Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series
Abstract
:1. Introduction
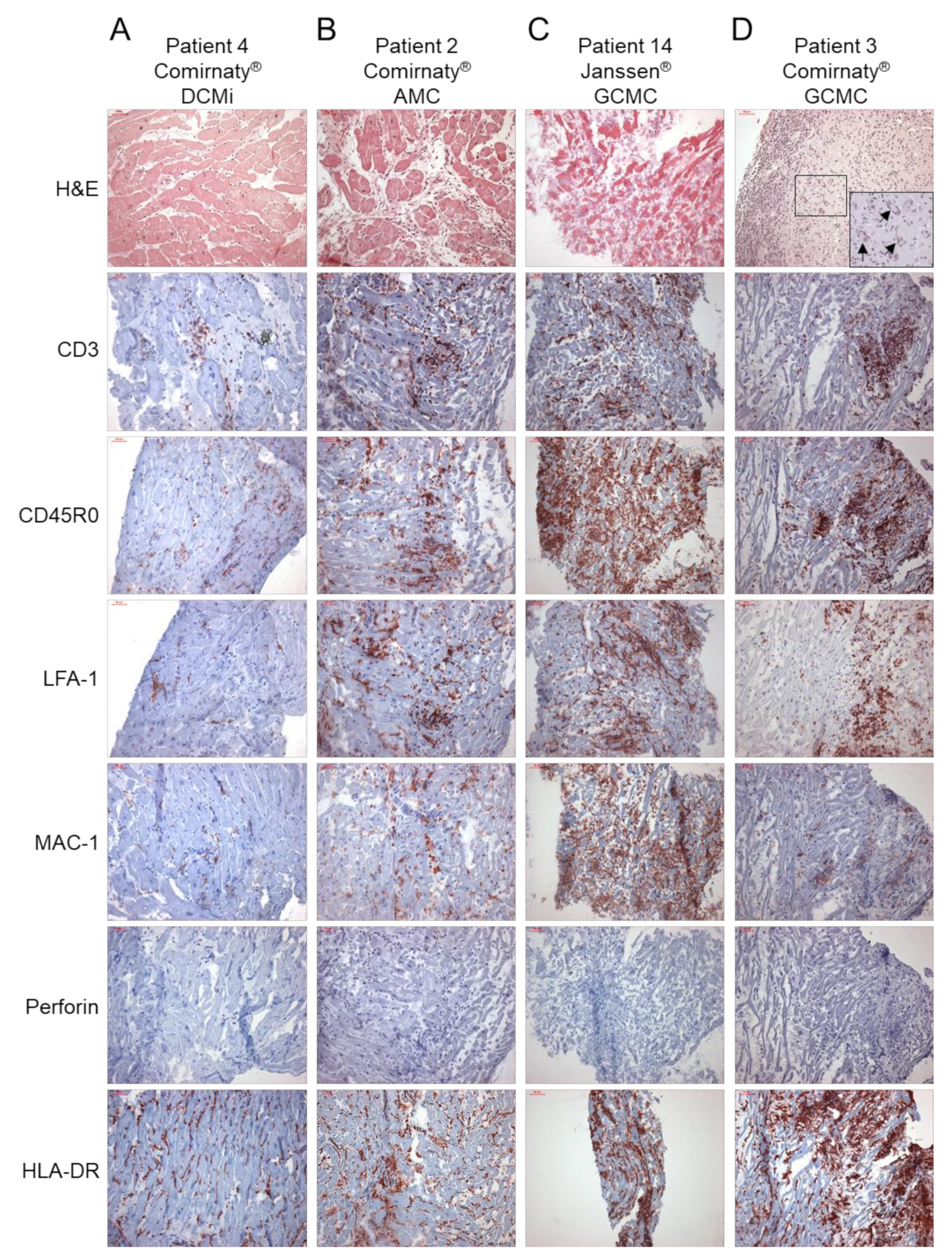
2. Results
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Patients and Clinical Investigations
4.2. Molecular Virology of EMBs
4.3. Histology, Immunohistochemistry and Digital Imaging Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC Centers for Disease Control and Prevention. Myocarditis and Pericarditis Following MRNA COVID-19 Vaccination. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html (accessed on 6 April 2022).
- Minocha, P.K.; Better, D.; Singh, R.K.; Hoque, T. Recurrence of Acute Myocarditis Temporally Associated with Receipt of the MRNA Coronavirus Disease 2019 (COVID-19) Vaccine in a Male Adolescent. J. Pediatr. 2021, 238, 321–323. [Google Scholar] [CrossRef]
- Muthukumar, A.; Narasimhan, M.; Li, Q.-Z.; Mahimainathan, L.; Hitto, I.; Fuda, F.; Batra, K.; Jiang, X.; Zhu, C.; Schoggins, J.; et al. In-Depth Evaluation of a Case of Presumed Myocarditis After the Second Dose of COVID-19 MRNA Vaccine. Circulation 2021, 144, 487–498. [Google Scholar] [CrossRef] [PubMed]
- McLean, K.; Johnson, T.J. Myopericarditis in a Previously Healthy Adolescent Male Following COVID-19 Vaccination: A Case Report. Acad. Emerg. Med. 2021, 28, 918–921. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, T.; Cattafi, A.; Carerj, M.L.; Booz, C.; Ascenti, G.; Cicero, G.; Blandino, A.; Mazziotti, S. Myocarditis After SARS-CoV-2 Vaccination: A Vaccine-Induced Reaction? Can. J. Cardiol. 2021, 37, 1665–1667. [Google Scholar] [CrossRef]
- Albert, E.; Aurigemma, G.; Saucedo, J.; Gerson, D.S. Myocarditis Following COVID-19 Vaccination. Radiol. Case Rep. 2021, 16, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Bautista García, J.; Peña Ortega, P.; Bonilla Fernández, J.A.; Cárdenes León, A.; Ramírez Burgos, L.; Caballero Dorta, E. Acute Myocarditis after Administration of the BNT162b2 Vaccine against COVID-19. Rev. Española Cardiol. 2021, 74, 812–814. [Google Scholar] [CrossRef]
- Nagasaka, T.; Koitabashi, N.; Ishibashi, Y.; Aihara, K.; Takama, N.; Ohyama, Y.; Yokoyama, T.; Kaneko, Y. Acute Myocarditis Associated with COVID-19 Vaccination: A Case Report. J. Cardiol. Cases 2021, 25, 285–288. [Google Scholar] [CrossRef]
- Matta, A.; Kunadharaju, R.; Osman, M.; Jesme, C.; McMiller, Z.; Johnson, E.M.; Matta, D.; Kallamadi, R.; Bande, D. Clinical Presentation and Outcomes of Myocarditis Post MRNA Vaccination: A Meta-Analysis and Systematic Review. Cureus 2021, 13, e19240. [Google Scholar] [CrossRef]
- European Medicine Agency Meeting Highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 29 November—2 December 2021. Available online: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-29-november-2-december-2021 (accessed on 1 April 2022).
- Verma, A.K.; Lavine, K.J.; Lin, C.-Y. Myocarditis after COVID-19 MRNA Vaccination. N. Engl. J. Med. 2021, 385, 1332–1334. [Google Scholar] [CrossRef]
- Viskin, D.; Topilsky, Y.; Aviram, G.; Mann, T.; Sadon, S.; Hadad, Y.; Flint, N.; Shmilovich, H.; Banai, S.; Havakuk, O. Myocarditis Associated With COVID-19 Vaccination: Echocardiography, Cardiac Tomography, and Magnetic Resonance Imaging Findings. Circ. Cardiovasc. Imaging 2021, 14, e013236. [Google Scholar] [CrossRef]
- Salah, H.M.; Mehta, J.L. COVID-19 Vaccine and Myocarditis. Am. J. Cardiol. 2021, 157, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Kadwalwala, M.; Chadha, B.; Ortoleva, J.; Joyce, M. Multimodality Imaging and Histopathology in a Young Man Presenting with Fulminant Lymphocytic Myocarditis and Cardiogenic Shock after MRNA-1273 Vaccination. BMJ Case Rep. 2021, 14, e246059. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P.; Klingel, K.; Ohlmann-Knafo, S.; Hüttinger, S.; Sood, N.; Pickuth, D.; Kindermann, M. Biopsy-Proven Lymphocytic Myocarditis Following First MRNA COVID-19 Vaccination in a 40-Year-Old Male: Case Report. Clin. Res. Cardiol. 2021, 110, 1855–1859. [Google Scholar] [CrossRef]
- Escher, F.; Pietsch, H.; Aleshcheva, G.; Bock, T.; Baumeier, C.; Elsaesser, A.; Wenzel, P.; Hamm, C.; Westenfeld, R.; Schultheiss, M.; et al. Detection of Viral SARS-CoV-2 Genomes and Histopathological Changes in Endomyocardial Biopsies. ESC Hear. Fail. 2020, 7, 2440–2447. [Google Scholar] [CrossRef] [PubMed]
- Witberg, G.; Barda, N.; Hoss, S.; Richter, I.; Wiessman, M.; Aviv, Y.; Grinberg, T.; Auster, O.; Dagan, N.; Balicer, R.D.; et al. Myocarditis after COVID-19 Vaccination in a Large Health Care Organization. N. Engl. J. Med. 2021, 385, 2132–2139. [Google Scholar] [CrossRef] [PubMed]
- Escher, F.; Aleshcheva, G.; Pietsch, H.; Baumeier, C.; Gross, U.M.; Schrage, B.N.; Westermann, D.; Bock, C.-T.; Schultheiss, H.-P. Transcriptional Active Parvovirus B19 Infection Predicts Adverse Long-Term Outcome in Patients with Non-Ischemic Cardiomyopathy. Biomedicines 2021, 9, 1898. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, H.; Escher, F.; Aleshcheva, G.; Lassner, D.; Bock, C.-T.; Schultheiss, H.-P. Detection of Parvovirus MRNAs as Markers for Viral Activity in Endomyocardial Biopsy-Based Diagnosis of Patients with Unexplained Heart Failure. Sci. Rep. 2020, 10, 22354. [Google Scholar] [CrossRef] [PubMed]
- Vdovenko, D.; Eriksson, U. Regulatory Role of CD4+ T Cells in Myocarditis. J. Immunol. Res. 2018, 2018, 4396351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Chen, Y.; Zhao, Y.; Lung, D.C.; Ye, Z.; Song, W.; Liu, F.-F.; Cai, J.-P.; Wong, W.-M.; Yip, C.C.-Y.; et al. Intravenous Injection of Coronavirus Disease 2019 (COVID-19) MRNA Vaccine Can Induce Acute Myopericarditis in Mouse Model. Clin. Infect. Dis. 2021, 73, 2372–2373. [Google Scholar] [CrossRef]
- Jurcova, I.; Rocek, J.; Bracamonte-Baran, W.; Zelizko, M.; Netuka, I.; Maluskova, J.; Kautzner, J.; Cihakova, D.; Melenovsky, V.; Maly, J. Complete Recovery of Fulminant Cytotoxic CD8 T-cell-mediated Myocarditis after ECMELLA Unloading and Immunosuppression. ESC Heart Failure 2020, 7, 1976–1981. [Google Scholar] [CrossRef]
- Escher, F.; Kühl, U.; Lassner, D.; Stroux, A.; Gross, U.; Westermann, D.; Pieske, B.; Poller, W.; Schultheiss, H. High Perforin-Positive Cardiac Cell Infiltration and Male Sex Predict Adverse Long-Term Mortality in Patients with Inflammatory Cardiomyopathy. J. Am. Heart Assoc. 2017, 6, e005352. [Google Scholar] [CrossRef]
- Gebhard, J.R.; Perry, C.M.; Harkins, S.; Lane, T.; Mena, I.; Asensio, V.C.; Campbell, I.L.; Whitton, J.L. Coxsackievirus B3-Induced Myocarditis. Am. J. Pathol. 1998, 153, 417–428. [Google Scholar] [CrossRef]
- Seko, Y.; Shinkai, Y.; Kawasaki, A.; Yagita, H.; Okumura, K.; Takaku, F.; Yazaki, Y. Expression of Perforin in Infiltrating Cells in Murine Hearts with Acute Myocarditis Caused by Coxsackievirus B3. Circulation 1991, 84, 788–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, L.; Joag, S.; Zheng, L.; Young, J.; Lee, C.; Lee, Y. Perforin-Mediated Myocardial Damage in Acute Myocarditis. Lancet 1990, 336, 1019–1021. [Google Scholar] [CrossRef]
- Bollano, E.; Bergh, N.; Dudás, A.; Bobbio, E.; Polte, C.L. Somatostatin Receptor Positron Emission Tomography/Computed Tomography in Myocarditis Following MRNA COVID-19 Vaccination. Eur. Hear. J. Case Rep. 2022, 6, ytac117. [Google Scholar] [CrossRef]
- Lampejo, T.; Durkin, S.M.; Bhatt, N.; Guttmann, O. Acute Myocarditis: Aetiology, Diagnosis and Management. Clin. Med. 2021, 21, e505–e510. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The Role of Endomyocardial Biopsy in the Management of Cardiovascular Disease. Circulation 2007, 116, 2216–2233. [Google Scholar] [CrossRef] [Green Version]
- Pietsch, H.; Escher, F.; Aleshcheva, G.; Baumeier, C.; Morawietz, L.; Elsaesser, A.; Schultheiss, H.-P. Proof of SARS-CoV-2 Genomes in Endomyocardial Biopsy with Latency after Acute Infection. Int. J. Infect. Dis. 2021, 102, 70–72. [Google Scholar] [CrossRef]
- Baumeier, C.; Escher, F.; Aleshcheva, G.; Pietsch, H.; Schultheiss, H.-P. Plasminogen Activator Inhibitor-1 Reduces Cardiac Fibrosis and Promotes M2 Macrophage Polarization in Inflammatory Cardiomyopathy. Basic Res. Cardiol. 2021, 116, 1. [Google Scholar] [CrossRef]
- Aretz, H.T. Myocarditis: The Dallas Criteria. Hum. Pathol. 1987, 18, 619–624. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current State of Knowledge on Aetiology, Diagnosis, Management, and Therapy of Myocarditis: A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Dal Ferro, M.; Bussani, R.; Paldino, A.; Nuzzi, V.; Collesi, C.; Zentilin, L.; Schneider, E.; Correa, R.; Silvestri, F.; Zacchigna, S.; et al. SARS-CoV-2, Myocardial Injury and Inflammation: Insights from a Large Clinical and Autopsy Study. Clin. Res. Cardiol. 2021, 110, 1822–1831. [Google Scholar] [CrossRef] [PubMed]


| Pat. No. | Sex | Age (y) | LVEF (%) | Vaccine | Manufacturer | Dose | Onset of Symptoms (Days) | Clinical Picture | Troponin (pg/mL) Normal <15 | BNP (pg/mL) Normal <125 | CK (U/L) Normal <171 | CRP (mg/dL) Normal <0.5 | Suspected Diagnosis | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | m | 23 | 45 | Comirnaty | Pfizer-BioNTech | 2nd | 20 | discomfort during exercise, reduced LVEF, dilated LV | <15 | <0.5 | AMC; myocarditis after vaccination | AMC | ||
| 2 | f | 31 | 20 | Comirnaty | Pfizer-BioNTech | 2nd | 0 | cardiac arrest during sports 6 h after the 2nd vaccination, resuscitation, dyspnea, hopotonia, reduced LVEF, dilated LV, NYHA III, intrapulmonary infiltrates, pericardial effusion, no signs of active myocarditis in cMRI | 1325 | 2183 | 784 | 7.3 | AMC; myocarditis after vaccination | AMC |
| 3 | m | 32 | 43 | Comirnaty | Pfizer-BioNTech | 1st | 1–3 | dyspnea, reduced exercise capacity, reduced LVEF, NYHA II | 576 | 2483 | 8.9 | AMC; myocarditis after vaccination | GCMC | |
| 4 | m | 52 | 45 | Comirnaty | Pfizer-BioNTech | 2nd | 3 | inpatient admission after resuscitation for ventricular fibrillation, reduced LVEF, LV latero-apical akinesia with wall thinning, no signs of active myocarditis in cMRI | 436 | 2.8 | ARVC; myocarditis after vaccination | DCMi | ||
| 5 | m | 18 | 12 | Comirnaty | Pfizer-BioNTech | 2nd | 21 | cardiac decompensation, reduced LVEF, NYHA II-III, no signs of active myocarditis in cMRI | 38.1 | 8430 | 181 | 0.7 | DCM; myocarditis after vaccination | DCMi |
| 6 | m | 59 | 38 | Comirnaty | Pfizer-BioNTech | 2nd | 56 | dyspnea, reduced LVEF | 58.3 | 669 | myocarditis after vaccination | DCMi | ||
| 7 | m | 24 | 30 | Comirnaty | Pfizer-BioNTech | 2nd | 2 | dyspnea, angina pectoris, reduced LVEF | 710 | 585 | 9.3 | AMC; myocarditis after vaccination | DCMi | |
| 8 | m | 39 | 5 | Comirnaty | Pfizer-BioNTech | 2nd | 4 | dyspnea, cardiac decompensation, reduced LVEF, signs of active myocarditis in cMRI | 935 | 68 | 1.47 | AMC; myocarditis after vaccination | DCMi | |
| 9 | m | 34 | 10 | Comirnaty | Pfizer-BioNTech | 1st | 14 | dyspnea, reduced exercise capacity, reduced LVEF, dilated LA, supraventricular tachycardia up to 140/min, atrial fibrillation, myocardial edema and signs of active myocarditis in cMRI | <15 | AMC; myocarditis after vaccination | DCMi | |||
| 10 | f | 38 | 40 | Comirnaty | Pfizer-BioNTech | 2nd | 14 | reduced LVEF, NYHA I | <15 | 84 | 35 | 2.8 | AMC; myocarditis after vaccination | DCMi |
| 11 | f | 52 | 15 | Comirnaty | Pfizer-BioNTech | 2nd | 1 | dyspnea on exertion, reduced LVEF, mitral valve insufficiency, hypertension, no signs of active myocarditis in cMRI | 1592 | 83 | 7.1 | AMC; myocarditis after vaccination | DCMi | |
| 12 | f | 59 | 37 | Vaxzevria | AstraZenica | 2nd | 14 | dyspnea, reduced LVEF | <15 | myocarditis after vaccination | DCM | |||
| 13 | f | 68 | 30 | Vaxzevria | AstraZenica | 1st | 1 | reduced LVEF | 582 | 1094 | AMC; DCMi; myocarditis after vaccination | DCMi | ||
| 14 | f | 31 | 35 | Janssen | Johnson & Johnsen | 1st | 28 | fulminant cardiogenic shock, reduced LVEF | 48 | 334 | 1557 | 35 | AMC; GCMC; Sarkoidosis; myocarditis after vaccination | GCMC |
| 15 | m | 45 | 10 | Janssen | Johnson & Johnsen | 1st | 14 | dyspnea, reduced LVEF, dilated LV, atrial fibrillation | 23.7 | 1670 | 90 | 1.4 | AMC; myocarditis after vaccination | DCMi |
| Pat. No. | Vaccine | Diagn. | Ventr. | Virology | CD3 (Cells/mm2) Normal <14 | CD45R0 (Cells/mm2) Normal <60 | LFA-1 (Cells/mm2) Normal <14 | MAC-1 (Cells/mm2) Normal <40 | Perforin (Cells/mm2) Normal <3.5 | HLA-DR (Area%) Normal <4.6 | ICAM-1 (Area%) Norma <2.8 | VCAM-1 (Area%) Normal <0.08 | CD4 to CD8 Ratio | SARS-CoV-2 Spike Protein |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Comirnaty | AMC | RV | B19V | 11.3 | 33.0 | 17.2 | 34.7 | 1.1 | 4.7 | 2.2 | 0.02 | 0.7 | - |
| 2 | Comirnaty | AMC | LV | - | 500 | 749 | 501 | 277 | 0.0 | 6.2 | 4.8 | 0.61 | 0.2 | + |
| 3 | Comirnaty | GCMC | LV | - | 274 | 682 | 439 | 80.6 | 0.0 | 12.7 | nd | nd | 0.7 | + |
| 4 | Comirnaty | DCMi | LV | B19V | 52.1 | 132.6 | 45.4 | 86.5 | 0.0 | 5.6 | 0.3 | 0.01 | 2.0 | - |
| 5 | Comirnaty | DCMi | LV | B19V | 4.8 | 51.6 | 18.3 | 38.3 | 0.0 | 3.4 | 0.7 | 0.01 | 1.0 | + |
| 6 | Comirnaty | DCMi | LV | B19V | 17.9 | 67.3 | 6.7 | 38.1 | 0.0 | 3.8 | nd | nd | 7.4 | - |
| 7 | Comirnaty | DCMi | LV | - | 21.4 | 110 | 95.5 | 53.3 | 0.0 | 5.0 | 2.1 | 0.04 | 1.0 | + |
| 8 | Comirnaty | DCMi | LV | B19V | 18.3 | 153 | 24.8 | 108 | 0.0 | 6.6 | 0.7 | 0.02 | 2.8 | - |
| 9 | Comirnaty | DCMi | RV | B19V | 48.7 | 62.2 | 72.8 | 84.3 | 0.0 | 8.0 | 2.2 | 0.02 | 0.6 | - |
| 10 | Comirnaty | DCMi | LV | B19V | 7.1 | 79.8 | 21.9 | 54.4 | 0.0 | 4.4 | nd | nd | 11.2 | + |
| 11 | Comirnaty | DCMi | RV | B19V | 10.5 | 76.7 | 28.4 | 61.3 | 2.5 | 8.2 | nd | nd | 1.0 | + |
| 12 | Vaxzevria | DCM | RV | B19V | 2.9 | 31.4 | 8.8 | 28.6 | 0.0 | 2.9 | 0.6 | 0.00 | 1.0 | + |
| 13 | Vaxzevria | DCMi | LV | - | 13.4 | 114 | 13.4 | 81.3 | 1.8 | 12.3 | nd | nd | 12.4 | + |
| 14 | Janssen | GCMC | LV | - | 800 | 987 | 713 | 360 | 0.0 | 23.1 | 6.4 | 0.37 | 0.6 | nd |
| 15 | Janssen | DCMi | RV | B19V | 52.9 | 108 | 54.0 | 100 | 0.0 | 8.0 | 3.7 | 0.06 | 1.0 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumeier, C.; Aleshcheva, G.; Harms, D.; Gross, U.; Hamm, C.; Assmus, B.; Westenfeld, R.; Kelm, M.; Rammos, S.; Wenzel, P.; et al. Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. Int. J. Mol. Sci. 2022, 23, 6940. https://doi.org/10.3390/ijms23136940
Baumeier C, Aleshcheva G, Harms D, Gross U, Hamm C, Assmus B, Westenfeld R, Kelm M, Rammos S, Wenzel P, et al. Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. International Journal of Molecular Sciences. 2022; 23(13):6940. https://doi.org/10.3390/ijms23136940
Chicago/Turabian StyleBaumeier, Christian, Ganna Aleshcheva, Dominik Harms, Ulrich Gross, Christian Hamm, Birgit Assmus, Ralf Westenfeld, Malte Kelm, Spyros Rammos, Philip Wenzel, and et al. 2022. "Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series" International Journal of Molecular Sciences 23, no. 13: 6940. https://doi.org/10.3390/ijms23136940
APA StyleBaumeier, C., Aleshcheva, G., Harms, D., Gross, U., Hamm, C., Assmus, B., Westenfeld, R., Kelm, M., Rammos, S., Wenzel, P., Münzel, T., Elsässer, A., Gailani, M., Perings, C., Bourakkadi, A., Flesch, M., Kempf, T., Bauersachs, J., Escher, F., & Schultheiss, H.-P. (2022). Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. International Journal of Molecular Sciences, 23(13), 6940. https://doi.org/10.3390/ijms23136940







