Changes in Rhizosphere Soil Fungal Communities of Pinus tabuliformis Plantations at Different Development Stages on the Loess Plateau
Abstract
:1. Introduction
2. Results
2.1. Soil Properties
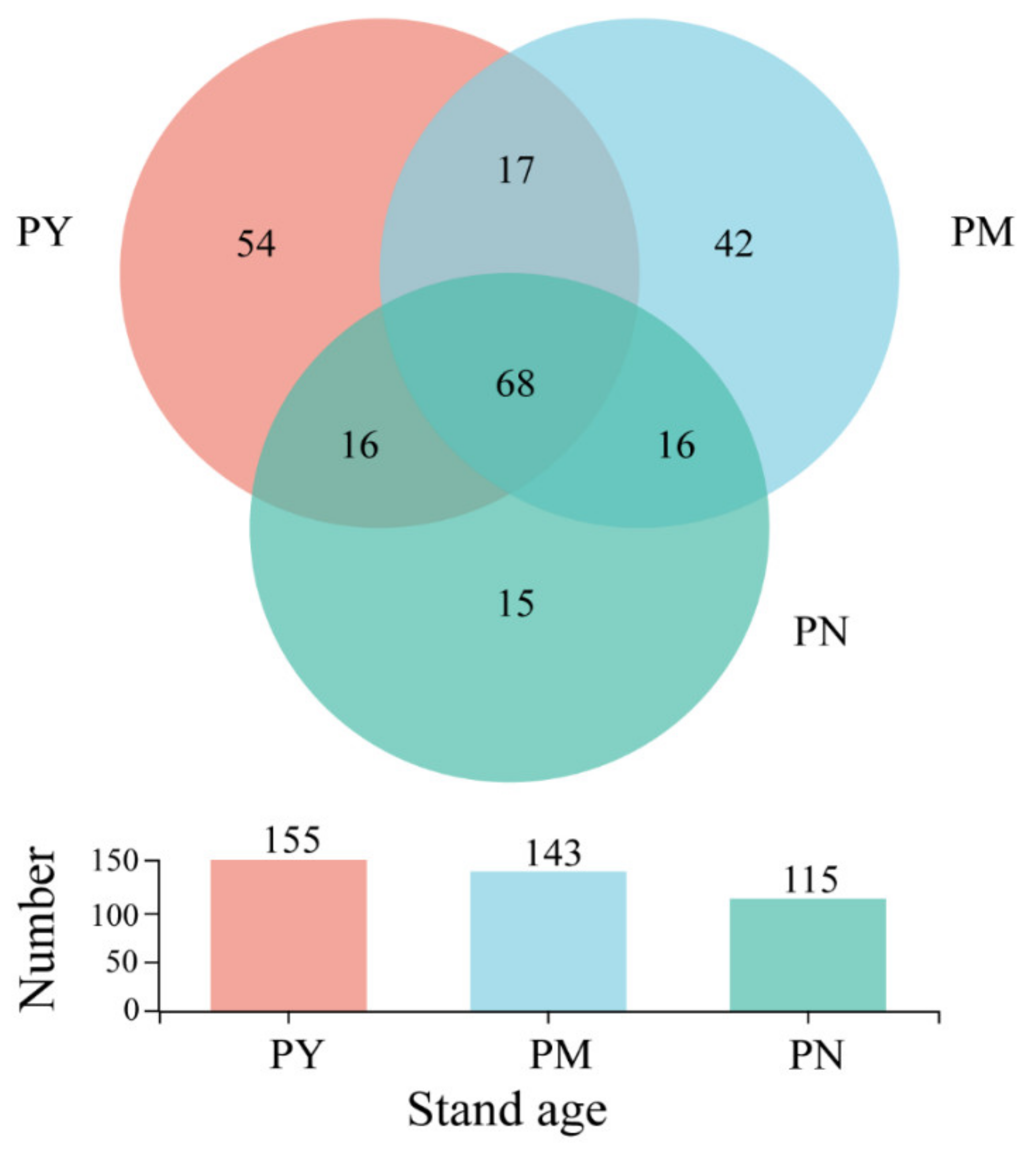
2.2. General Characterization of the Fungal Community
2.3. Fungal Community Diversity and Structure
2.4. Associations between Fungal Community and Soil Properties
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Collection and Analysis of Soil Samples
4.3. Sampling and Molecular Characterization of Fungi
4.4. Data Preprocessing and Bioinformatics Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, M.A.; Wang, Y.; Xia, Y.; Jia, X. Soil drought and water carrying capacity for vegetation in the critical zone of the Loess Plateau: A Review. Vadose Zone J. 2018, 17, 170077. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, M. Spatial variability of soil physical properties in a region of the Loess Plateau of PR China subject to wind and water erosion. Land Degrad. Dev. 2013, 24, 296–304. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Yang, R.; Gao, J.; Liu, Y.; Zhang, H.; Wang, Y.; Tang, M. Contribution of Suillus variegatus to the ecological restoration of 10-year-old Pinus tabuliformis on the Loess Plateau. Appl. Soil Ecol. 2021, 167, 104044. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, L.; Zhao, X.; Wu, P. Feedbacks between vegetation restoration and local precipitation over the Loess Plateau in China. Sci. China Earth Sci. 2021, 64, 920–931. [Google Scholar] [CrossRef]
- Chen, K.; Abbott, R.J.; Milne, R.I.; Tian, X.M.; Liu, J. Phylogeography of Pinus tabulaeformis Carr. (Pinaceae), a dominant species of coniferous forest in northern China. Mol. Ecol. 2008, 17, 4276–4288. [Google Scholar] [CrossRef]
- Zhou, Z.; Shangguan, Z. Vertical distribution of fine roots in relation to soil factors in Pinus tabulaeformis Carr. forest of the Loess Plateau of China. Plant Soil 2007, 291, 119–129. [Google Scholar] [CrossRef]
- Feng, X.; Fu, B.; Piao, S.; Wang, S.; Ciais, P.; Zeng, Z.; Lü, Y.; Zeng, Y.; Li, Y.; Jiang, X.; et al. Revegetation in China’s Loess Plateau is approaching sustainable water resource limits. Nat. Clim. Chang. 2016, 6, 1019–1022. [Google Scholar] [CrossRef]
- Hu, Y.; Niu, Z.; Zeng, D.; Wang, C. Soil amendment improves tree growth and soil carbon and nitrogen pools in Mongolian pine plantations on post-mining land in Northeast China. Land Degrad. Dev. 2015, 26, 807–812. [Google Scholar] [CrossRef]
- Dang, P.; Vu, N.H.; Shen, Z.; Liu, J.; Zhao, F.; Zhu, H.; Yu, X.; Zhao, Z. Changes in soil fungal communities and vegetation following afforestation with Pinus tabulaeformis on the Loess Plateau. Ecosphere 2018, 9, e02401. [Google Scholar] [CrossRef] [Green Version]
- Castaño, C.; Lindahl, B.D.; Alday, J.G.; Hagenbo, A.; de Aragon, J.M.; Parlade, J.; Pera, J.; Bonet, J.A. Soil microclimate changes affect soil fungal communities in a Mediterranean pine forest. New Phytol. 2018, 220, 1211–1221. [Google Scholar] [CrossRef] [Green Version]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; De Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou Nourou, S.; Wijesundera, R.; Ruiz Luis, V.; Vasco-Palacios Aída, M.; Thu Pham, Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Read, D.J.; Perez-Moreno, J. Mycorrhizas and nutrient cycling in ecosystems–a journey towards relevance? N. Phytol. 2003, 157, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.D.; Ihrmark, K.; Boberg, J.; Trumbore, S.E.; Högberg, P.; Stenlid, J.; Finlay, R.D. Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. N. Phytol. 2007, 173, 611–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: London, UK, 2008. [Google Scholar] [CrossRef]
- Courty, P.-E.; Buée, M.; Diedhiou, A.G.; Frey-Klett, P.; Le Tacon, F.; Rineau, F.; Turpault, M.-P.; Uroz, S.; Garbaye, J. The role of ectomycorrhizal communities in forest ecosystem processes: New perspectives and emerging concepts. Soil Biol. Biochem. 2010, 42, 679–698. [Google Scholar] [CrossRef]
- Dong, H.; Ge, J.; Sun, K.; Wang, B.; Xue, J.; Wakelin, S.A.; Wu, J.; Sheng, W.; Liang, C.; Xu, Q.; et al. Change in root-associated fungal communities affects soil enzymatic activities during Pinus massoniana forest development in subtropical China. For. Ecol. Manag. 2021, 482, 118817. [Google Scholar] [CrossRef]
- Guo, M.; Ding, G.; Gao, G.; Zhang, Y.; Cao, H.; Ren, Y. Community composition of ectomycorrhizal fungi associated with Pinus sylvestris var. mongolica plantations of various ages in the Horqin Sandy Land. Ecol. Indic. 2020, 110, 105860. [Google Scholar] [CrossRef]
- He, F.; Yang, B.; Wang, H.; Yan, Q.; Cao, Y.; He, X. Changes in composition and diversity of fungal communities along Quercus mongolica forests developments in Northeast China. Appl. Soil Ecol. 2016, 100, 162–171. [Google Scholar] [CrossRef]
- Leake, J.R.; Donnelly, D.P.; Boddy, L. Interactions between ecto-mycorrhizal and saprotrophic fungi. In Mycorrhizal Ecology; Heijden, M.G.A., Sanders, I.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 345–372. [Google Scholar] [CrossRef]
- Canini, F.; Zucconi, L.; Pacelli, C.; Selbmann, L.; Onofri, S.; Geml, J. Vegetation, pH and water content as main factors for shaping fungal richness, community composition and functional guilds distribution in soils of Western Greenland. Front. Microbiol. 2019, 10, 2348. [Google Scholar] [CrossRef]
- Shanmugam, S.G.; Kingery, W.L. Changes in soil microbial community structure in relation to plant succession and soil properties during 4000 years of pedogenesis. Eur. J. Soil Biol. 2018, 88, 80–88. [Google Scholar] [CrossRef]
- Bastida, F.; López-Mondéjar, R.; Baldrian, P.; Andrés-Abellán, M.; Jehmlich, N.; Torres, I.F.; García, C.; López-Serrano, F.R. When drought meets forest management: Effects on the soil microbial community of a Holm oak forest ecosystem. Sci. Total Environ. 2019, 662, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.J.; Weintraub, M.N.; Hewins, C.R.; Kalisz, S. Relationship between soil enzyme activities, nutrient cycling and soil fungal communities in a northern hardwood forest. Soil Biol. Biochem. 2011, 43, 795–803. [Google Scholar] [CrossRef]
- Nagati, M.; Roy, M.; Manzi, S.; Richard, F.; Desrochers, A.; Gardes, M.; Bergeron, Y. Impact of local forest composition on soil fungal communities in a mixed boreal forest. Plant Soil 2018, 432, 345–357. [Google Scholar] [CrossRef]
- Wu, Y.; Wubet, T.; Trogisch, S.; Both, S.; Scholten, T.; Bruelheide, H.; Buscot, F. Forest age and plant species composition determine the soil fungal community composition in a Chinese subtropical forest. PLoS ONE 2013, 8, e66829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuire, K.L.; D’Angelo, H.; Brearley, F.Q.; Gedallovich, S.M.; Babar, N.; Yang, N.; Gillikin, C.M.; Gradoville, R.; Bateman, C.; Turner, B.L.; et al. Responses of soil fungi to logging and oil palm agriculture in southeast Asian tropical forests. Microb. Ecol. 2015, 69, 733–747. [Google Scholar] [CrossRef] [Green Version]
- Sepp, S.-K.; Davison, J.; Jairus, T.; Vasar, M.; Moora, M.; Zobel, M.; Öpik, M. Non-random association patterns in a plant-mycorrhizal fungal network reveal host-symbiont specificity. Mol. Ecol. 2019, 28, 365–378. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, N.; Liu, C.; Yang, H.; Li, M.; Yu, G.; Wilcox, K.; Yu, Q.; He, N. C:N:P stoichiometry in China’s forests: From organs to ecosystems. Funct. Ecol. 2018, 32, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, P.G.; Mielke, L.A.; Nguyen, N.H. Ecological responses to forest age, habitat, and host vary by mycorrhizal type in boreal peatlands. Mycorrhiza 2018, 28, 315–328. [Google Scholar] [CrossRef]
- Kolaříková, Z.; Kohout, P.; Krüger, C.; Janoušková, M.; Mrnka, L.; Rydlová, J. Root-associated fungal communities along a primary succession on a mine spoil: Distinct ecological guilds assemble differently. Soil Biol. Biochem. 2017, 113, 143–152. [Google Scholar] [CrossRef]
- Lodge, D.J.; Cantrell, S. Fungal communities in wet tropical forests: Variation in time and space. Can. J. Bot. 1995, 73, 1391–1398. [Google Scholar] [CrossRef]
- Mohan, J.E.; Cowden, C.C.; Baas, P.; Dawadi, A.; Frankson, P.T.; Helmick, K.; Hughes, E.; Khan, S.; Lang, A.; Machmuller, M.; et al. Mycorrhizal fungi mediation of terrestrial ecosystem responses to global change: Mini-review. Fungal Ecol. 2014, 10, 3–19. [Google Scholar] [CrossRef]
- Sheng, M.; Chen, X.; Zhang, X.; Hamel, C.; Cui, X.; Chen, J.; Chen, H.; Tang, M. Changes in arbuscular mycorrhizal fungal attributes along a chronosequence of black locust (Robinia pseudoacacia) plantations can be attributed to the plantation-induced variation in soil properties. Sci. Total Environ. 2017, 599, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhao, F.; Kang, D.; Yang, G.; Han, X.; Tong, X.; Feng, Y.; Ren, G. Linkages of C:N:P stoichiometry and bacterial community in soil following afforestation of former farmland. For. Ecol. Manag. 2016, 376, 59–66. [Google Scholar] [CrossRef]
- Vesterdal, L.; Ritter, E.; Gundersen, P. Change in soil organic carbon following afforestation of former arable land. For. Ecol. Manag. 2002, 169, 137–147. [Google Scholar] [CrossRef]
- Dang, P.; Gao, Y.; Liu, J.; Yu, S.; Zhao, Z. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Sci. Total Environ. 2018, 630, 171–180. [Google Scholar] [CrossRef]
- Wang, W.; Page-Dumroese, D.; Lv, R.; Xiao, C.; Li, G.; Liu, Y. Soil enzyme activities in Pinus tabuliformis (Carriére) plantations in northern China. Forests 2016, 7, 112. [Google Scholar] [CrossRef] [Green Version]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Castaño, C.; Dejene, T.; Mediavilla, O.; Geml, J.; Oria-de-Rueda, J.A.; Martín-Pinto, P. Changes in fungal diversity and composition along a chronosequence of Eucalyptus grandis plantations in Ethiopia. Fungal Ecol. 2019, 39, 328–335. [Google Scholar] [CrossRef]
- Dickie, I.A.; Martínez-García, L.B.; Koele, N.; Grelet, G.A.; Tylianakis, J.M.; Peltzer, D.A.; Richardson, S.J. Mycorrhizas and mycorrhizal fungal communities throughout ecosystem development. Plant Soil 2013, 367, 11–39. [Google Scholar] [CrossRef]
- Kyaschenko, J.; Clemmensen, K.E.; Hagenbo, A.; Karltun, E.; Lindahl, B.D. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. ISME J. 2017, 11, 863–874. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z.; Meng, S.; Wu, F. Soil properties and microbial community in the rhizosphere of Populus alba var. pyramidalis along a chronosequence. Microbiol. Res. 2021, 250, 126812. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, T.; Hattori, M.; Nara, K. Ectomycorrhizal fungal communities in alpine relict forests of Pinus pumila on Mt. Norikura, Japan. Mycorrhiza 2018, 28, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, M.; Oria-De-Rueda, J.A.; Martín-Pinto, P. Post-fire fungal succession in a Mediterranean ecosystem dominated by Cistus ladanifer L. For. Ecol. Manag. 2013, 289, 48–57. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Boeraeve, M.; Honnay, O.; Jacquemyn, H. Effects of host species, environmental filtering and forest age on community assembly of ectomycorrhizal fungi in fragmented forests. Fungal Ecol. 2018, 36, 89–98. [Google Scholar] [CrossRef]
- James, T.Y.; Kauff, F.; Schoch, C.L.; Matheny, P.B.; Hofstetter, V.; Cox, C.J.; Celio, G.; Gueidan, C.; Fraker, E.; Miadlikowska, J.; et al. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 2006, 443, 818–822. [Google Scholar] [CrossRef]
- Alem, D.; Dejene, T.; Oria-De-Rueda, J.A.; Geml, J.; Martín-Pinto, P. Soil fungal communities under Pinus patula Schiede ex Schltdl. & Cham. plantation forests of different ages in Ethiopia. Forests 2020, 11, 1109. [Google Scholar] [CrossRef]
- Policelli, N.; Bruns, T.D.; Vilgalys, R.; Nuñez, M.A. Suilloid fungi as global drivers of pine invasions. N. Phytol. 2019, 222, 714–725. [Google Scholar] [CrossRef]
- Davey, M.; Blaalid, R.; Vik, U.; Carlsen, T.; Kauserud, H.; Eidesen, P.B. Primary succession of Bistorta vivipara (L.) Delabre (Polygonaceae) root-associated fungi mirrors plant succession in two glacial chronosequences. Environ. Microbiol. 2015, 17, 2777–2790. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Ploetz, R.C.; Wingfield, M.J.; Wingfield, B.D.; Steenkamp, E.T. Mango malformation disease and the associated Fusarium Species. Phytopathology 2006, 96, 667–672. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Zhang, C.; Wang, Q. Analysis of potential fumonisin-producing Fusarium species in corn products from three main maize-producing areas in eastern China. J. Sci. Food Agric. 2013, 93, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.; Barsoum, N.; Lilleskov, E.A.; Bidartondo, M.I. Nitrogen availability is a primary determinant of conifer mycorrhizas across complex environmental gradients. Ecol. Lett. 2010, 13, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Crowther, T.W.; Stanton, D.W.G.; Thomas, S.M.; A’Bear, A.D.; Hiscox, J.; Jones, T.H.; Voříšková, J.; Baldrian, P.; Boddy, L. Top-down control of soil fungal community composition by a globally distributed keystone consumer. Ecology 2013, 94, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Koide, R.T.; Fernandez, C.; Malcolm, G. Determining place and process: Functional traits of ectomycorrhizal fungi that affect both community structure and ecosystem function. N. Phytol. 2014, 201, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Kranabetter, J.M.; Durall, D.M.; Mackenzie, W.H. Diversity and species distribution of ectomycorrhizal fungi along productivity gradients of a southern boreal forest. Mycorrhiza 2009, 19, 99–111. [Google Scholar] [CrossRef]
- Cairney, J.W.G. Ectomycorrhizal fungi: The symbiotic route to the root for phosphorus in forest soils. Plant Soil 2011, 344, 51–71. [Google Scholar] [CrossRef]
- Pampolina, N.M.; Dell, B.; Malajczuk, N. Dynamics of ectomycorrhizal fungi in an Eucalyptus globulus plantation: Effect of phosphorus fertilization. For. Ecol. Manag. 2002, 158, 291–304. [Google Scholar] [CrossRef]
- Clausing, S.; Likulunga, L.E.; Janz, D.; Feng, H.Y.; Schneider, D.; Daniel, R.; Krüger, J.; Lang, F.; Polle, A. Impact of nitrogen and phosphorus addition on resident soil and root mycobiomes in beech forests. Biol. Fertil. Soils 2021, 57, 1031–1052. [Google Scholar] [CrossRef]
- Bremner, J.; Tabatabai, M. Use of an ammonia electrode for determination of ammonium in Kjeldahl analysis of soils. Commun. Soil Sci. Plant Anal. 1972, 3, 159–165. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic forms. In Methods of Soil Analysis: Part 2—Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy; Soil Science Society of America: Madison, WI, USA, 1983; pp. 643–698. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Stevenson, F.J. Nitrogen—Organic forms. In Methods of Soil Analysis: Part 2—Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy; Soil Science Society of America: Madison, WI, USA, 1982; pp. 625–641. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3—Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; American Society of Agronomy; Soil Science Society of America: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Gardner, W.H. Water content. In Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; Klute, A., Ed.; American Society of Agronomy; Soil Science Society of America: Madison, WI, USA, 1986; pp. 493–544. [Google Scholar]
- Hendershot, W.; Lalande, H.; Duquette, M. Ion exchange and exchangeable cations. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; CRC Press: Boca Raton, FL, USA, 1993; Volume 19, pp. 167–176. [Google Scholar]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, W.; Shao, Y.; Li, Y.-J.; Lin, L.-A.; Zhang, Y.-J.; Han, H.; Chen, Z.-J. Miscanthus cultivation shapes rhizosphere microbial community structure and function as assessed by Illumina MiSeq sequencing combined with PICRUSt and FUNGUIld analyses. Arch. Microbiol. 2020, 202, 1157–1171. [Google Scholar] [CrossRef] [PubMed]









| Sites | TN (mg/kg) | AN (mg/kg) | NN (mg/kg) | TP (mg/kg) | AP (mg/kg) | TK (g/kg) | AK (mg/kg) | SOC (g/kg) | SWC (%) | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| PY | 173.10 ± 11.97a | 5.97 ± 0.82c | 1.88 ± 0.07b | 157.43 ± 14.27a | 13.97 ±1.71a | 13.34 ± 1.25b | 43.76 ± 5.67a | 5.66 ± 0.51b | 4.23 ± 0.47b | 8.31 ± 0.07a |
| PM | 179.97 ± 23.40a | 10.50 ± 1.03b | 2.01 ± 0.09b | 174.49 ±11.77a | 11.34 ± 0.45b | 13.59 ± 1.61b | 40.80 ± 8.12a | 4.66 ± 0.44b | 3.85 ± 0.96b | 8.38 ± 0.03a |
| PN | 245.43 ± 33.03a | 16.71 ± 1.17a | 2.87 ± 0.31a | 160.54 ± 9.72a | 10.16 ±1.29c | 17.31 ± 1.49a | 54.60 ± 2.97a | 12.18 ± 1.48a | 6.38 ± 0.86a | 8.34 ± 0.05a |
| p value | ns | Linear regression (p < 0.01, R2 = 0.93) | Linear regression (p < 0.01, R2 = 0.67) | ns | Linear regression (p < 0.05, R2 = 0.52) | Linear regression (p < 0.05, R2 = 0.41) | ns | Linear regression (p < 0.05, R2 = 0.53) | ns | ns |
| Sites | Sobs Index | Shannon Index | Simpson Index |
|---|---|---|---|
| PY | 94.7 ± 22.1a | 2.7 ± 0.1a | 0.14 ± 0.03a |
| PM | 81.7 ± 12.8a | 3.1 ± 0.2a | 0.06 ± 0.02b |
| PN | 72.3 ± 9.5a | 3.0 ± 0.2a | 0.06 ± 0.01b |
| p value | 0.562 | 0.704 | p < 0.001 |
| Sites | Stand Age | Height (m) | Diameter at Breast Height (cm) | Canopy Density | Planting Density (N/ha) |
|---|---|---|---|---|---|
| PY | 10a | 4.32 ± 0.71 | 10.54 ± 0.31 | 0.46 ± 0.05 | 954 ± 52 |
| PM | 20a | 9.15 ± 0.47 | 12.36 ± 0.60 | 0.53 ± 0.09 | 1003 ± 63 |
| PN | 30a | 12.33 ± 0.26 | 14.73 ± 0.85 | 0.63 ± 0.08 | 808 ± 86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Gao, J.; Zhang, H.; Tang, M. Changes in Rhizosphere Soil Fungal Communities of Pinus tabuliformis Plantations at Different Development Stages on the Loess Plateau. Int. J. Mol. Sci. 2022, 23, 6753. https://doi.org/10.3390/ijms23126753
Wang J, Gao J, Zhang H, Tang M. Changes in Rhizosphere Soil Fungal Communities of Pinus tabuliformis Plantations at Different Development Stages on the Loess Plateau. International Journal of Molecular Sciences. 2022; 23(12):6753. https://doi.org/10.3390/ijms23126753
Chicago/Turabian StyleWang, Jiaxing, Jing Gao, Haoqiang Zhang, and Ming Tang. 2022. "Changes in Rhizosphere Soil Fungal Communities of Pinus tabuliformis Plantations at Different Development Stages on the Loess Plateau" International Journal of Molecular Sciences 23, no. 12: 6753. https://doi.org/10.3390/ijms23126753
APA StyleWang, J., Gao, J., Zhang, H., & Tang, M. (2022). Changes in Rhizosphere Soil Fungal Communities of Pinus tabuliformis Plantations at Different Development Stages on the Loess Plateau. International Journal of Molecular Sciences, 23(12), 6753. https://doi.org/10.3390/ijms23126753






