In Situ Collection and Rapid Detection of Pathogenic Bacteria Using a Flexible SERS Platform Combined with a Portable Raman Spectrometer
Abstract
:1. Introduction
2. Results and Discussion
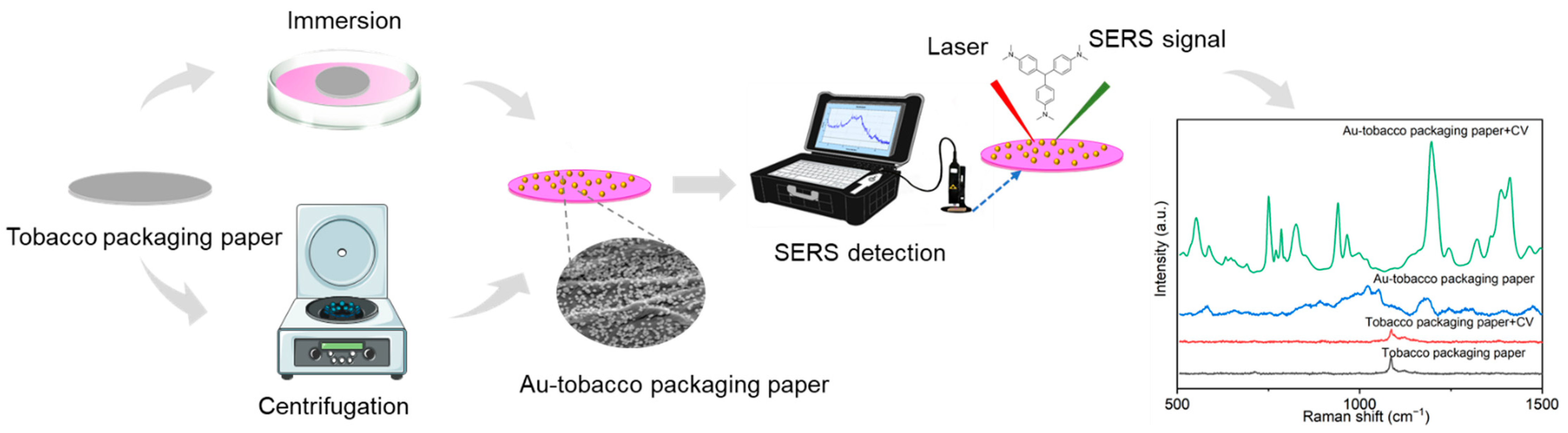
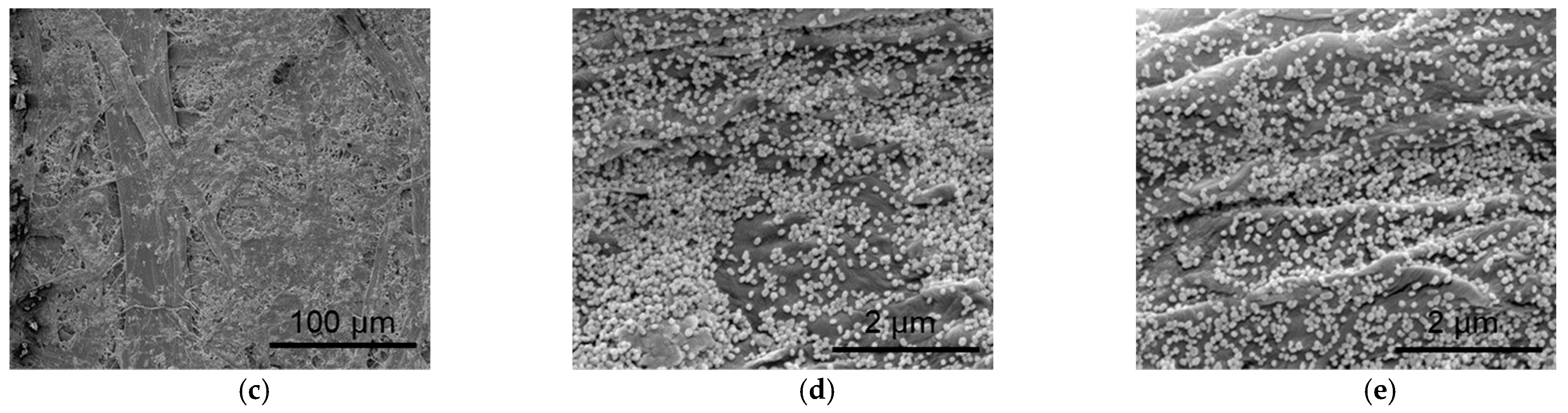
2.1. Preparation and Characterization of Au NPs and Au-TPP Substrates
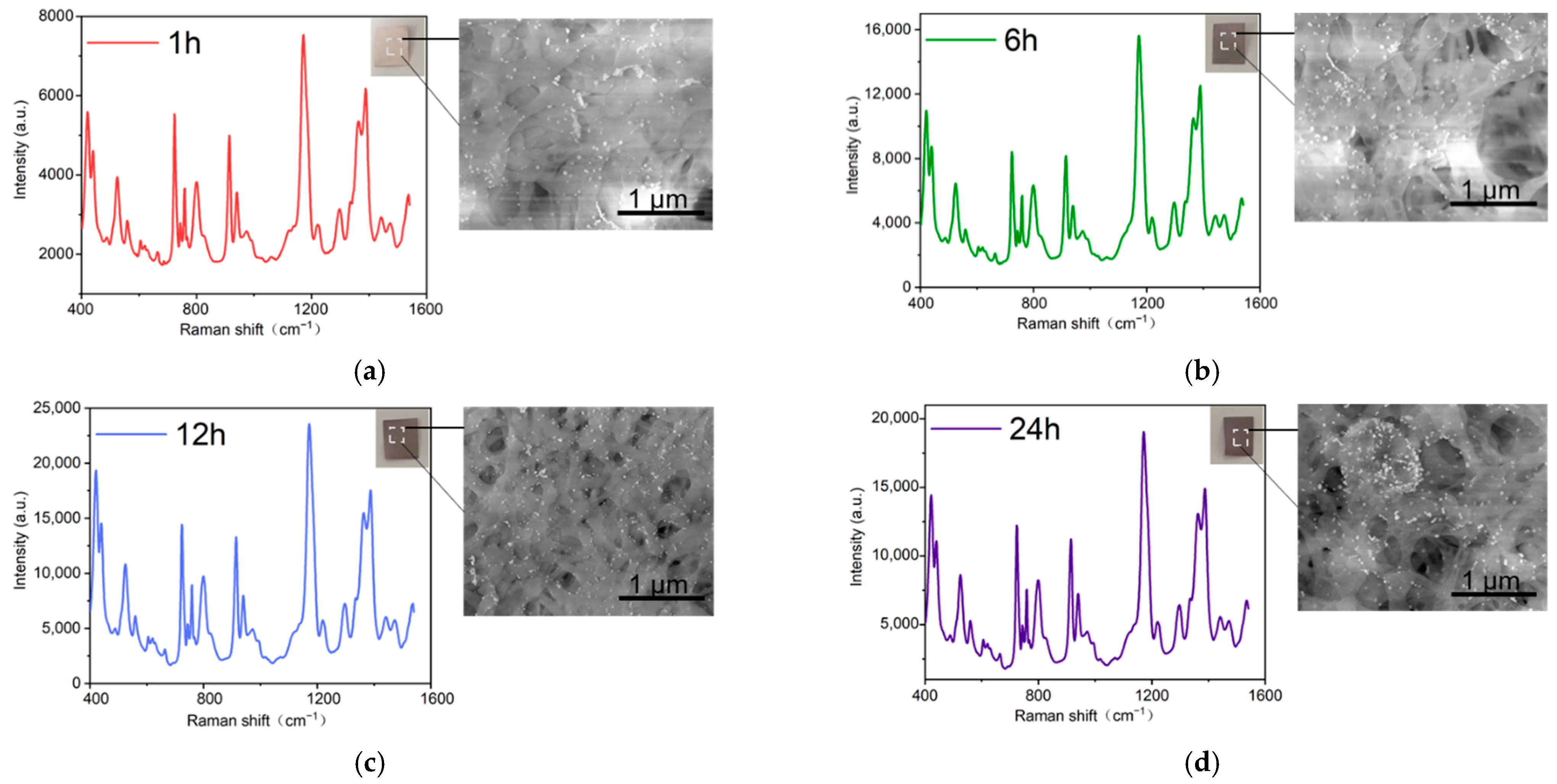
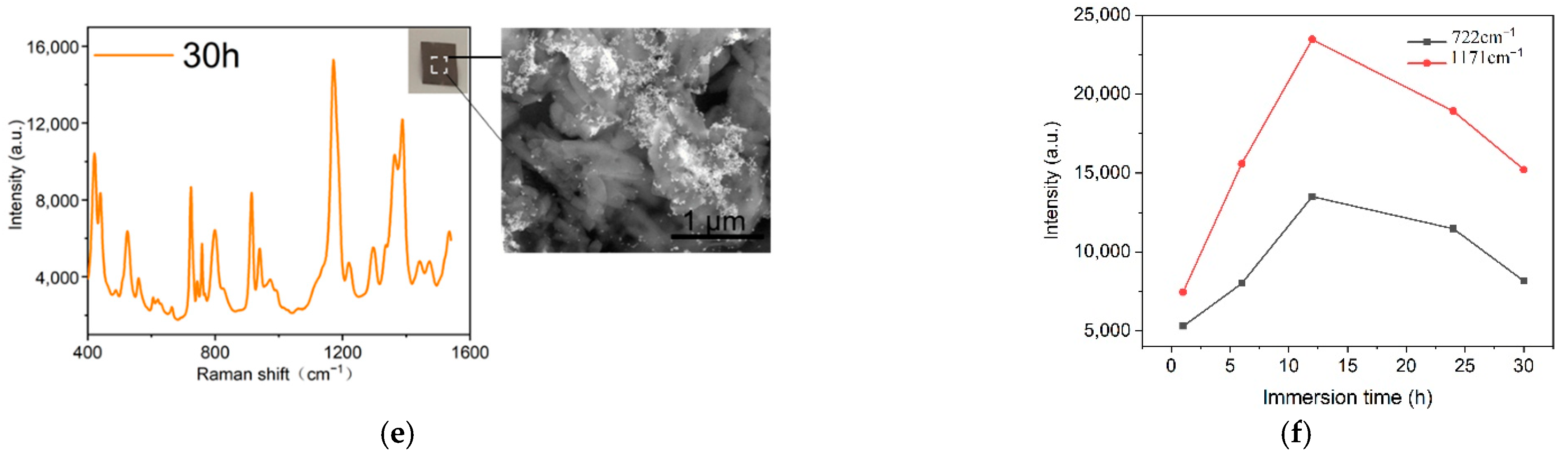
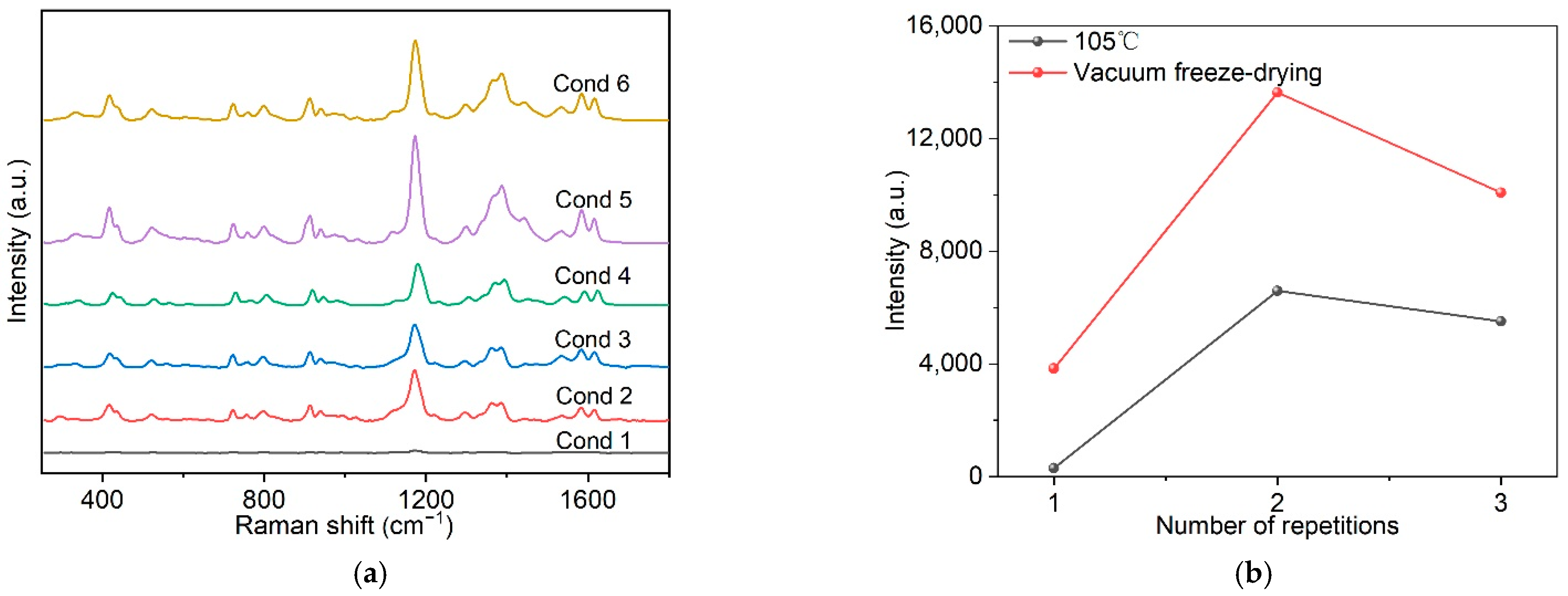
2.2. Optimization of Preparation Conditions
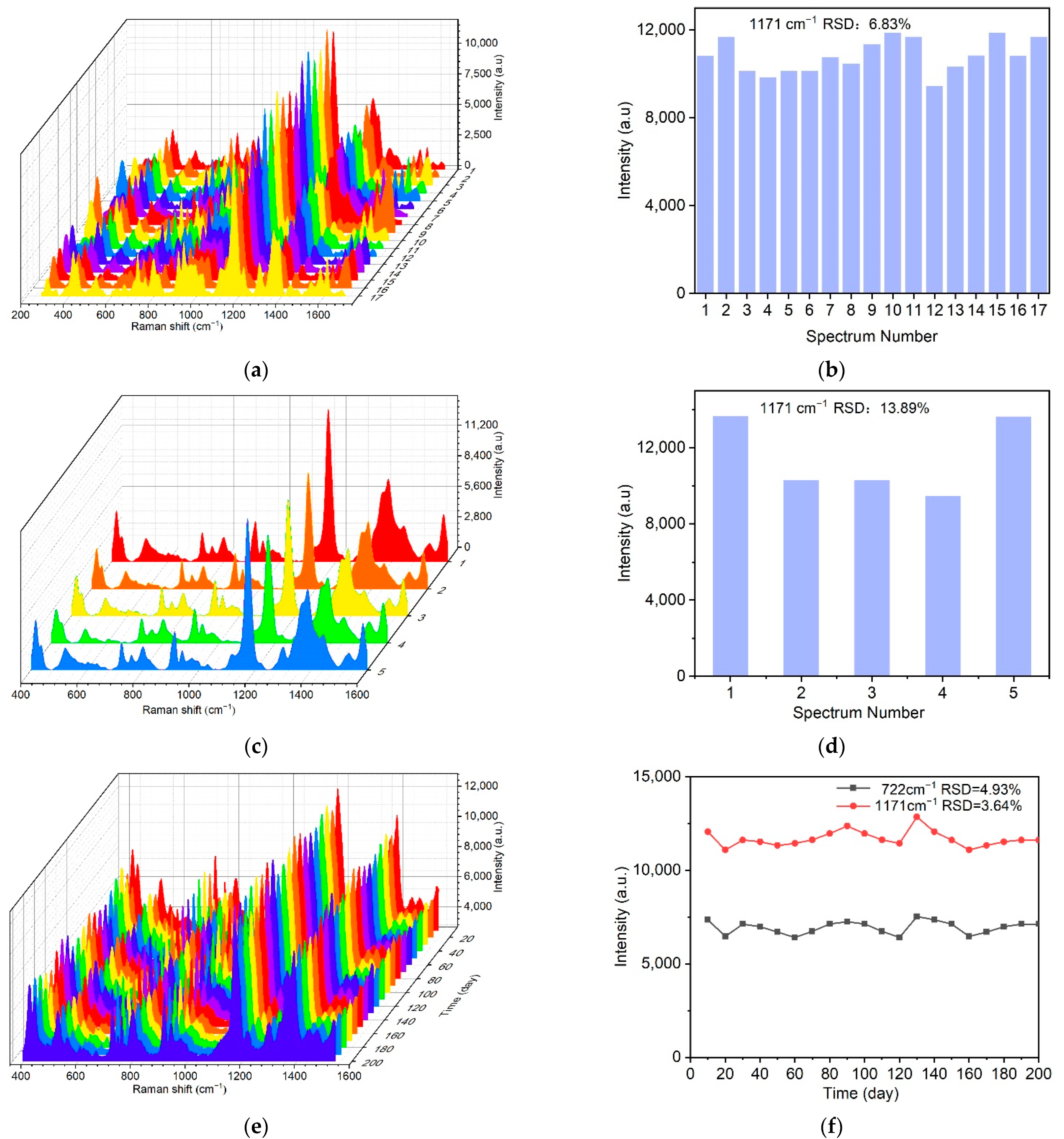
2.3. Uniformity, Reproducibility and Stability of the Au-TPP Substrate
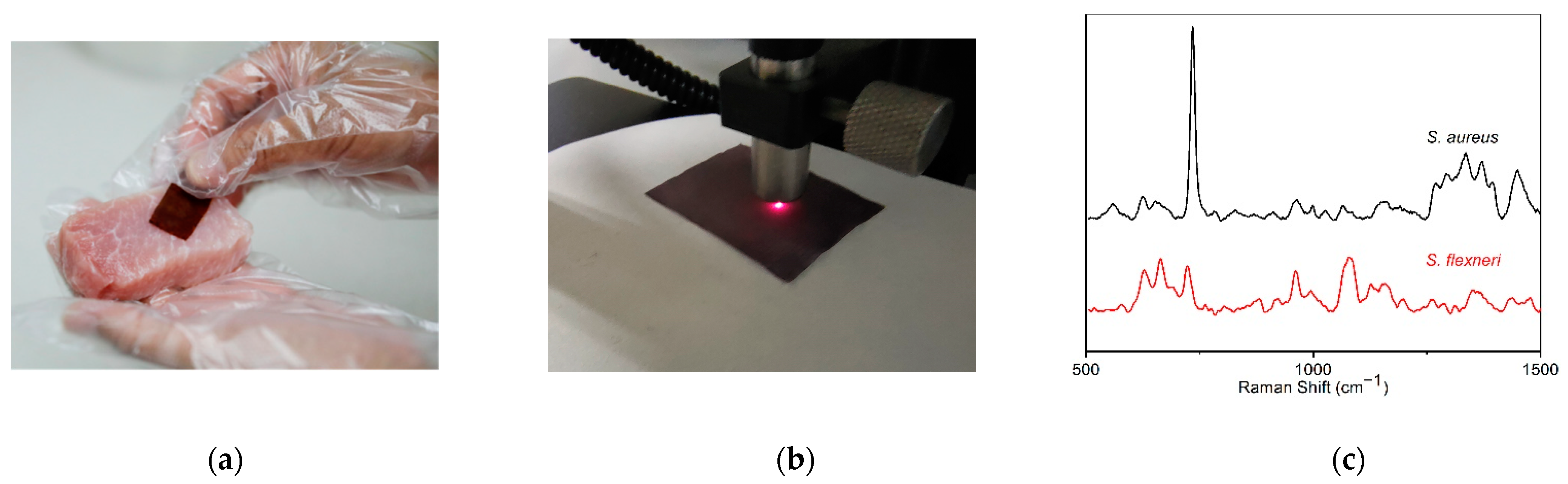
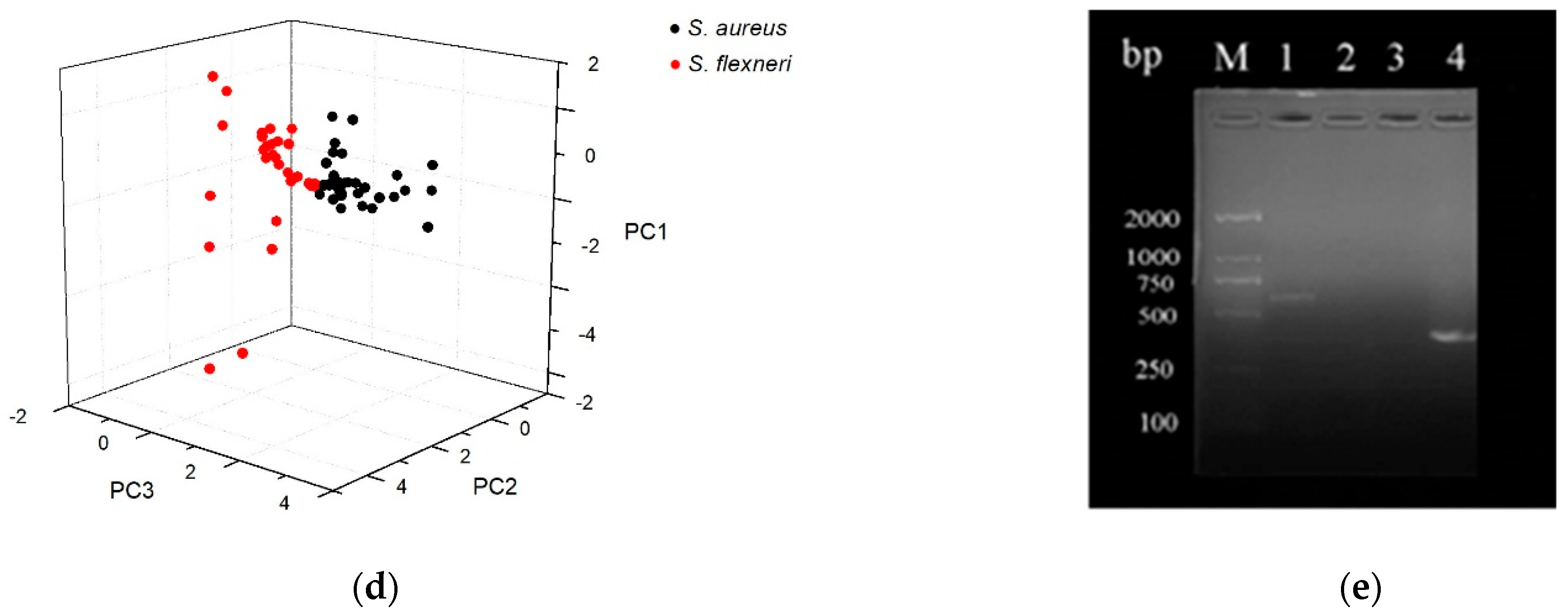
2.4. Application in the Detection of Pathogenic Bacteria
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Au-TPP Substrates
3.3. Characterization of Au NPs and the Au-TPP Substrate
3.4. SERS Detection
3.5. PCR Technology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Hassan, M.M.; Zhu, A.; Li, H.; Chen, Q. Dual-mode of magnetic assisted Au@Ag SERS tags and cationic conjugated UCNPs for qualitative and quantitative analysis of multiple foodborne pathogens. Sens. Actuators B Chem. 2021, 344, 130305. [Google Scholar] [CrossRef]
- Li, D.; Kumari, B.; Makabenta, J.M.; Gupta, A.; Rotello, V. Effective detection of bacteria using metal nanoclusters. Nanoscale 2019, 11, 22172–22181. [Google Scholar] [CrossRef]
- Xu, Y.; Hassan, M.M.; Sharma, A.S.; Li, H.; Chen, Q. Recent advancement in nano-optical strategies for detection of pathogenic bacteria and their metabolites in food safety. Crit. Rev. Food Sci. Nutr. 2021, 1–19. [Google Scholar] [CrossRef]
- You, S.; Luo, K.; Jung, J.; Jeong, K.; Lee, E.; Oh, M.; Kim, Y. Gold nanoparticle-coated starch magnetic beads for the separation, concentration, and SERS-based detection of E. Coli O157:H7. ACS Appl. Mater. Inter. 2020, 12, 18292–18300. [Google Scholar] [CrossRef]
- Trnčíková, T.; Hrušková, V.; Oravcová, K.; Pangallo, D.; Kaclíková, E. Rapid and sensitive detection of staphylococcus aureus in food using selective enrichment and real-time PCR targeting a new gene marker. Food Anal. Method. 2009, 2, 241–250. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, C.; Li, L.; Song, X.; Xu, K.; Wang, J.; Liu, Y.; Fu, K.; Bao, H.; Song, D.; et al. Development of a low-cost paper-based ELISA method for rapid Escherichia coli O157:H7 detection. Anal. Biochem. 2018, 542, 58–62. [Google Scholar] [CrossRef]
- Shih, C.M.; Chang, C.L.; Hsu, M.Y.; Lin, J.Y.; Kuan, C.M.; Wang, H.K.; Huang, C.T.; Chung, M.C.; Huang, K.C.; Hsu, C.E.; et al. Paper-based ELISA to rapidly detect Escherichia coli. Talanta 2015, 145, 2–5. [Google Scholar] [CrossRef]
- Settanni, L.; Corsetti, A. The use of multiplex PCR to detect and differentiate food- and beverage-associated microorganisms: A review. J. Microbiol. Meth. 2007, 69, 1–22. [Google Scholar] [CrossRef]
- Pieczonka, N.P.W.; Aroca, R.F. Single molecule analysis by surfaced-enhanced Raman scattering. Chem. Soc. Rev. 2008, 37, 946. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Puebla, R.A.; Liz-Marzan, L.M. Traps and cages for universal SERS detection. Chem. Soc. Rev. 2012, 41, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, M.; Xu, Z. Detection of foodborne pathogens by surface enhanced Raman spectroscopy. Front. Microbiol. 2018, 9, 1236. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Girmatsion, M.; Li, H.W.; Xie, Y.; Yao, W.; Qian, H.; Abraha, B.; Mahmud, A. Rapid and ultrasensitive detection of food contaminants using surface-enhanced Raman spectroscopy-based methods. Crit. Rev. Food Sci. Nutr. 2021, 61, 3555–3568. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Ying, Y.; Yu, R.; Long, Y. In situ food-borne pathogen sensors in a nanoconfined space by surface enhanced Raman scattering. Microchim. Acta. 2021, 188, 201. [Google Scholar] [CrossRef]
- Jarvis, R.M.; Goodacre, R. Characterisation and identification of bacteria using SERS. Chem. Soc. Rev. 2008, 37, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Dina, N.E.; Gherman, A.M.R.; Colniță, A.; Marconi, D.; Sârbu, C. Fuzzy characterization and classification of bacteria species detected at single-cell level by surface-enhanced Raman scattering. Spectrochim. Acta A 2021, 247, 119149. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, S.; Petersen, M.; Wang, S.; Lu, X. Detection and characterization of antibiotic-resistant bacteria using surface-enhanced Raman spectroscopy. Nanomaterials 2018, 8, 762. [Google Scholar] [CrossRef] [Green Version]
- Kashif, M.; Majeed, M.I.; Nawaz, H.; Rashid, N.; Abubakar, M.; Ahmad, S.; Ali, S.; Hyat, H.; Bashir, S.; Batool, F.; et al. Surface-enhanced Raman spectroscopy for identification of food processing bacteria. Spectrochim. Acta A 2021, 261, 119989. [Google Scholar] [CrossRef]
- Trebolazabala, J.; Maguregui, M.; Morillas, H.; de Diego, A.; Madariaga, J.M. Portable Raman spectroscopy for an in-situ monitoring the ripening of tomato (Solanum lycopersicum) fruits. Spectrochim. Acta A 2017, 180, 138–143. [Google Scholar] [CrossRef]
- Jehlička, J.; Culka, A.; Mana, L.; Oren, A. Comparison of miniaturized Raman spectrometers for discrimination of carotenoids of halophilic microorganisms. Front. Microbiol. 2019, 10, 1155. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Jiang, X.; He, Y. Highly sensitive and reproducible silicon-based surface-enhanced Raman scattering sensors for real applications. Analyst 2016, 141, 5010–5019. [Google Scholar] [CrossRef]
- Liang, P.; Zhou, Y.F.; Zhang, D.; Chang, Y.; Dong, Q.; Huang, J.; Rao, B.; Chen, B.; Yu, Z.; Ni, D.; et al. SERS based determination of vanillin and its methyl and ethyl derivatives using flower-like silver nanoparticles on a silicon wafer. Microchim. Acta 2019, 186, 302. [Google Scholar] [CrossRef] [PubMed]
- Bassi, B.; Albini, B.; Agostino, D.A.; Dacarro, G.; Pallavicini, P.; Galinetto, P.; Taglietti, A. Robust, reproducible, recyclable SERS substrates: Monolayers of gold nanostars grafted on glass and coated with a thin silica layer. Nanotechnology 2019, 30, 25302. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, A.; Giovannozzi, A.M.; Mandrile, L.; Sacco, A.; Rossi, A.M.; Taglietti, A. In situ seed-growth synthesis of silver nanoplates on glass for the detection of food contaminants by surface enhanced Raman scattering. Talanta 2020, 216, 120936. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Hasi, W.; Han, S.; Lin, X.; Wang, L. A dual-functional PDMS-assisted paper-based SERS platform for the reliable detection of thiram residue both on fruit surfaces and in juice. Anal. Methods 2020, 12, 2571–2579. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.; Xiao, X.; Zhang, T.; Yang, H.; Zhao, Y.; Wang, J.; Jiang, K.; Fan, S.; Li, Q. Flexible, transparent and highly sensitive SERS substrates with cross-nanoporous structures for fast on-site detection. Nanoscale 2018, 10, 15195–15204. [Google Scholar] [CrossRef]
- Xie, T.; Cao, Z.; Li, Y.; Li, Z.; Zhang, F.L.; Gu, Y.; Han, C.; Yang, G.; Qu, L. Highly sensitive SERS substrates with multi-hot spots for on-site detection of pesticide residues. Food Chem. 2022, 381, 132208. [Google Scholar] [CrossRef]
- Zong, C.; Ge, M.; Pan, H.; Wang, J.; Nie, X.; Zhang, Q.; Zhao, W.; Liu, X.; Yu, Y. In situ synthesis of low-cost and large-scale flexible metal nanoparticle-polymer composite films as highly sensitive SERS substrates for surface trace analysis. RSC Adv. 2019, 9, 2857–2864. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Santhanam, V. Paper swab based SERS detection of non-permitted colourants from dals and vegetables using a portable spectrometer. Anal. Chim. Acta 2019, 1090, 106–113. [Google Scholar] [CrossRef]
- Lee, C.H.; Tian, L.; Singamaneni, S. Paper-based SERS swab for rapid trace detection on real-world surfaces. ACS Appl. Mater. Inter. 2010, 2, 3429–3435. [Google Scholar] [CrossRef]
- Li, Z.Y.; Huang, X.; Lu, G. Recent developments of flexible and transparent SERS substrates. J. Mater. Chem. C 2020, 8, 3956–3969. [Google Scholar] [CrossRef]
- Zhang, D.; Pu, H.; Huang, L.; Sun, D. Advances in flexible surface-enhanced Raman scattering (SERS) substrates for nondestructive food detection: Fundamentals and recent applications. Trends Food Sci. Tech. 2021, 109, 690–701. [Google Scholar] [CrossRef]
- Zhang, Z.; Si, T.; Liu, J.; Zhou, G. In-situ grown silver nanoparticles on nonwoven fabrics based on mussel-inspired polydopamine for highly sensitive SERS carbaryl pesticides detection. Nanomaterials 2019, 9, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalachyova, Y.; Erzina, M.; Postnikov, P.; Svorcik, V.; Lyutakov, O. Flexible SERS substrate for portable Raman analysis of biosamples. Appl. Surf. Sci. 2018, 458, 95–99. [Google Scholar] [CrossRef]
- He, D.; Hu, B.; Yao, Q.; Wang, K.; Yu, S. Large-scale synthesis of flexible free-standing SERS substrates with high sensitivity: Electrospun PVA nanofibers embedded with controlled alignment of silver nanoparticles. ACS Nano 2009, 3, 3993–4002. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, S.M.; Burgess, I.J. Electrochemical and infrared spectroscopy studies of 4-mercaptobenzoic acid SAMs on gold surfaces. Electrochim. Acta 2008, 53, 6759–6767. [Google Scholar] [CrossRef]
- Rippa, M.; Castagna, R.; Tkachenko, V.; Zhou, J.; Petti, L. Engineered nanopatterned substrates for high-sensitive localized surface plasmon resonance: An assay on biomacromolecules. J. Mater. Chem. B 2017, 5, 5473–5478. [Google Scholar] [CrossRef] [PubMed]
- Orendorff, C.J.; Gole, A.; Sau, T.K.; Murphy, C.J. Surface-enhanced Raman spectroscopy of self-assembled monolayers: sandwich architecture and nanoparticle shape dependence. Anal. Chem. 2005, 77, 3261–3266. [Google Scholar] [CrossRef]
- Geng, Z.; Zheng, J.; Li, Y.; Chen, Y.; Wang, P.; Han, C.; Yang, G.; Qu, L. A disposable paper-based hydrophobic substrate for highly sensitive surface-enhanced Raman scattering detection. Talanta 2020, 220, 121340. [Google Scholar] [CrossRef]
- Lin, S.; Lin, X.; Han, S.; Liu, Y.; Hasi, W.; Wang, L. Flexible fabrication of a paper-fluidic SERS sensor coated with a monolayer of core–shell nanospheres for reliable quantitative SERS measurements. Anal. Chim. Acta 2020, 1108, 167–176. [Google Scholar] [CrossRef]
- Wu, M.; Li, P.; Zhu, Q.; Wu, M.; Li, H.; Lu, F. Functional paper-based SERS substrate for rapid and sensitive detection of sudan dyes in herbal medicine. Spectrochim. Acta A 2018, 196, 110–116. [Google Scholar] [CrossRef]
- Teixeira, C.A.; Poppi, R.J. Paper-based SERS substrate and one-class classifier to monitor thiabendazole residual levels in extracts of mango peels. Spectrochim. Acta A 2020, 229, 117913. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xi, J.; Chen, H.; Li, S.; Zhang, L.; Li, P.; Wu, W. Flexible 2D nanocellulose-based SERS substrate for pesticide residue detection. Carbohyd. Polym. 2022, 277, 118890. [Google Scholar] [CrossRef] [PubMed]
- Villa, J.E.L.; Quiñones, N.R.; Fantinatti-Garboggini, F.; Poppi, R.J. Fast discrimination of bacteria using a filter paper-based SERS platform and PLS-DA with uncertainty estimation. Anal. Bioanal. Chem. 2019, 411, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.J.; Quaresma, P.; Peixoto De Almeida, M.; Araújo, A.; Pereira, E.; Fortunato, E.; Martins, R.; Franco, R.; Águas, H. Office paper decorated with silver nanostars—An alternative cost effective platform for trace analyte detection by SERS. Sci. Rep. 2017, 7, 2480. [Google Scholar] [CrossRef] [PubMed]
- Torul, H.; Çiftçi, H.; Çetin, D.; Suludere, Z.; Boyacı, I.H.; Tamer, U. PAPER membrane-based SERS platform for the determination of glucose in blood samples. Anal. Bioanal. Chem. 2015, 407, 8243–8251. [Google Scholar] [CrossRef]
- Huang, W.; Cheng, K.; Shyub, J. Flexible SERS substrate of silver nanoparticles on cotton swabs for rapid in situ detection of melamine. Nanoscale Adv. 2022, 4, 1164–1172. [Google Scholar] [CrossRef]
- Wang, T.; Barveen, N.R.; Liu, Z.; Chen, C.; Chou, M. Transparent, flexible plasmonic Ag NP/PMMA substrates using chemically patterned ferroelectric crystals for detecting pesticides on curved surfaces. ACS Appl. Mater. Inter. 2021, 13, 34910–34922. [Google Scholar] [CrossRef]
- Restaino, S.M.; White, I.M. A critical review of flexible and porous SERS sensors for analytical chemistry at the point-of-sample. Anal. Chim. Acta 2019, 1060, 17–29. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, W.; Zhong, H.; Yang, F.; Fu, W.; Ling, Y.; Zhang, Z. Robust quantitative SERS analysis with relative Raman scattering intensities. Talanta 2021, 221, 121465. [Google Scholar] [CrossRef]
- Accardo, A.; Gentile, F.; Mecarini, F.; De Angelis, F.; Burghammer, M.; Di Fabrizio, E.; Riekel, C. In situ X-ray scattering studies of protein solution droplets drying on micro- and nanopatterned superhydrophobic PMMA surfaces. Langmuir 2010, 26, 15057–15064. [Google Scholar] [CrossRef]
- Zhang, C.; Yi, P.; Peng, L.; Lai, X.; Chen, J.; Huang, M.; Ni, J. Continuous fabrication of nanostructure arrays for flexible surface enhanced Raman scattering substrate. Sci. Rep. 2017, 7, 39814. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.; Caro, C.; Mendes, M.J.; Nunes, D.; Fortunato, E.; Franco, R.; Águas, H.; Martins, R. Highly efficient nanoplasmonic SERS on cardboard packaging substrates. Nanotechnology 2014, 25, 415202. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Luo, C.; Xu, L.; Ji, H.; Ouyang, Q.; Yu, D.; Chen, Y. Artificial lotus leaf by nanocasting. Langmuir 2005, 21, 8978–8981. [Google Scholar] [CrossRef] [PubMed]
- Reyssat, M.; Pépin, A.; Marty, F.; Chen, Y.; Quéré, D. Bouncing transitions on microtextured materials. Europhys. Lett. 2006, 74, 306–312. [Google Scholar] [CrossRef]
- Sun, J.; Gong, L.; Wang, W.; Gong, Z.; Wang, D.; Fan, M. Surface-enhanced Raman spectroscopy for on-site analysis: A review of recent developments. Luminescence 2020, 35, 808–820. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X. Nanostructure-based surface-enhanced Raman spectroscopy techniques for pesticide and veterinary drug residues screening. B Environ. Contam. Tox. 2021, 107, 194–205. [Google Scholar] [CrossRef]
- Fu, Z.; Zhou, S.; Xia, L.; Mao, Y.; Zhu, L.; Cheng, Y.; Wang, A.; Zhang, C.; Xu, W. Juncus effusus fiber-based cellulose cigarette filter with 3D hierarchically porous structure for removal of PAHs from mainstream smoke. Carbohyd. Polym. 2020, 241, 116308. [Google Scholar] [CrossRef]
- Altuntaş, E.E.; Sümer, Z. Biocompatibility evaluation of cigarette and carbon papers used in repair of traumatic tympanic membrane perforations: Experimental study. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 81–86. [Google Scholar] [CrossRef]
- Shen, J.; Li, J.; Qian, X.; Ren, W.; Fatehi, P. A review on engineering of cellulosic cigarette paper to reduce carbon monoxide delivery of cigarettes. Carbohyd. Polym. 2014, 101, 769–775. [Google Scholar] [CrossRef]
- Zumbado, M.; Luzardo, O.P.; Rodríguez-Hernández, Á.; Boada, L.D.; Henríquez-Hernández, L.A. Differential exposure to 33 toxic elements through cigarette smoking, based on the type of tobacco and rolling paper used. Environ. Res. 2019, 169, 368–376. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Yi, Z.; Glushenkov, A.M.; Kong, L. Plasmonic substrates for surface enhanced Raman scattering. Anal. Chim. Acta 2017, 984, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, J.; Li, Y.; Wang, B.; Yang, S.; Chen, W.; Wu, X.; Guo, J.; Ma, X. SERS substrate fabrication for biochemical sensing: Towards point-of-care diagnostics. J. Mater. Chem. B 2021, 9, 8378–8388. [Google Scholar] [CrossRef] [PubMed]
- Abu Hatab, N.A.; Oran, J.M.; Sepaniak, M.J. Surface-enhanced Raman spectroscopy substrates created via electron beam lithography and nanotransfer printing. ACS Nano 2008, 2, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Mahurin, S.M.; John, J.; Sepaniak, M.J.; Dai, S. A reusable surface-enhanced Raman scattering (SERS) substrate prepared by atomic layer deposition of alumina on a multi-layer gold and silver film. Appl. Spectrosc. 2011, 65, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, L.; Khan, I.M.; Wang, Z.; Ma, X. Flexible paper-based SERS substrate strategy for rapid detection of methyl parathion on the surface of fruit. Spectrochim. Acta A 2020, 231, 118104. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.; Liu, C.; Foda, M.F.; Zhu, Y. Strawberry-like SiO2/Ag nanocomposites immersed filter paper as SERS substrate for acrylamide detection. Food Chem. 2020, 328, 127106. [Google Scholar] [CrossRef]
- Chen, J.; Huang, M.; Kong, L.; Lin, M. Jellylike flexible nanocellulose SERS substrate for rapid in-situ non-invasive pesticide detection in fruits/vegetables. Carbohydr. Polym. 2019, 205, 596–600. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Hu, Z.; Yu, G.; Yang, D.; Zhao, J. Label and label-free based surface-enhanced Raman scattering for pathogen bacteria detection: A review. Biosens. Bioelectron. 2017, 94, 131–140. [Google Scholar] [CrossRef]
- Wei, X.D.; Zheng, D.W.; Zhang, P.; Lin, T.F.; Wang, H.Q.; Zhu, Y.W. Surface-enhanced Raman scattering investigation of bovine serum albumin by Au nanoparticles with different sizes. J. Appl. Biomater. Func. 2018, 16, 157–162. [Google Scholar]

| Sample | Prediction Group | Total | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| S. aureus | S. flexneri | ||||
| S. aureus | 34 | 0 | 34 | 100 | 100 |
| S. flexneri | 0 | 30 | 30 | 100 | 100 |
| Primer | Sequence (5′-3′) | Length |
|---|---|---|
| nuc-1 | CGGTTTCGAAAGGGCAATACGCAAAGAGG | 607 bp |
| nuc-2 | CGATTGACCTGAATCAGCGTTGTCTTCGC | |
| ipaH-1 | CGTCCGATACCGTCTCTGCACGCAATA | 406 bp |
| ipaH-2 | CGCCGACACGCCATAGAAACGCATTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Zheng, D.; Wang, H.; Lin, T.; Liu, W.; Wang, X.; Lu, W.; Liu, M.; Liu, W.; Zhang, Y.; et al. In Situ Collection and Rapid Detection of Pathogenic Bacteria Using a Flexible SERS Platform Combined with a Portable Raman Spectrometer. Int. J. Mol. Sci. 2022, 23, 7340. https://doi.org/10.3390/ijms23137340
Zhao H, Zheng D, Wang H, Lin T, Liu W, Wang X, Lu W, Liu M, Liu W, Zhang Y, et al. In Situ Collection and Rapid Detection of Pathogenic Bacteria Using a Flexible SERS Platform Combined with a Portable Raman Spectrometer. International Journal of Molecular Sciences. 2022; 23(13):7340. https://doi.org/10.3390/ijms23137340
Chicago/Turabian StyleZhao, Huimin, Dawei Zheng, Huiqin Wang, Taifeng Lin, Wei Liu, Xiaoli Wang, Wenjing Lu, Mengjia Liu, Wenbo Liu, Yumiao Zhang, and et al. 2022. "In Situ Collection and Rapid Detection of Pathogenic Bacteria Using a Flexible SERS Platform Combined with a Portable Raman Spectrometer" International Journal of Molecular Sciences 23, no. 13: 7340. https://doi.org/10.3390/ijms23137340





