Dually Responsive Nanoparticles for Drug Delivery Based on Quaternized Chitosan
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterization of Quaternized Chitosan Modified with Phenylboronic Acid
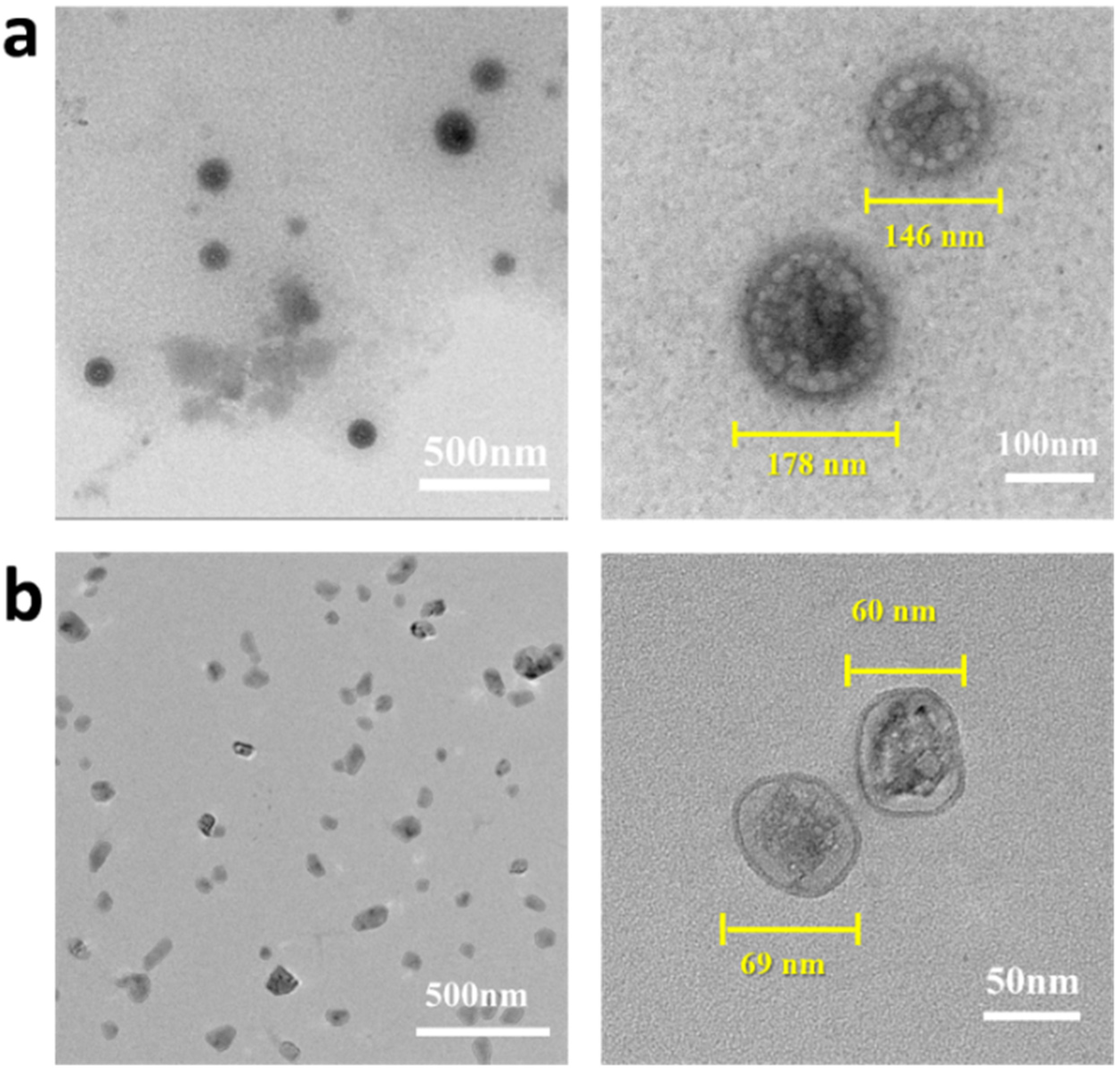
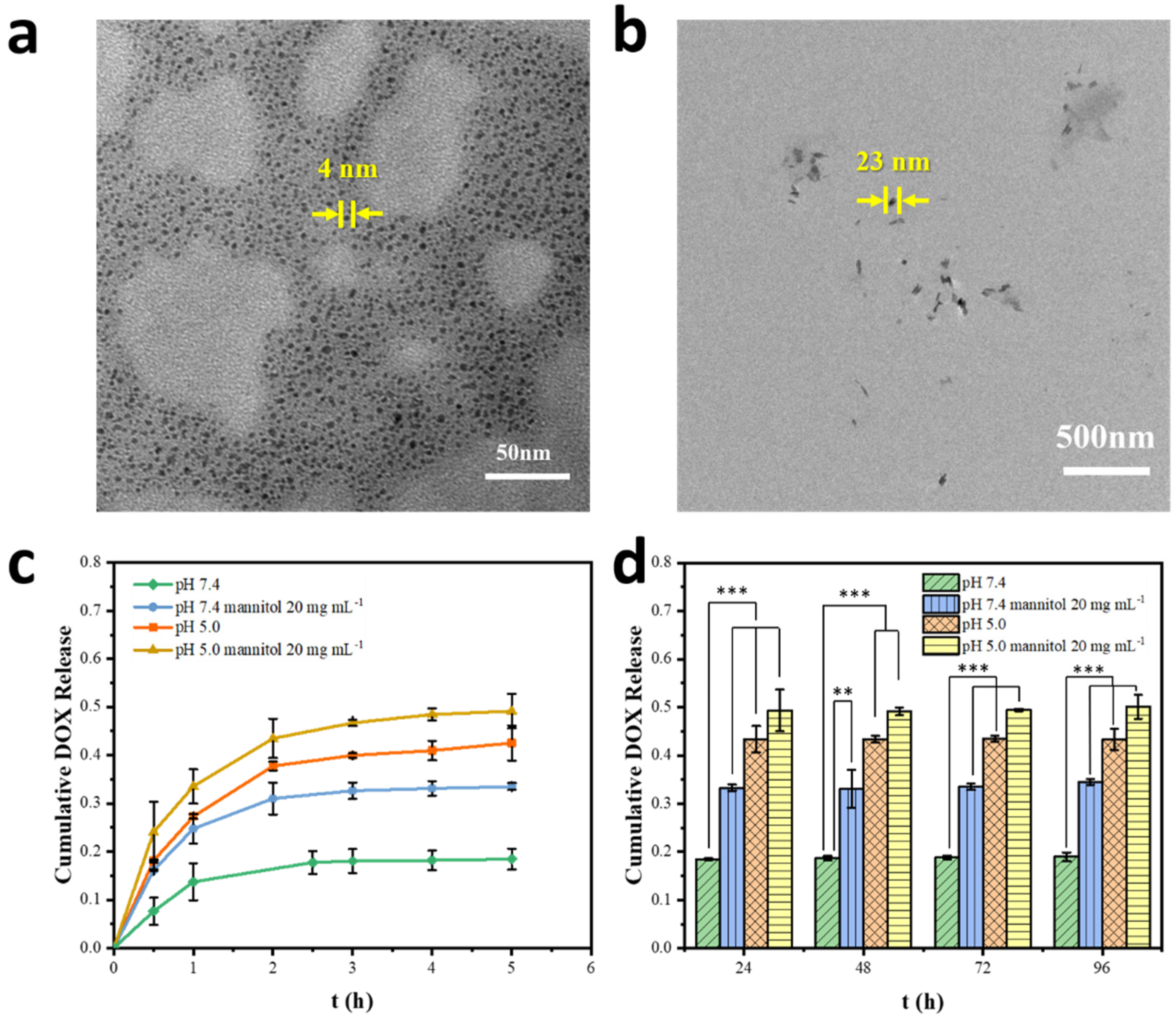
2.2. Preparation and Characterization of the Drug-Loaded Complex Nanoparticles
2.3. Dually Responsive Drug Release In Vitro
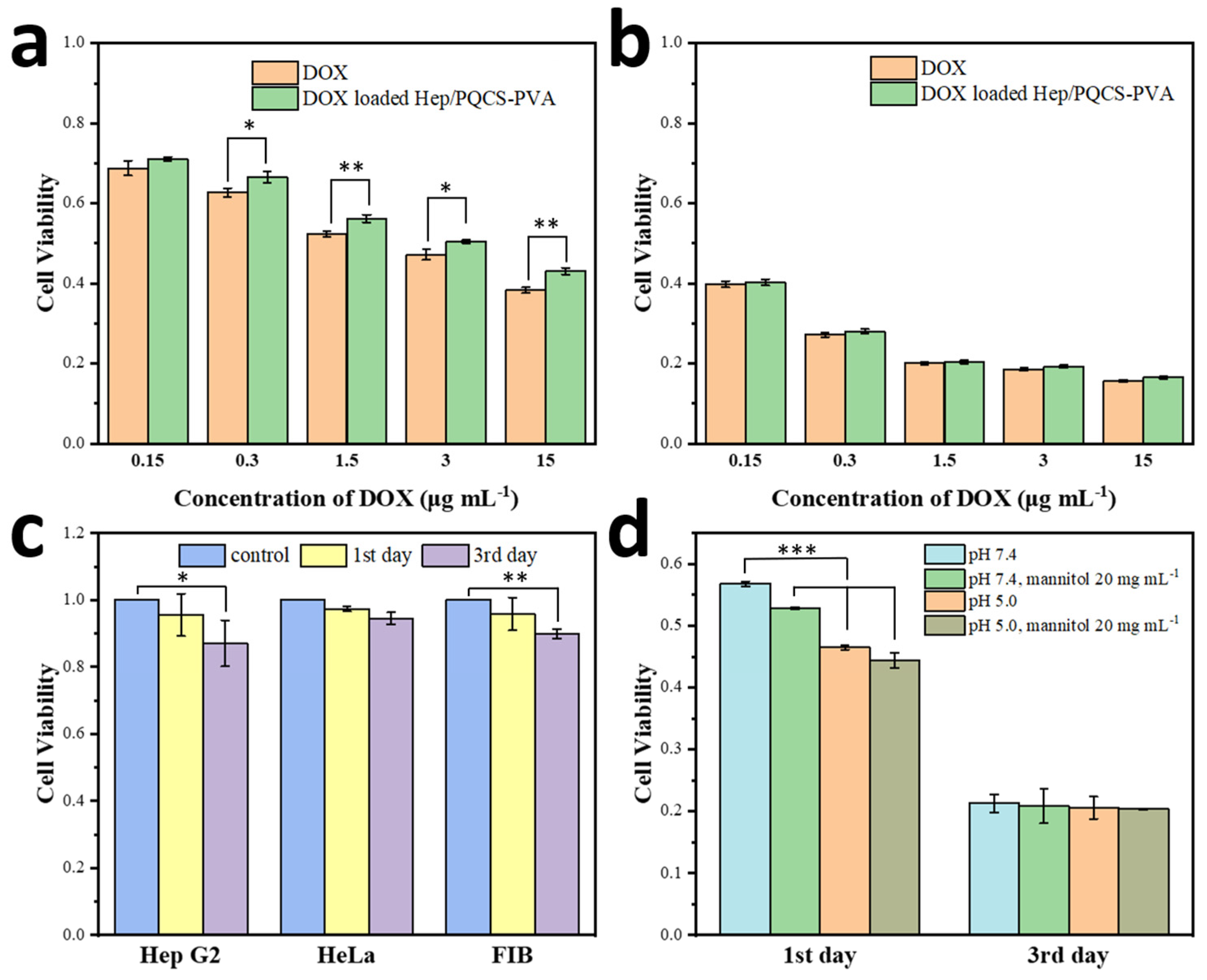
2.4. Cell Viability
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Synthesis of PQCS
4.3. Preparation of Hep/PQCS-PVA and Drug-Loaded Hep/PQCS-PVA NPs
4.4. Drug Release Estimation
4.5. Cell Toxicity Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, M.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.K.; Inamdar, N.N. Chitosan-Modifications and Applications: Opportunities Galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and Chitosan Polymers: Chemistry, Solubility and Fiber Formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Fathi, M.; Majidi, S.; Zangabad, P.S.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Chitosan-based multifunctional nanomedicines and theranostics for targeted therapy of cancer. Med. Res. Rev. 2018, 38, 2110–2136. [Google Scholar] [CrossRef]
- Mohebbi, S.; Nezhad, M.N.; Zarrintaj, P.; Jafari, S.H.; Gholizadeh, S.S.; Saeb, M.R.; Mozafari, M. Chitosan in Biomedical Engineering: A Critical Review. Curr. Stem. Cell Res. Ther. 2019, 14, 93–116. [Google Scholar] [CrossRef]
- Wang, Y.J.; Nie, J.Y.; Fang, W.; Yang, L.; Hu, Q.L.; Wang, Z.K.; Sun, J.Z.; Tang, B.Z. Sugar-Based Aggregation-Induced Emission Luminogens: Design, Structures, and Applications. Chem. Rev. 2020, 120, 4534–4577. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wu, Y.X.; Zhao, X.C.; Wang, Z.K. Fabrication and applications of bioactive chitosan-based organic-inorganic hybrid materials: A review. Carbohydr. Polym. 2021, 267, 118179. [Google Scholar] [CrossRef]
- Madamsetty, V.S.; Tavakol, S.; Moghassemi, S.; Dadashzadeh, A.; Schneible, J.D.; Fatemi, I.; Shirvani, A.; Zarrabi, A.; Azedi, F.; Dehshahri, A.; et al. Chitosan: A versatile bio-platform for breast cancer theranostics. J. Control. Release 2022, 341, 733–752. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhang, H.; Chen, K.W.; Jin, M.; Vu, S.H.; Jung, S.; He, N.N.; Zheng, Z.; Lee, M.S. Application of chitosan/alginate nanoparticle in oral drug delivery systems: Prospects and challenges. Drug Deliv. 2022, 29, 1142–1149. [Google Scholar] [CrossRef]
- Fabiano, A.; Beconcini, D.; Migone, C.; Piras, A.M.; Zambito, Y. Quaternary Ammonium Chitosans: The Importance of the Positive Fixed Charge of the Drug Delivery Systems. Int. J. Mol. Sci. 2020, 21, 6617. [Google Scholar] [CrossRef]
- Rinaudo, M. Main Properties and Current Applications of Some Polysaccharides as Biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Wang, W.Q.; Meng, Q.Y.; Li, Q.; Liu, J.B.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Inter. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naskar, S.; Koutsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target. 2019, 27, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.R.; Hua, S.Y.; Tian, Y.; Liu, J.Y. Chemical and physical chitosan hydrogels as prospective carriers for drug delivery: A review. J. Mater. Chem. B 2020, 8, 10050–10064. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef]
- Cao, S.; Deng, Y.; Zhang, L.; Aleahmad, M. Chitosan nanoparticles, as biological macromolecule-based drug delivery systems to improve the healing potential of artificial neural guidance channels: A review. Int. J. Biol. Macromol. 2022, 201, 569–579. [Google Scholar] [CrossRef]
- Pontillo, A.R.N.; Detsi, A. Nanoparticles for ocular drug delivery: Modified and non-modified chitosan as a promising biocompatible carrier. Nanomedicine 2019, 14, 1889–1909. [Google Scholar] [CrossRef]
- Qiao, F.; Ke, L.; Liu, Y.; Pei, B.; Hu, Q.; Tang, B.Z.; Wang, Z. Cationic quaternized chitosan bioconjugates with aggregation-induced emission features for cell imaging. Carbohydr. Polym. 2020, 230, 115614. [Google Scholar] [CrossRef]
- Almasi, M.; Matiasova, A.A.; Sulekova, M.; Benova, E.; Sevc, J.; Vahovska, L.; Lisnichuk, M.; Girman, V.; Zelenakova, A.; Hudak, A.; et al. In vivo study of light-driven naproxen release from gated mesoporous silica drug delivery system. Sci. Rep. 2021, 11, 20191. [Google Scholar] [CrossRef] [PubMed]
- Mihalache, C.; Rata, D.M.; Cadinoiu, A.N.; Patras, X.; Sindilar, E.V.; Bacaita, S.E.; Popa, M.; Atanase, L.I.; Daraba, O.M. Bupivacaine-loaded chitosan hydrogels for topical anesthesia in dentistry. Polym. Int. 2020, 69, 1152–1160. [Google Scholar] [CrossRef]
- Dellali, K.Z.; Dellali, M.; Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Spataru, M.-C.; Solcan, C. Assessment of Physicochemical and In Vivo Biological Properties of Polymeric Nanocapsules Based on Chitosan and Poly(N-vinyl pyrrolidone-alt-itaconic anhydride). Polymers 2022, 14, 1811. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Hu, G.; Zhao, K. Mannose-anchored quaternized chitosan/thiolated carboxymethyl chitosan composite NPs as mucoadhesive carrier for drug delivery. Carbohydr. Polym. 2022, 283, 119174. [Google Scholar] [CrossRef] [PubMed]
- Bilensoy, E. Cationic NPs for cancer therapy. Expert Opin. Drug Deliv. 2010, 7, 795–809. [Google Scholar] [CrossRef]
- Yang, G.B.; Xu, L.G.; Xu, J.; Zhang, R.; Song, G.S.; Chao, Y.; Feng, L.Z.; Han, F.X.; Dong, Z.L.; Li, B.; et al. Smart Nanoreactors for pH-Responsive Tumor Homing, Mitochondria-Targeting, and Enhanced Photodynamic-Immunotherapy of Cancer. Nano Lett. 2018, 18, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fei, J.B.; Yan, H.X.; Wang, A.H.; Li, J.B. Enzyme-Responsive Release of Doxorubicin from Monodisperse Dipeptide-Based Nanocarriers for Highly Efficient Cancer Treatment In Vitro. Adv. Funct. Mater. 2015, 25, 1193–1204. [Google Scholar] [CrossRef]
- Ashjari, M.; Kazemi, M.; Abi, M.N.; Mohammadi, M.; Rafiezadeh, S. Poly(isopropyl-oxazoline) micelle nanocarrier as dual-responsive prodrug for targeted doxorubicin delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 8. [Google Scholar] [CrossRef]
- Song, X.Y.; Yan, T.; Tian, F.; Li, F.Y.; Ren, L.L.; Li, Q.; Zhang, S.S. Aptamer Functionalized Upconversion Nanotheranostic Agent with Nuclear Targeting as the Highly Localized Drug-Delivery System of Doxorubicin. Front. Bioeng. Biotechnol. 2021, 9, 12. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, J.; Song, P.A.; Li, J.G.; Cao, S.K. Chitosan/hyaluronic acid based hollow microcapsules equipped with MXene/gold nanorods for synergistically enhanced near infrared responsive drug delivery. Int. J. Biol. Macromol. 2021, 183, 870–879. [Google Scholar] [CrossRef]
- Garg, S.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, G.; Long, Z.; Yang, G.; Wang, B. Controllable layer-by-layer assembly of PVA and phenylboronic acid-derivatized chitosan. Carbohydr. Polym. 2016, 140, 228–232. [Google Scholar] [CrossRef]
- Li, J.; Hu, W.; Zhang, Y.; Tan, H.; Yan, X.; Zhao, L.; Liang, H. pH and glucose dually responsive injectable hydrogel prepared by in situ crosslinking of phenylboronic modified chitosan and oxidized dextran. J. Polym. Sci. A Polym. Chem. 2015, 53, 1235–1244. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, W.; Xiao, K.; Berti, L.; Luo, J.; Tseng, H.P.; Fung, G.; Lam, K.S. Well-defined, reversible boronate crosslinked nano-carriers for targeted drug delivery in response to acidic pH values and cis-diols. Angew. Chem. Int. Ed. Engl. 2012, 51, 2864–2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerweck, L.E.; Seetharaman, K. Cellular pH gradient in tumor versus normal tissue: Potential exploitation for the treatment of cancer. Cancer Res. 1996, 56, 1194–1198. [Google Scholar] [PubMed]
- Overly, C.C.; Lee, K.D.; Berthiaume, E.; Hollenbeck, P.J. Quantitative Measurement of Intraorganelle pH in the Endosomal Lysosomal Pathway in Neurons by Using Ratiometric Imaging with Pyranine. Proc. Natl. Acad. Sci. USA 1995, 92, 3156–3160. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Ding, H. pH-Responsive Nanoparticles for Cancer Immunotherapy: A Brief Review. Nanomaterials 2020, 10, 1613. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, G.J.; Shih, Y.V.; Kim, T.I.; Varghese, S. Phenylboronic Acid-polymers for Biomedical Applications. Curr. Med. Chem. 2019, 26, 6797–6816. [Google Scholar] [CrossRef]
- Chao, S.; Lv, X.; Ma, N.; Shen, Z.; Zhang, F.; Pei, Y.; Pei, Z. A supramolecular nanoprodrug based on a boronate ester linked curcumin complexing with water-soluble pillar[5]arene for synergistic chemotherapies. Chem. Commun. 2020, 56, 8861–8864. [Google Scholar] [CrossRef]
- Morey, M.; Srivastava, A.; Pandit, A. Glucose-Responsive Gene Delivery at Physiological pH through Tertiary-Amine Stabilized Boronate-PVA Particles Synthesized by One-Pot Reaction. Pharmaceutics 2021, 13, 62. [Google Scholar] [CrossRef]
- Wu, J.-Z.; Williams, G.R.; Li, H.-Y.; Wang, D.-X.; Li, S.-D.; Zhu, L.-M. Insulin-loaded PLGA microspheres for glucose-responsive release. Drug Deliv. 2017, 24, 1513–1525. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Sun, H.; Alt, K.; Tardy, B.L.; Richardson, J.J.; Suma, T.; Ejima, H.; Cui, J.; Hagemeyer, C.E.; Caruso, F. Boronate-Phenolic Network Capsules with Dual Response to Acidic pH and cis-Diols. Adv. Healthc. Mater. 2015, 4, 1796–1801. [Google Scholar] [CrossRef]
- Atallah, J.; Khachfe, H.H.; Berro, J.; Assi, H.I. The use of heparin and heparin-like molecules in cancer treatment: A review. Cancer Treat. Res. Commun. 2020, 24, 100192. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-N.; Mao, Z.-X.; Wu, Y.; Liang, M.-X.; Wang, D.-D.; Chen, X.; Chang, P.-a.; Zhang, W.; Tang, J.-H. The anti-cancer properties of heparin and its derivatives: A review and prospect. Cell Adhes. Migr. 2020, 14, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Sun, Y.; Wang, P.; Zhang, R.; Huo, C.; Gao, T.; Song, C.; Xing, J.; Dong, Y. Mucoadhesive nanoparticles-based oral drug delivery systems enhance ameliorative effects of low molecular weight heparin on experimental colitis. Carbohydr. Polym. 2020, 246, 116660. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-J.; Young, Y.-A.; Tsai, T.-N.; Cheng, K.-M.; Chen, X.-A.; Chen, Y.-C.; Chen, C.-C.; Young, J.-J.; Hong, P.-D. Positively charged gold nanoparticles capped with folate quaternary chitosan: Synthesis, cytotoxicity, and uptake by cancer cells. Carbohydr. Polym. 2018, 183, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Kajani, A.A.; Javanmard, S.H.; Asadnia, M.; Razmjou, A. Recent Advances in Nanomaterials Development for Nanomedicine and Cancer. ACS Appl. Bio Mater. 2021, 4, 5908–5925. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Vu, T.T.; Karthika, V.; Jo, S.-H.; Jo, Y.-J.; Seo, J.-W.; Oh, C.-W.; Park, S.-H.; Lim, K.T. Dual cross-linked chitosan/alginate hydrogels prepared by Nb-Tz ’click’ reaction for pH responsive drug delivery. Carbohydr. Polym. 2022, 288, 119389. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Tang, C.; Yin, C. Dual stimulus-responsive chitosan-based nanoparticles co-delivering doxorubicin and quercetin for cancer therapy. Mater. Lett. 2021, 305, 130826. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Y.; Fei, W.; Zhao, Y.; Liu, Y.; Yan, J.; Chen, Y.; Zheng, C.; Zhang, M. Redox-Responsive and Electrically Neutral PLGA Nanoparticles for siRNA Delivery in Human Cervical Carcinoma Cells. J. Pharm. Innov. 2022, 1–13. [Google Scholar] [CrossRef]
- Veselov, V.V.; Nosyrev, A.E.; Jicsinszky, L.; Alyautdin, R.N.; Cravotto, G. Targeted Delivery Methods for Anticancer Drugs. Cancers 2022, 14, 622. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Shao, J.; Liang, H.; Na, H.; Zhu, J. Folate-conjugated dually responsive micelles for targeted anticancer drug delivery. RSC Adv. 2016, 6, 35658–35667. [Google Scholar] [CrossRef]
- Wang, X.; Wei, B.; Cheng, X.; Wang, J.; Tang, R. 3-Carboxyphenylboronic acid-modified carboxymethyl chitosan nanoparticles for improved tumor targeting and inhibitory. Eur. J. Pharm. Biopharm. 2017, 113, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Smoum, R.; Rubinstein, A.; Srebnik, M. Chitosan-pentaglycine-phenylboronic acid conjugate: A potential colon-specific platform for calcitonin. Bioconjugate Chem. 2006, 17, 1000–1007. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, F.; Jiang, Z.; Fang, W.; Sun, J.; Hu, Q. Dually Responsive Nanoparticles for Drug Delivery Based on Quaternized Chitosan. Int. J. Mol. Sci. 2022, 23, 7342. https://doi.org/10.3390/ijms23137342
Qiao F, Jiang Z, Fang W, Sun J, Hu Q. Dually Responsive Nanoparticles for Drug Delivery Based on Quaternized Chitosan. International Journal of Molecular Sciences. 2022; 23(13):7342. https://doi.org/10.3390/ijms23137342
Chicago/Turabian StyleQiao, Fenghui, Zhiqi Jiang, Wen Fang, Jingzhi Sun, and Qiaoling Hu. 2022. "Dually Responsive Nanoparticles for Drug Delivery Based on Quaternized Chitosan" International Journal of Molecular Sciences 23, no. 13: 7342. https://doi.org/10.3390/ijms23137342




