Antidepressant Effect of Intermittent Long-Term Systemic Administration of Irisin in Mice
Abstract
:1. Introduction
2. Results
2.1. Irisin Long-Term Systemic Administration Effect on Mouse Weight
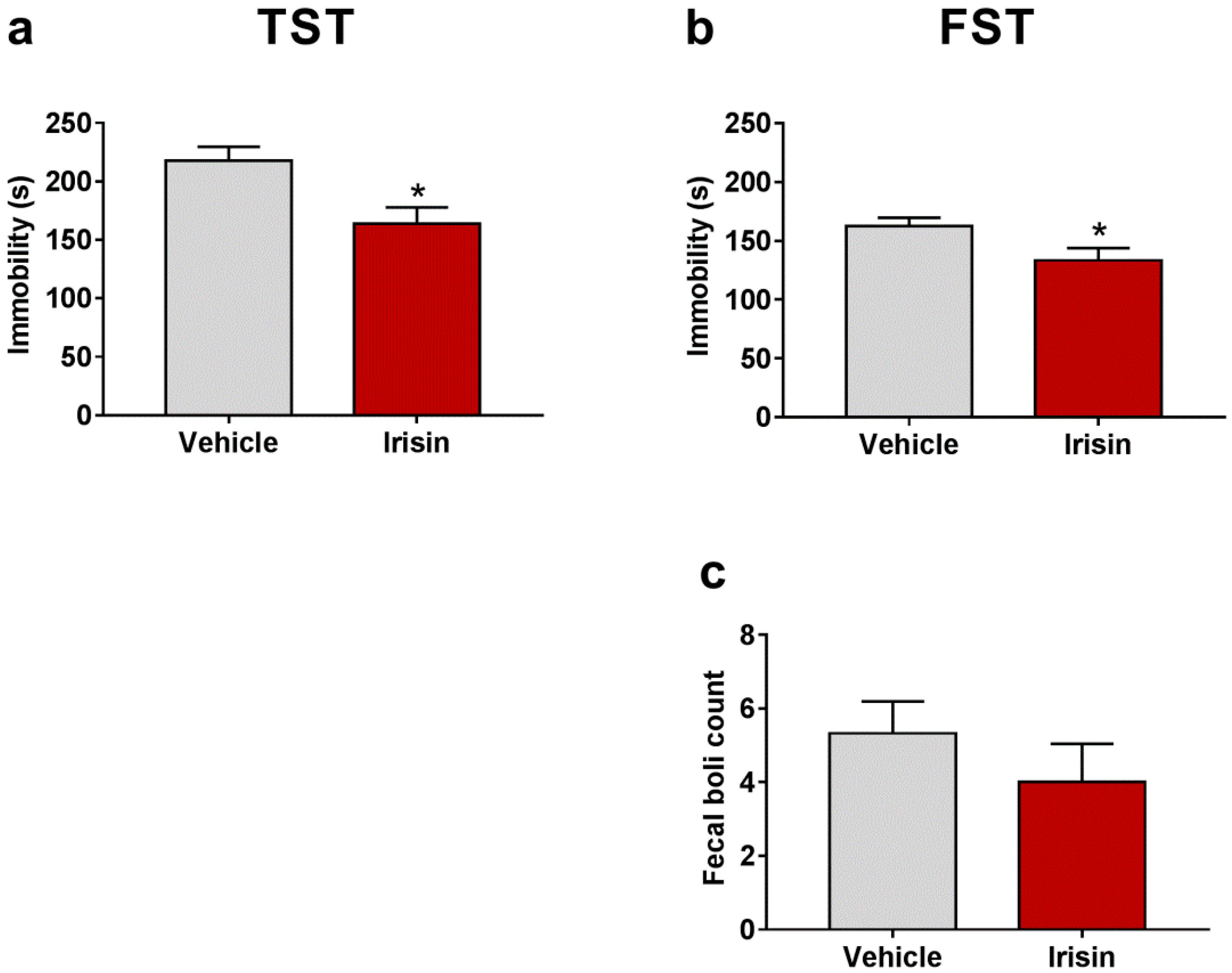
2.2. Irisin Long-Term Systemic Administration Effect on Mouse Behavior in the FST and TST
2.3. Irisin Long-Term Systemic Administration Effect on Locomotor Activity in the OFT
2.4. Irisin Effect on Serum Cortisol Levels
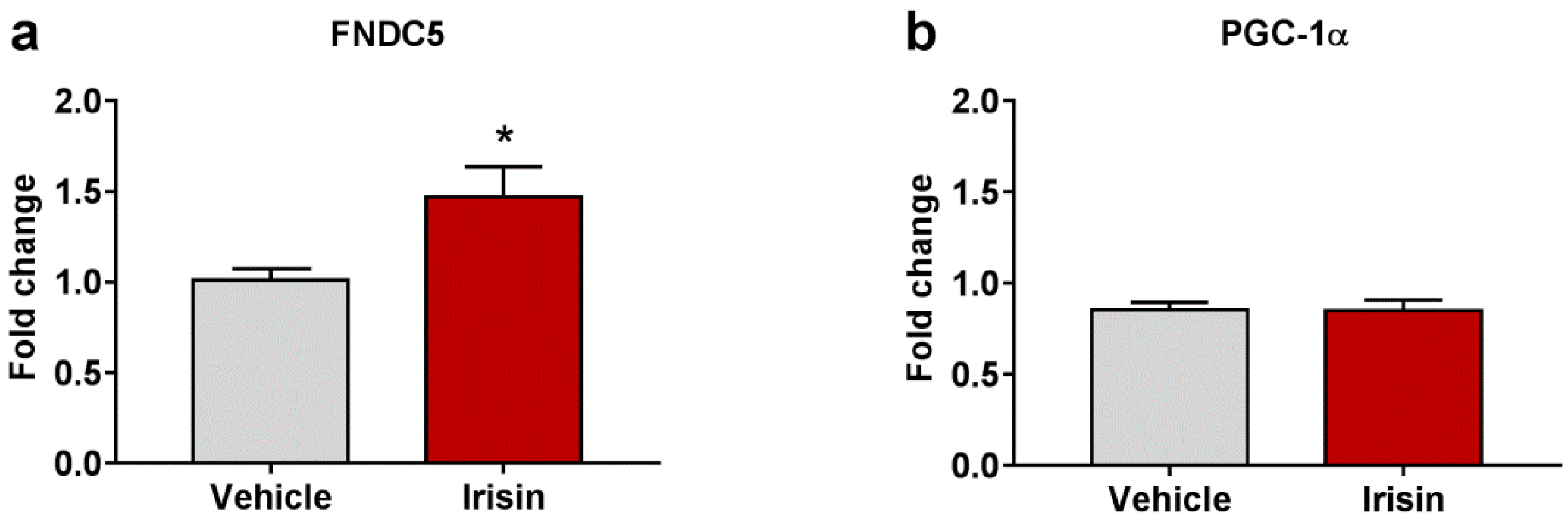
2.5. Irisin Long-Term Systemic Administration Effect on FNDC5 and PGC-1α Gene Expression in Brain
2.6. Irisin Impact on Neurotrophic/Growth Factors Expression
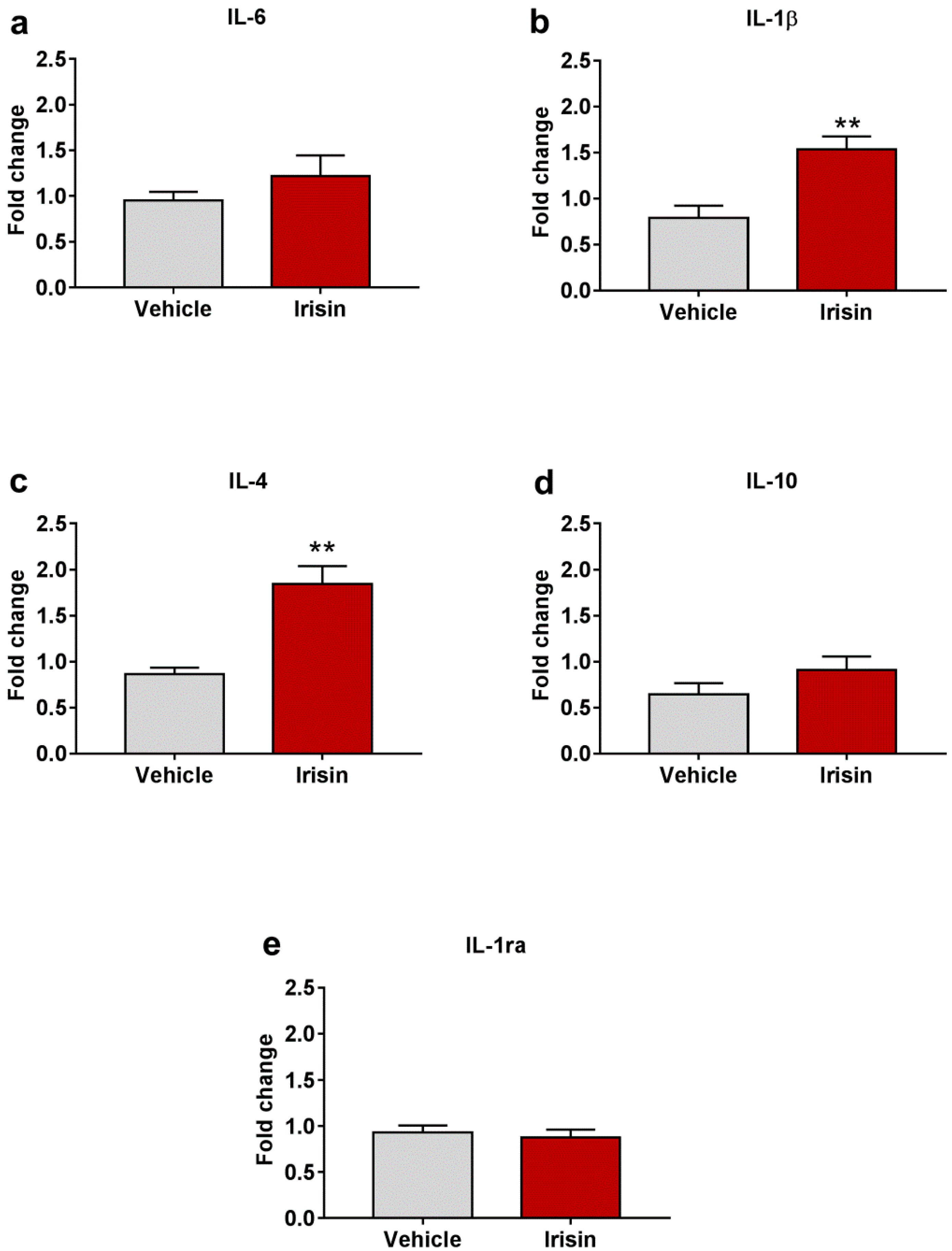
2.7. Irisin Influence on Cytokine Expression in Brain
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Irisin Treatment
4.3. Behavioral Tests
4.3.1. Forced Swim Test (FST)
4.3.2. Tail Suspension Test (TST)
4.3.3. Open Field Test (OFT)
4.4. Cortisol Determination
4.5. RNA Extraction, cDNA Synthesis, and qRT-PCR Assays on Brain Tissues
4.6. Data Processing and Statistical Analyses
5. Conclusions
6. Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Depression and other Common Mental Disorders: Global Health Estimates 2017. Available online: https://apps.who.int/iris/handle/10665/254610 (accessed on 31 May 2022).
- Gładka, A.; Zatoński, T.; Rymaszewska, J. Association between the long-term exposure to air pollution and depression. Adv. Clin. Exp. Med. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Carrera-González, M.D.P.; Cantón-Habas, V.; Rich-Ruiz, M. Aging, depression and dementia: The inflammatory process. Adv. Clin. Exp. Med. 2022, 31, 469–473. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Hunt, C.; Cordeiro, T.M.e.; Suchting, R.; de Dios, C.; Leal, V.A.C.; Soares, J.C.; Dantzer, R.; Teixeira, A.L.; Selvaraj, S. Effect of immune activation on the kynurenine pathway and depression symptoms—A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 514–523. [Google Scholar] [CrossRef]
- Tanaka, M.; Spekker, E.; Szabó, Á.; Polyák, H.; Vécsei, L. Modelling the neurodevelopmental pathogenesis in neuropsychiatric disorders. Bioactive kynurenines and their analogues as neuroprotective agents-in celebration of 80th birthday of Professor Peter Riederer. J. Neural Transm. 2022, 129, 627–642. [Google Scholar] [CrossRef]
- Erabi, H.; Okada, G.; Shibasaki, C.; Setoyama, D.; Kang, D.; Takamura, M.; Yoshino, A.; Fuchikami, M.; Kurata, A.; Kato, T.A.; et al. Kynurenic acid is a potential overlapped biomarker between diagnosis and treatment response for depression from metabolome analysis. Sci. Rep. 2020, 10, 16822. [Google Scholar] [CrossRef]
- Spekker, E.; Tanaka, M.; Szabó, Á.; Vécsei, L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines 2021, 10, 76. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Monitoring the kynurenine system: Concentrations, ratios or what else? Adv. Clin. Exp. Med. 2021, 30, 775–778. [Google Scholar] [CrossRef]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines 2022, 10, 849. [Google Scholar] [CrossRef]
- Zhang, F.-F.; Peng, W.; Sweeney, J.A.; Jia, Z.-Y.; Gong, Q.-Y. Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci. Ther. 2018, 24, 994–1003. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, S.; Fabius, J.H.; Moravkova, K.; Fracasso, A.; Borgomaneri, S. The Neurobiological Correlates of Gaze Perception in Healthy Individuals and Neurologic Patients. Biomedicines 2022, 10, 627. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Garofalo, S.; di Pellegrino, G.; Starita, F. Revaluing the Role of vmPFC in the Acquisition of Pavlovian Threat Conditioning in Humans. J. Neurosci. 2020, 40, 8491–8500. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Mol. Psychiatry 2022, 27, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S. Neurobiological advances of learned fear in humans. Adv. Clin. Exp. Med. 2022, 31, 217–221. [Google Scholar] [CrossRef]
- Battaglia, S.; Thayer, J.F. Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends Neurosci. 2022, 45, 504–506. [Google Scholar] [CrossRef]
- Battaglia, S.; Orsolini, S.; Borgomaneri, S.; Barbieri, R.; Diciotti, S.; di Pellegrino, G. Characterizing cardiac autonomic dynamics of fear learning in humans. Psychophysiology 2022, e14122. [Google Scholar] [CrossRef]
- Chen, C. Recent advances in the study of the comorbidity of depressive and anxiety disorders. Adv. Clin. Exp. Med. 2022, 31, 355–358. [Google Scholar] [CrossRef]
- Al-Harbi, K.S. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient. Prefer Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef] [Green Version]
- Martinsen, E.W. Physical activity in the prevention and treatment of anxiety and depression. Nord. J. Psychiatry 2008, 62, 25–29. [Google Scholar] [CrossRef]
- Hu, M.X.; Turner, D.; Generaal, E.; Bos, D.; Ikram, M.K.; Ikram, M.A.; Cuijpers, P.; Penninx, B.W.J.H. Exercise interventions for the prevention of depression: A systematic review of meta-analyses. BMC Public Health 2020, 20, 1255. [Google Scholar] [CrossRef]
- Duman, R.S. Neurotrophic factors and regulation of mood: Role of exercise, diet and metabolism. Neurobiol. Aging 2005, 26, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Valaris, S.; Young, M.F.; Haley, E.B.; Luo, R.; Bond, S.F.; Mazuera, S.; Kitchen, R.R.; Caldarone, B.J.; Bettio, L.E.B.; et al. Exercise hormone irisin is a critical regulator of cognitive function. Nat. Metab. 2021, 3, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Pignataro, P.; Dicarlo, M.; Zerlotin, R.; Zecca, C.; Dell’Abate, M.T.; Buccoliero, C.; Logroscino, G.; Colucci, S.; Grano, M. FNDC5/Irisin System in Neuroinflammation and Neurodegenerative Diseases: Update and Novel Perspective. Int. J. Mol. Sci. 2021, 22, 1605. [Google Scholar] [CrossRef]
- Wang, S.; Pan, J. Irisin ameliorates depressive-like behaviors in rats by regulating energy metabolism. Biochem. Biophys. Res. Commun. 2016, 474, 22–28. [Google Scholar] [CrossRef]
- Siteneski, A.; Cunha, M.P.; Lieberknecht, V.; Pazini, F.L.; Gruhn, K.; Brocardo, P.S.; Rodrigues, A.L.S. Central irisin administration affords antidepressant-like effect and modulates neuroplasticity-related genes in the hippocampus and prefrontal cortex of mice. Prog. Neuro-Psychopharmacol. Biol. 2018, 84, 294–303. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Gong, S.; Miao, Y.L.; Jiao, G.Z.; Sun, M.J.; Li, H.; Lin, J.; Luo, M.J.; Tan, J.H. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS ONE 2015, 10, e0117503. [Google Scholar] [CrossRef]
- Bschor, T.; Adli, M. Treatment of depressive disorders. Dtsch. Ärzteblatt Int. 2008, 105, 782–792. [Google Scholar] [CrossRef]
- Tanaka, M.; Schally, A.V.; Telegdy, G. Neurotransmission of the antidepressant-like effects of the growth hormone-releasing hormone antagonist MZ-4-71. Behav. Brain. Res. 2012, 228, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Telegdy, G. Neurotransmissions of antidepressant-like effects of neuromedin U-23 in mice. Behav. Brain. Res. 2014, 259, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Belozertseva, I.V.; Tamkovich, N.V.; Baranov, K.O.; Kudryavtseva, N.N. Chronic Lithium Treatment Affects Anxious Behaviors and the Expression of Serotonergic Genes in Midbrain Raphe Nuclei of Defeated Male Mice. Biomedicines 2021, 9, 1293. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Török, N.; Vécsei, L. Novel Pharmaceutical Approaches in Dementia. In NeuroPsychopharmacotherapy; Riederer, P., Laux, G., Nagatsu, T., Le, W., Riederer, C., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–18. [Google Scholar]
- Pacheco, F.; Guiomar, R.; Brunoni, A.R.; Buhagiar, R.; Evagorou, O.; Roca-Lecumberri, A.; Poleszczyk, A.; Lambregtse-van den Berg, M.; Caparros-Gonzalez, R.A.; Fonseca, A.; et al. Efficacy of non-invasive brain stimulation in decreasing depression symptoms during the peripartum period: A systematic review. J. Psychiatr. Res. 2021, 140, 443–460. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neurosci. Biobehav. Rev. 2021, 127, 334–352. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Avenanti, A.; Pellegrino, G.D. Don’t Hurt Me No More: State-dependent Transcranial Magnetic Stimulation for the treatment of specific phobia. J. Affect. Disord. 2021, 286, 78–79. [Google Scholar] [CrossRef]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Leopardi, R. Stress and depression: Preclinical research and clinical implications. PLoS ONE 2009, 4, e4265. [Google Scholar] [CrossRef] [Green Version]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [Green Version]
- Sturman, O.; Germain, P.L.; Bohacek, J. Exploratory rearing: A context- and stress-sensitive behavior recorded in the open-field test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Zimcikova, E.; Simko, J.; Karesova, I.; Kremlacek, J.; Malakova, J. Behavioral effects of antiepileptic drugs in rats: Are the effects on mood and behavior detectable in open-field test? Seizure 2017, 52, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiller, J.W. Depression and anxiety. Med. J. Aust. 2013, 199, S28–S31. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004, 5, 11–25. [Google Scholar] [CrossRef]
- Duman, R.S.; Li, N. A neurotrophic hypothesis of depression: Role of synaptogenesis in the actions of NMDA receptor antagonists. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2475–2484. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.B.; Park, S.C. Neural Circuitry-Neurogenesis Coupling Model of Depression. Int. J. Mol. Sci. 2021, 22, 2468. [Google Scholar] [CrossRef]
- Wrigley, S.; Arafa, D.; Tropea, D. Insulin-Like Growth Factor 1: At the Crossroads of Brain Development and Aging. Front. Cell. Neurosci. 2017, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Levada, O.A.; Troyan, A.S. Insulin-like growth factor-1: A possible marker for emotional and cognitive disturbances, and treatment effectiveness in major depressive disorder. Ann. Gen. Psychiatry 2017, 16, 38. [Google Scholar] [CrossRef]
- Hoshaw, B.A.; Malberg, J.E.; Lucki, I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005, 1037, 204–208. [Google Scholar] [CrossRef]
- Smith, R.S. The macrophage theory of depression. Med. Hypotheses 1991, 35, 298–306. [Google Scholar] [CrossRef]
- Roque, S.; Correia-Neves, M.; Mesquita, A.R.; Palha, J.A.; Sousa, N. Interleukin-10: A key cytokine in depression? Cardiovasc. Psychiatry Neurol. 2009, 2009, 187894. [Google Scholar] [CrossRef] [Green Version]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, M. Evidence for an immune response in major depression: A review and hypothesis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1995, 19, 11–38. [Google Scholar] [CrossRef]
- Małyszczak, K.; Inglot, M.; Frydecka, D.; Hadryś, T.; Pawłowski, T. Biological and psychological components of depression in patients receiving IFN-alpha therapy for hepatitis C. Adv. Clin. Exp. Med. 2019, 28, 1217–1222. [Google Scholar] [PubMed] [Green Version]
- Peña-Vargas, C.; Armaiz-Peña, G.; & Castro-Figueroa, E. A Biopsychosocial Approach to Grief, Depression, and the Role of Emotional Regulation. Behav. Sci. 2021, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Ogłodek, E. Changes in the Serum Levels of Cytokines: IL-1β, IL-4, IL-8 and IL-10 in Depression with and without Posttraumatic Stress Disorder. Brain Sci. 2022, 12, 387. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Shim, H.S.; An, K.; Starkweather, A.; Kim, K.S.; Shim, I. IL-4 Inhibits IL-1β-Induced Depressive-Like Behavior and Central Neurotransmitter Alterations. Mediat. Inflamm. 2015, 2015, 941413. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, R.H.; Silver, R. Neuroimmune Signaling: Cytokines and the CNS. In Neuroscience in the 21st Century, from Basic to Clinical; Pfaff, D., Volkow, N., Rubenstein, J., Eds.; Springer: New York, NY, USA, 2015; pp. 1–41. [Google Scholar]
- Szelényi, J. Cytokines and the central nervous system. Brain Res. Bull. 2001, 54, 329–338. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]
- Broadhurst, P.L. Determinants of emotionality in the rat. I. Situational factors. Br. J. Psychol. 1957, 48, 1–12. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar] [CrossRef] [Green Version]
- Planchez, B.; Surget, A.; Belzung, C. Animal models of major depression: Drawbacks and challenges. J. Neural. Transm. 2019, 126, 1383–1408. [Google Scholar] [CrossRef] [Green Version]
- Garro-Martínez, E.; Fullana, M.N.; Florensa-Zanuy, E.; Senserrich, J.; Paz, V.; Ruiz-Bronchal, E.; Adell, A.; Castro, E.; Díaz, Á.; Pazos, Á.; et al. mTOR Knockdown in the Infralimbic Cortex Evokes A Depressive-like State in Mouse. Int. J. Mol. Sci. 2021, 22, 8671. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue “Crosstalk between Depression, Anxiety, and Dementia: Comorbidity in Behavioral Neurology and Neuropsychiatry”. Biomedicines 2021, 9, 517. [Google Scholar] [CrossRef]



| Test | Parameter | Vehicle-Treated Mice (Mean ± SEM) | Irisin-Treated Mice (Mean ± SEM) | p-Value |
|---|---|---|---|---|
| TST | Immobility (s) | 217.80 ± 11.88 | 163.80 ± 14 | * p < 0.05 |
| FST | Immobility (s) | 162.33 ± 7.409 | 133.44 ± 10.39 | * p < 0.05 |
| Fecal boli count | 5.33 ± 0.866 | 4 ± 1.041 | p = 0.339 | |
| OFT | Wall rearing count | 21.75 ± 3.35 | 22 ± 1.414 | p = 0.9373 |
| Rearing count | 17 ± 3.769 | 23.25 ± 1.75 | p = 0.1211 | |
| Fecal boli count | 1.25 ± 0.4532 | 1.333 ± 0.2887 | p = 0.8283 | |
| Time in inner zone (s) | 30 ± 8.426 | 45.42 ± 11.7 | p = 0.3472 | |
| Jumping count | 3.25 ± 2.358 | 0 ± 0 | p = 0.1333 |
| Gene Expression | Fold Change (Mean ± SEM) | Fold Change (Mean ± SEM) | p-Value |
|---|---|---|---|
| FNDC5 | 1.011 ± 0.062 | 1.471 ± 0.166 | * p < 0.05 |
| PGC-1α | 0.852 ± 0.042 | 0.850 ± 0.057 | p = 0.525 |
| BDNF | 0.562 ± 0.045 | 0.810 ± 0.016 | *** p < 0.001 |
| IGF-1 | 0.302 ± 0.015 | 0.638 ± 0.086 | ** p < 0.01 |
| NGF | 1.248 ± 0.123 | 1.288 ± 0.149 | p = 0.839 |
| FGF-2 | 0.761 ± 0.057 | 0.691 ± 0.073 | p = 0.464 |
| IL-6 | 0.950 ± 0.097 | 1.220 ± 0.224 | p = 0.267 |
| IL-1β | 0.789 ± 0.133 | 1.534 ± 0.141 | ** p < 0.01 |
| IL-4 | 0.863 ± 0.073 | 1.843 ± 0.196 | ** p < 0.01 |
| IL-10 | 0.645 ± 0.121 | 0.907 ± 0.149 | p = 0.202 |
| IL-1ra | 0.931 ± 0.076 | 0.874 ± 0.087 | p = 0.630 |
| Gene Name | Gene Bank Number | Primer sequence (5′-3′) | Product Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| Gapdh | NM_001289726.1 | Forward ACACCAGTAGACTCCACGACA Reverse ACGGCAAATTCAACGGCACAG | 145 | 60.48 62.59 |
| Fndc5 | NM_027402.4 | Forward GTGCTGATCATTGTTGTGGTCC Reverse ATCATATCTTGCTGCGGAGGAG | 169 | 60.10 60.03 |
| Pgc-1α | NM_008904.3 | Forward CCCTGCCATTGTTAAGACC Reverse TGCTGCTGTTCCTGTTTTC | 161 | 55.87 56.35 |
| Bdnf | NM_001048139.1 | Forward TGAAGTTGGCTTCCTAGCGG Reverse CCTGGTGGAACTTCTTTGCG | 146 | 60.04 59.41 |
| Fgf-2 | NM_008006.2 | Forward GCTGCTGGCTTCTAAGTGTG Reverse GTCCAGGTCCCGTTTTGGAT | 158 | 59.20 59.96 |
| Igf-1 | NM_001111276.1 | Forward TGCCTGGGTGTCCAAATGTA Reverse TGTATCTTTATTGCAGGTGCGG | 170 | 59.23 59.06 |
| Ngf | NM_001112698.2 | Forward GGAGCGCATCGAGTTTTGG Reverse CCTCACTGCGGCCAGTATAG | 136 | 59.57 59.97 |
| Il-4 | NM_021283.2 | Forward TCACAGCAACGAAGAACACCA Reverse CAGGCATCGAAAAGCCCGAA | 158 | 60.41 61.31 |
| Il-10 | NM_010548.2 | Forward GTAGAAGTGATGCCCCAGGC Reverse CACCTTGGTCTTGGAGCTTATT | 187 | 60.46 58.31 |
| Il-1ra | NM_001039701.3 | Forward GTGGCCTCGGGATGGAAAT Reverse TGGTTAGTATCCCAGATTCTGAAGG | 148 | 59.77 59.63 |
| Il-6 | NM_001314054.1 | Forward CCAAGAGATAAGCTGGAGTCACA Reverse CGCACTAGGTTTGCCGAGTA | 121 | 59.80 60.11 |
| Il-1β | NM_008361.4 | Forward TGCCACCTTTTGACAGTGATG Reverse ATGTGCTGCTGCGAGATTTG | 136 | 59.04 59.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pignataro, P.; Dicarlo, M.; Zerlotin, R.; Storlino, G.; Oranger, A.; Sanesi, L.; Lovero, R.; Buccoliero, C.; Mori, G.; Colaianni, G.; et al. Antidepressant Effect of Intermittent Long-Term Systemic Administration of Irisin in Mice. Int. J. Mol. Sci. 2022, 23, 7596. https://doi.org/10.3390/ijms23147596
Pignataro P, Dicarlo M, Zerlotin R, Storlino G, Oranger A, Sanesi L, Lovero R, Buccoliero C, Mori G, Colaianni G, et al. Antidepressant Effect of Intermittent Long-Term Systemic Administration of Irisin in Mice. International Journal of Molecular Sciences. 2022; 23(14):7596. https://doi.org/10.3390/ijms23147596
Chicago/Turabian StylePignataro, Patrizia, Manuela Dicarlo, Roberta Zerlotin, Giuseppina Storlino, Angela Oranger, Lorenzo Sanesi, Roberto Lovero, Cinzia Buccoliero, Giorgio Mori, Graziana Colaianni, and et al. 2022. "Antidepressant Effect of Intermittent Long-Term Systemic Administration of Irisin in Mice" International Journal of Molecular Sciences 23, no. 14: 7596. https://doi.org/10.3390/ijms23147596
APA StylePignataro, P., Dicarlo, M., Zerlotin, R., Storlino, G., Oranger, A., Sanesi, L., Lovero, R., Buccoliero, C., Mori, G., Colaianni, G., Colucci, S., & Grano, M. (2022). Antidepressant Effect of Intermittent Long-Term Systemic Administration of Irisin in Mice. International Journal of Molecular Sciences, 23(14), 7596. https://doi.org/10.3390/ijms23147596











