 | 1722 | 8.87 |
| PvLAC28 | Pavir.5NG053100 | 589 | 65.5 | / | / | 1770 | 6.60 |
| PvLAC29 | Pavir.6KG211600 | 516 | 55.8 | / | / | 1551 | 6.24 |
| PvLAC30 | Pavir.7NG016200 | 564 | 62.2 | GHA-AT | 18 | 1695 | 8.76 |
| PvLAC31 | Pavir.8KG104100 | 637 | 68.7 | / | / | 1914 | 9.77 |
| PvLAC32 | Pavir.8KG112100 | 567 | 62.3 | GRA-AI | 19 | 1704 | 9.05 |
| PvLAC33 | Pavir.8KG177500 | 573 | 61.9 | VVA-KE | 25 | 1722 | 6.43 |
| PvLAC34 | Pavir.8KG103400 | 583 | 62.8 | AEA-ET | 29 | 1752 | 7.67 |
| PvLAC35 | Pavir.8KG057100 | 563 | 61.5 | / | / | 1692 | 5.72 |
| PvLAC36 | Pavir.8KG368500 | 576 | 63.3 | AMA-AV | 28 | 1731 | 5.86 |
| PvLAC37 | Pavir.8KG368600 | 587 | 64.7 | GEA-AV | 22 | 1764 | 6.04 |
| PvLAC38 | Pavir.8NG320100 | 589 | 65.0 | GEA-AV | 24 | 1770 | 6.33 |
| PvLAC39 | Pavir.8NG328000 | 596 | 65.2 | AAG-AV | 28 | 1791 | 6.43 |
| PvLAC40 | Pavir.8NG353900 | 565 | 61.4 | VVA-KE | 22 | 1698 | 6.62 |
| PvLAC41 | Pavir.8NG327600 | 621 | 69.3 | AVA-AS | 24 | 1866 | 6.13 |
| PvLAC42 | Pavir.8NG327500 | 589 | 65.3 | GEA-AV | 24 | 1770 | 6.11 |
| PvLAC43 | Pavir.8NG321100 | 617 | 68.8 | AVA-AS | 23 | 1854 | 6.10 |
| PvLAC44 | Pavir.8NG003600 | 565 | 61.4 | VVA-KE | 22 | 1698 | 6.62 |
| PvLAC45 | Pavir.9KG472600 | 607 | 66.0 | ALA-EE | 28 | 1824 | 8.58 |
| PvLAC46 | Pavir.9KG493900 | 571 | 62.3 | SHG-AT | 22 | 1716 | 8.64 |
| PvLAC47 | Pavir.9NG438800 | 585 | 63.0 | AEA-ET | 32 | 1758 | 6.92 |
| PvLAC48 | Pavir.9NG711200 | 601 | 65.3 | ALA-EE | 26 | 1806 | 8.43 |
| PvLAC49 | Pavir.9NG700300 | 571 | 62.3 | SHG-AT | 22 | 1716 | 8.74 |
| Gene Name | Hormones-Responsive | Stress-Responsive | Flavonoid Biosynthetic | Circadian Control | Light Response | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MeJA | Auxin | Abscisic Acid | Gibberellin | Salicylic Acid | Low Temperature | Stress and Defense | Drought-Inducibility | ||||
| PvLAC1 | + | + | + | + | + | ||||||
| PvLAC2 | + | + | + | + | + | ||||||
| PvLAC3 | + | + | + | + | + | + | |||||
| PvLAC4 | + | + | + | + | + | ||||||
| PvLAC5 | + | + | + | + | + | + | + | + | + | + | |
| PvLAC6 | + | + | + | + | + | ||||||
| PvLAC7 | + | + | + | + | + | + | + | + | |||
| PvLAC8 | + | + | + | + | + | ||||||
| PvLAC9 | + | + | + | + | + | + | + | ||||
| PvLAC10 | + | + | + | + | + | + | + | ||||
| PvLAC11 | + | + | + | + | + | + | |||||
| PvLAC12 | + | + | + | + | + | + | + | + | + | + | |
| PvLAC13 | + | + | + | + | + | + | + | + | |||
| PvLAC14 | + | + | + | + | + | ||||||
| PvLAC15 | + | + | + | + | + | + | + | ||||
| PvLAC16 | + | + | + | + | + | ||||||
| PvLAC17 | + | + | + | + | + | + | + | + | |||
| PvLAC18 | + | + | + | + | + | ||||||
| PvLAC19 | + | + | + | + | + | + | + | + | |||
| PvLAC20 | + | + | + | + | + | + | + | ||||
| PvLAC21 | + | + | + | + | + | ||||||
| PvLAC22 | + | + | + | + | + | + | + | + | + | ||
| PvLAC23 | + | + | + | + | + | ||||||
| PvLAC24 | + | + | + | + | + | + | + | ||||
| PvLAC25 | + | + | + | + | + | + | |||||
| PvLAC26 | + | + | + | + | + | + | + | ||||
| PvLAC27 | + | + | + | + | + | + | |||||
| PvLAC28 | + | + | + | + | + | ||||||
| PvLAC29 | + | + | + | + | + | ||||||
| PvLAC30 | + | + | + | + | + | + | + | ||||
| PvLAC31 | + | + | + | + | + | + | + | ||||
| PvLAC32 | + | + | + | + | + | + | |||||
| PvLAC33 | + | + | + | + | + | + | |||||
| PvLAC34 | + | + | + | + | + | + | + | ||||
| PvLAC35 | + | + | + | + | + | + | + | + | + | ||
| PvLAC36 | + | + | + | + | + | + | |||||
| PvLAC37 | + | + | + | + | + | + | + | ||||
| PvLAC38 | + | + | + | + | + | + | + | ||||
| PvLAC39 | + | + | + | + | + | ||||||
| PvLAC40 | + | + | + | + | + | ||||||
| PvLAC41 | + | + | + | + | + | + | |||||
| PvLAC42 | + | + | + | + | + | ||||||
| PvLAC43 | + | + | + | + | + | + | |||||
| PvLAC44 | + | + | + | + | + | ||||||
| PvLAC45 | + | + | + | + | + | ||||||
| PvLAC46 | + | + | + | + | + | + | |||||
| PvLAC47 | + | + | + | + | + | + | + | ||||
| PvLAC48 | + | + | + | + | + | + | |||||
| PvLAC49 | + | + | + | + | + | + | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Zhao, Y.; Sun, Z.; Wu, Z.; Wang, H.; Fu, C.; Zhao, H.; He, F. Genome-Wide Identification of Switchgrass Laccases Involved in Lignin Biosynthesis and Heavy-Metal Responses. Int. J. Mol. Sci. 2022, 23, 6530. https://doi.org/10.3390/ijms23126530
Li R, Zhao Y, Sun Z, Wu Z, Wang H, Fu C, Zhao H, He F. Genome-Wide Identification of Switchgrass Laccases Involved in Lignin Biosynthesis and Heavy-Metal Responses. International Journal of Molecular Sciences. 2022; 23(12):6530. https://doi.org/10.3390/ijms23126530
Chicago/Turabian StyleLi, Rui, Yan Zhao, Zhen Sun, Zhenying Wu, Honglun Wang, Chunxiang Fu, Hongbo Zhao, and Feng He. 2022. "Genome-Wide Identification of Switchgrass Laccases Involved in Lignin Biosynthesis and Heavy-Metal Responses" International Journal of Molecular Sciences 23, no. 12: 6530. https://doi.org/10.3390/ijms23126530
APA StyleLi, R., Zhao, Y., Sun, Z., Wu, Z., Wang, H., Fu, C., Zhao, H., & He, F. (2022). Genome-Wide Identification of Switchgrass Laccases Involved in Lignin Biosynthesis and Heavy-Metal Responses. International Journal of Molecular Sciences, 23(12), 6530. https://doi.org/10.3390/ijms23126530






