Probiotic Supplementation Suppresses Tumor Growth in an Experimental Colorectal Cancer Liver Metastasis Model
Abstract
:1. Introduction
2. Results
2.1. General Data and Blood Counts
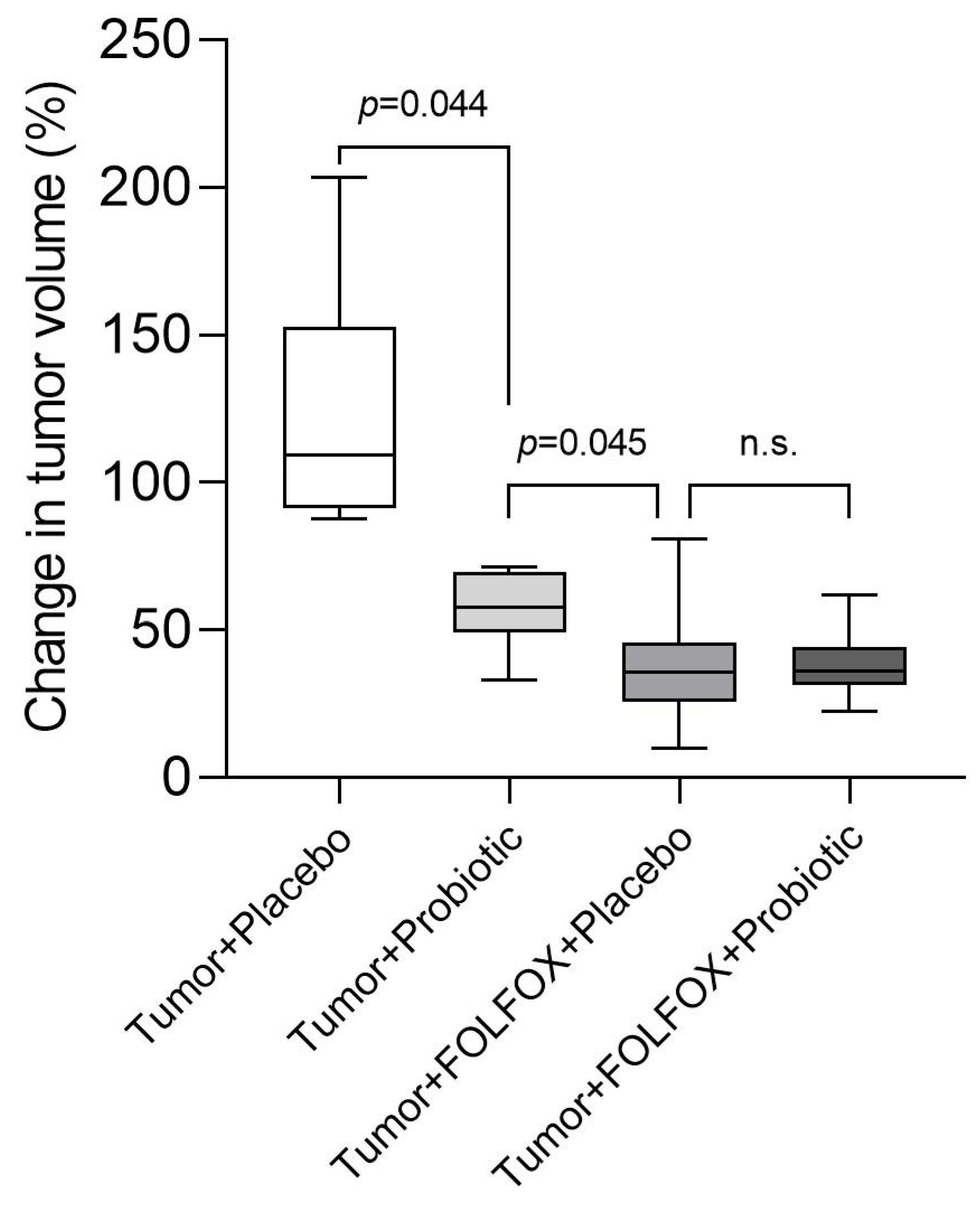
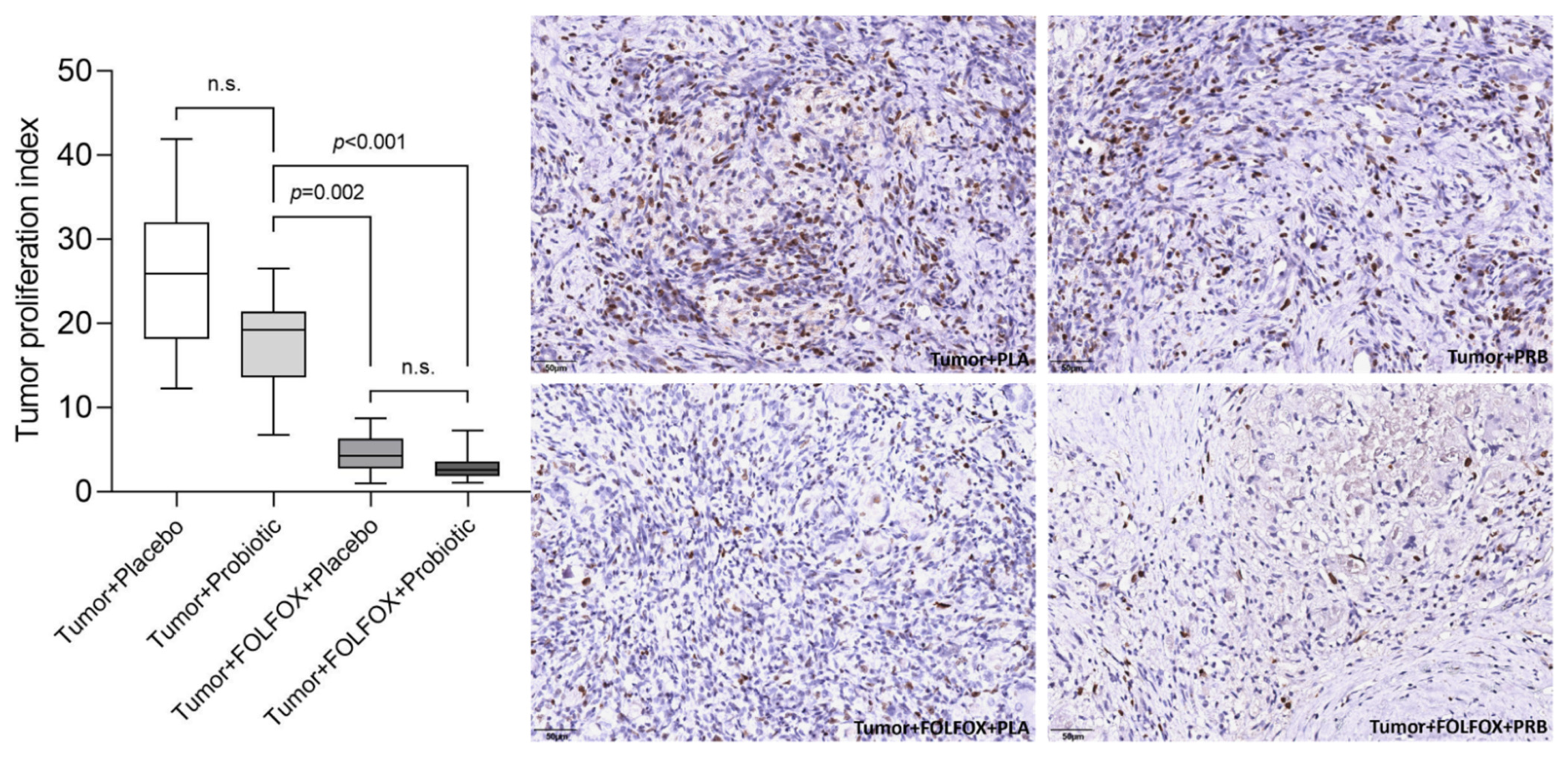
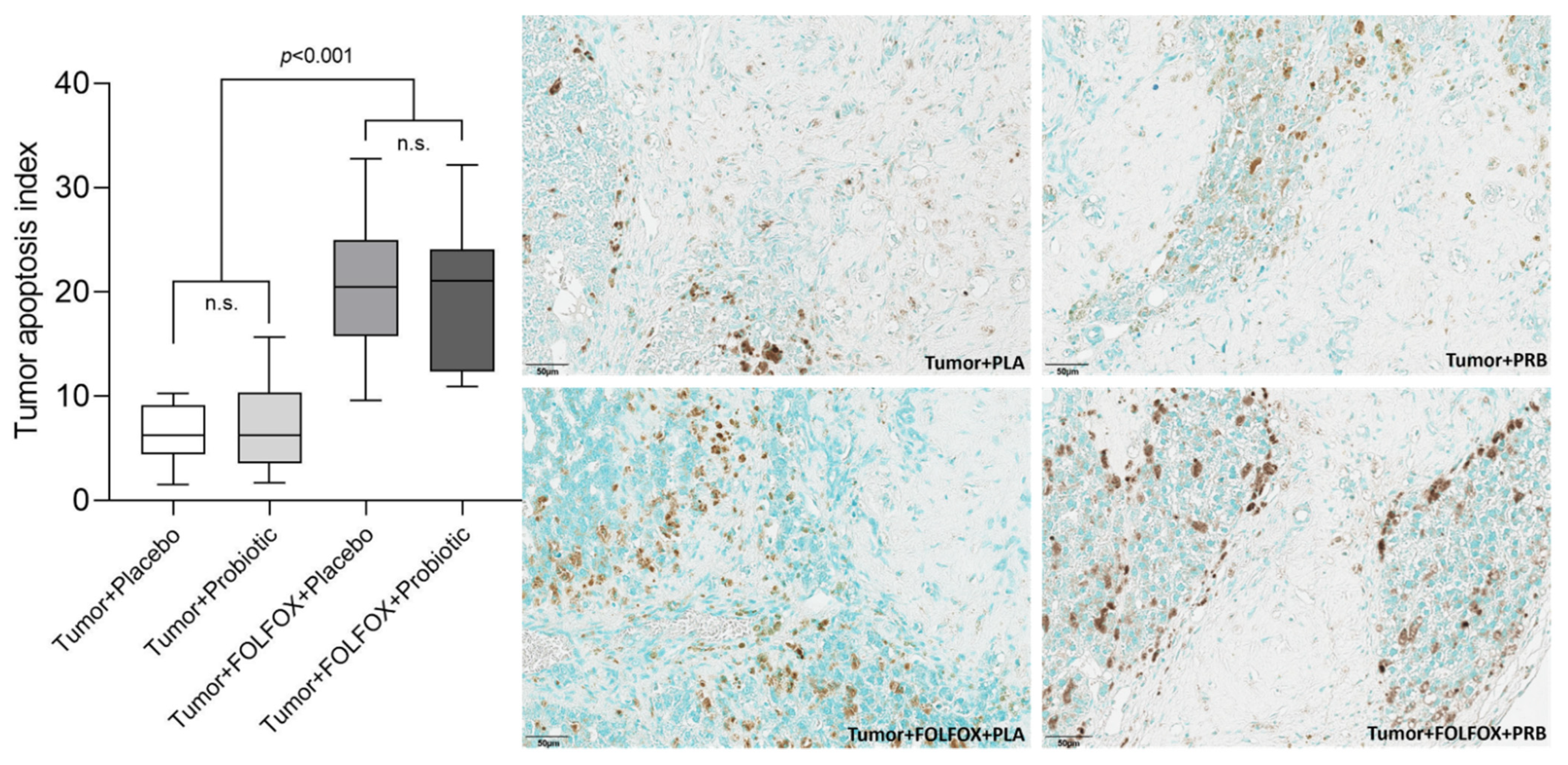
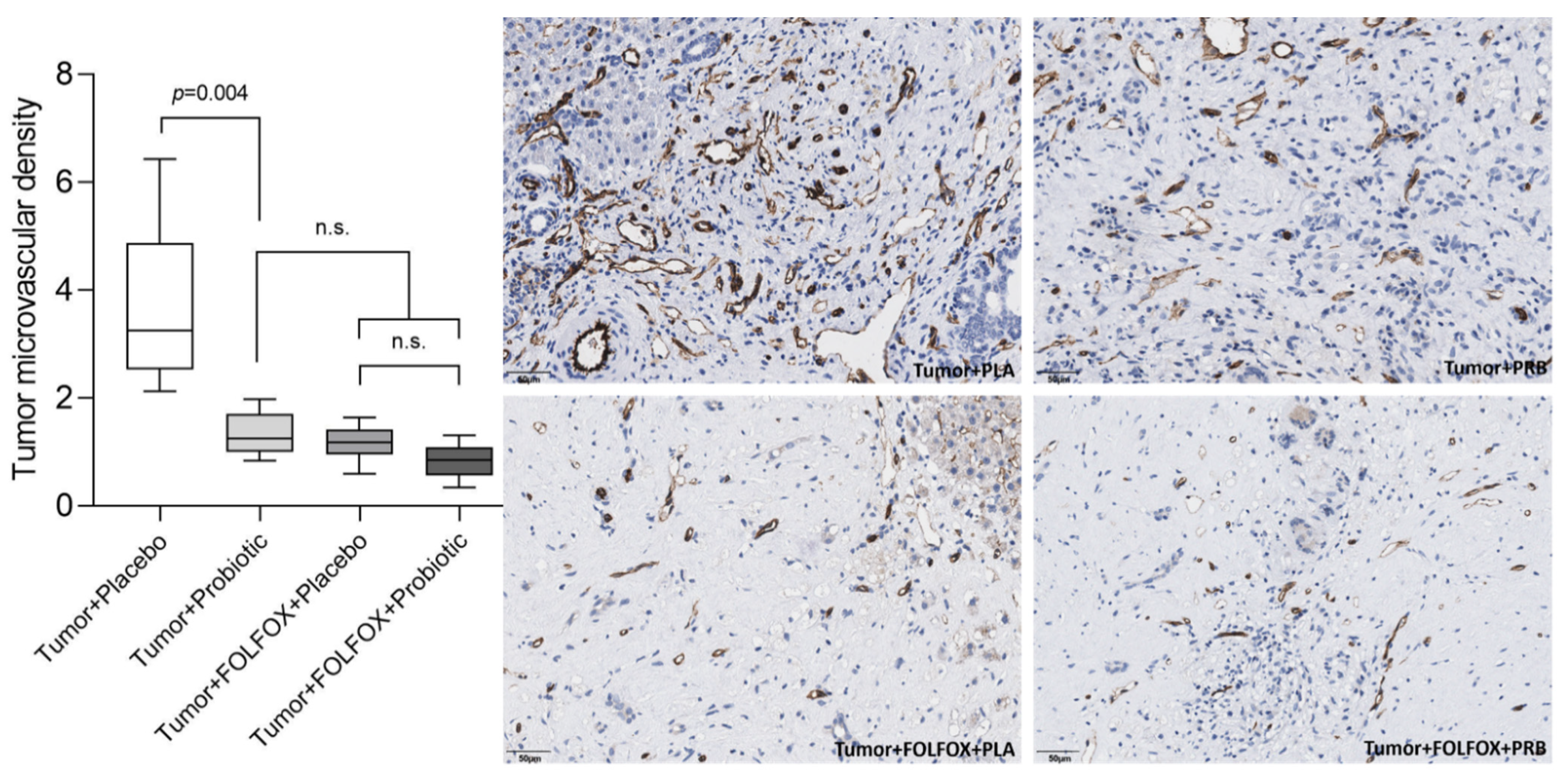
2.2. Tumor Analysis
3. Discussion
4. Materials and Methods
4.1. Animals
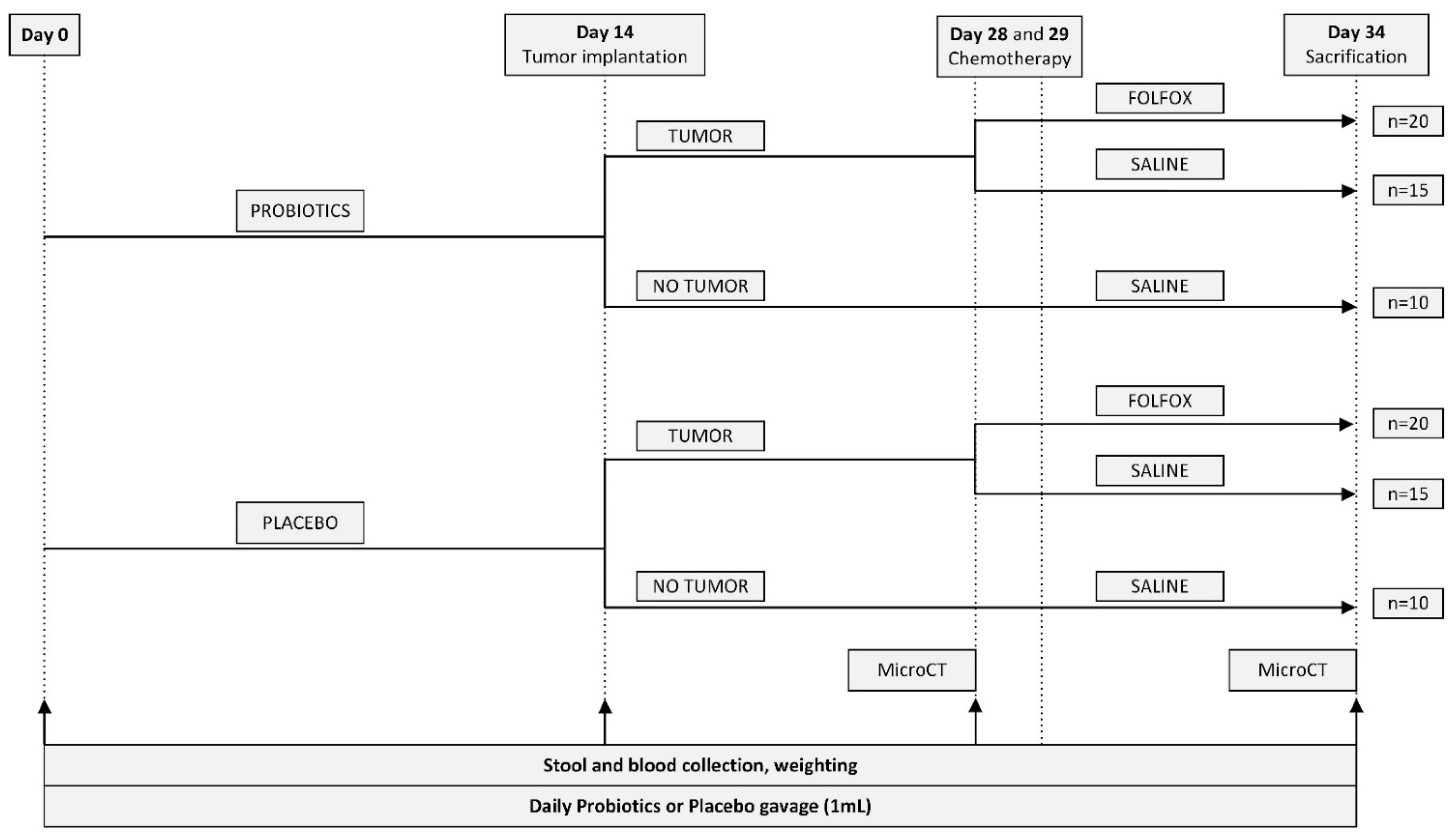
4.2. Experimental Groups and Study Design
4.3. Probiotics
4.4. Tumor Implantation
4.5. Micro-CT Scanning and Image Analysis
4.6. Chemotherpy
4.7. Blood Sample Analysis
4.8. Immunohistochemical Staining
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical Activity, Sedentary Behaviour, Diet, and Cancer: An Update and Emerging New Evidence. Lancet Oncol. 2017, 18, e457–e471. [Google Scholar] [CrossRef]
- Rasool, S.; Kadla, S.A.; Rasool, V.; Ganai, B.A. A Comparative Overview of General Risk Factors Associated with the Incidence of Colorectal Cancer. Tumor Biol. 2013, 34, 2469–2476. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [Green Version]
- Brenner, H.; Stock, C.; Hoffmeister, M. Effect of Screening Sigmoidoscopy and Screening Colonoscopy on Colorectal Cancer Incidence and Mortality: Systematic Review and Meta-Analysis of Randomised Controlled Trials and Observational Studies. BMJ 2014, 348, g2467. [Google Scholar] [CrossRef] [Green Version]
- Van Gestel, Y.R.B.M.; de Hingh, I.H.J.T.; van Herk-Sukel, M.P.P.; van Erning, F.N.; Beerepoot, L.V.; Wijsman, J.H.; Slooter, G.D.; Rutten, H.J.T.; Creemers, G.-J.M.; Lemmens, V.E.P.P. Patterns of Metachronous Metastases after Curative Treatment of Colorectal Cancer. Cancer Epidemiol. 2014, 38, 448–454. [Google Scholar] [CrossRef]
- Van der Geest, L.G.M.; Lam-Boer, J.; Koopman, M.; Verhoef, C.; Elferink, M.A.G.; de Wilt, J.H.W. Nationwide Trends in Incidence, Treatment and Survival of Colorectal Cancer Patients with Synchronous Metastases. Clin. Exp. Metastasis 2015, 32, 457–465. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Nordlinger, B.; Arnold, D. Metastatic Colorectal Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2014, 25, iii1–iii9. [Google Scholar] [CrossRef]
- Kow, A.W.C. Hepatic Metastasis from Colorectal Cancer. J. Gastrointest. Oncol. 2019, 10, 1274–1298. [Google Scholar] [CrossRef]
- Schadde, E.; Grunhagen, D.J.; Verhoef, C.; Krzywon, L.; Metrakos, P. Limitations in Resectability of Colorectal Liver Metastases 2020—A Systematic Approach for Clinicians and Patients. Semin. Cancer Biol. 2021, 71, 10–20. [Google Scholar] [CrossRef]
- Kim, J.H. Chemotherapy for Colorectal Cancer in the Elderly. WJG 2015, 21, 5158. [Google Scholar] [CrossRef]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.-A.; Haywood, P.; Brown, C.; Ward, R. Incidence and Severity of Self-Reported Chemotherapy Side Effects in Routine Care: A Prospective Cohort Study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef]
- Van Der Jeught, K.V.; Xu, H.-C.; Li, Y.-J.; Lu, X.-B.; Ji, G. Drug Resistance and New Therapies in Colorectal Cancer. WJG 2018, 24, 3834–3848. [Google Scholar] [CrossRef]
- Boussios, S.; Ozturk, M.; Moschetta, M.; Karathanasi, A.; Zakynthinakis-Kyriakou, N.; Katsanos, K.; Christodoulou, D.; Pavlidis, N. The Developing Story of Predictive Biomarkers in Colorectal Cancer. J. Pers. Med. 2019, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Williams, N.T. Probiotics. Am. J. Health-Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef]
- Markowiak, P.; Slizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Panebianco, C.; Latiano, T.; Pazienza, V. Microbiota Manipulation by Probiotics Administration as Emerging Tool in Cancer Prevention and Therapy. Front. Oncol. 2020, 10, 679. [Google Scholar] [CrossRef]
- Miknevicius, P.; Zulpaite, R.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. The Impact of Probiotics on Intestinal Mucositis during Chemotherapy for Colorectal Cancer: A Comprehensive Review of Animal Studies. Int. J. Mol. Sci. 2021, 22, 9347. [Google Scholar] [CrossRef]
- Folkman, J. Role of Angiogenesis in Tumor Growth and Metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef]
- Owens, J.A.; Saeedi, B.J.; Naudin, C.R.; Hunter-Chang, S.; Barbian, M.E.; Eboka, R.U.; Askew, L.; Darby, T.M.; Robinson, B.S.; Jones, R.M. Lactobacillus Rhamnosus GG Orchestrates an Antitumor Immune Response. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1311–1327. [Google Scholar] [CrossRef]
- Shang, Y.; Regassa, A.; Kim, J.H.; Kim, W.K. The Effect of Dietary Fructooligosaccharide Supplementation on Growth Performance, Intestinal Morphology, and Immune Responses in Broiler Chickens Challenged with Salmonella Enteritidis Lipopolysaccharides. Poult. Sci. 2015, 94, 2887–2897. [Google Scholar] [CrossRef]
- Li, J.; Sung, C.Y.J.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics Modulated Gut Microbiota Suppresses Hepatocellular Carcinoma Growth in Mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef] [Green Version]
- Aindelis, G.; Tiptiri-Kourpeti, A.; Lampri, E.; Spyridopoulou, K.; Lamprianidou, E.; Kotsianidis, I.; Ypsilantis, P.; Pappa, A.; Chlichlia, K. Immune Responses Raised in an Experimental Colon Carcinoma Model Following Oral Administration of Lactobacillus Casei. Cancers 2020, 12, 368. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; et al. Clostridium Butyricum, a Butyrate-Producing Probiotic, Inhibits Intestinal Tumor Development through Modulating Wnt Signaling and Gut Microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Ye, L.; Wang, C. The Anti-Cancer Effects and Mechanisms of Lactic Acid Bacteria Exopolysaccharides in Vitro: A Review. Carbohydr. Polym. 2021, 253, 117308. [Google Scholar] [CrossRef]
- Garbacz, K. Anticancer Activity of Lactic Acid Bacteria. Semin. Cancer Biol. 2022; in press. [Google Scholar] [CrossRef]
- Kvietkauskas, M.; Zitkute, V.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. Dietary Melatonin and Glycine Decrease Tumor Growth through Antiangiogenic Activity in Experimental Colorectal Liver Metastasis. Nutrients 2021, 13, 2035. [Google Scholar] [CrossRef]
- Maneikyte, J.; Bausys, A.; Leber, B.; Horvath, A.; Feldbacher, N.; Hoefler, G.; Strupas, K.; Stiegler, P.; Schemmer, P. Dietary Glycine Decreases Both Tumor Volume and Vascularization in a Combined Colorectal Liver Metastasis and Chemotherapy Model. Int. J. Biol. Sci. 2019, 15, 1582–1590. [Google Scholar] [CrossRef] [Green Version]
- Lazaris, A.; Amri, A.; Petrillo, S.K.; Zoroquiain, P.; Ibrahim, N.; Salman, A.; Gao, Z.-H.; Vermeulen, P.B.; Metrakos, P. Vascularization of Colorectal Carcinoma Liver Metastasis: Insight into Stratification of Patients for Anti-Angiogenic Therapies: Vascularization of Colorectal Carcinoma Liver Metastasis. J. Path Clin. Res. 2018, 4, 184–192. [Google Scholar] [CrossRef]
- Alberts, S.R.; Horvath, W.L.; Sternfeld, W.C.; Goldberg, R.M.; Mahoney, M.R.; Dakhil, S.R.; Levitt, R.; Rowland, K.; Nair, S.; Sargent, D.J.; et al. Oxaliplatin, Fluorouracil, and Leucovorin for Patients With Unresectable Liver-Only Metastases From Colorectal Cancer: A North Central Cancer Treatment Group Phase II Study. JCO 2005, 23, 9243–9249. [Google Scholar] [CrossRef]
- Delaunoit, T.; Alberts, S.R.; Sargent, D.J.; Green, E.; Goldberg, R.M.; Krook, J.; Fuchs, C.; Ramanathan, R.K.; Williamson, S.K.; Morton, R.F.; et al. Chemotherapy Permits Resection of Metastatic Colorectal Cancer: Experience from Intergroup N9741. Ann. Oncol. 2005, 16, 425–429. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [Green Version]




| Gavage | CONTROL Tumor (−)/FOLFOX (−) | Non-FOLFOX Tumor (+)/FOLFOX (−) | FOLFOX Tumor (+)/FOLFOX (+) | |||
|---|---|---|---|---|---|---|
| Placebo | Probiotics | Placebo | Probiotics | Placebo | Probiotics | |
| Started (n) | 10 | 10 | 15 | 15 | 20 | 20 |
| Finished (n) | 10 | 10 | 15 | 15 | 20 | 19 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubauskas, M.; Jakubauskiene, L.; Leber, B.; Horvath, A.; Strupas, K.; Stiegler, P.; Schemmer, P. Probiotic Supplementation Suppresses Tumor Growth in an Experimental Colorectal Cancer Liver Metastasis Model. Int. J. Mol. Sci. 2022, 23, 7674. https://doi.org/10.3390/ijms23147674
Jakubauskas M, Jakubauskiene L, Leber B, Horvath A, Strupas K, Stiegler P, Schemmer P. Probiotic Supplementation Suppresses Tumor Growth in an Experimental Colorectal Cancer Liver Metastasis Model. International Journal of Molecular Sciences. 2022; 23(14):7674. https://doi.org/10.3390/ijms23147674
Chicago/Turabian StyleJakubauskas, Matas, Lina Jakubauskiene, Bettina Leber, Angela Horvath, Kestutis Strupas, Philipp Stiegler, and Peter Schemmer. 2022. "Probiotic Supplementation Suppresses Tumor Growth in an Experimental Colorectal Cancer Liver Metastasis Model" International Journal of Molecular Sciences 23, no. 14: 7674. https://doi.org/10.3390/ijms23147674
APA StyleJakubauskas, M., Jakubauskiene, L., Leber, B., Horvath, A., Strupas, K., Stiegler, P., & Schemmer, P. (2022). Probiotic Supplementation Suppresses Tumor Growth in an Experimental Colorectal Cancer Liver Metastasis Model. International Journal of Molecular Sciences, 23(14), 7674. https://doi.org/10.3390/ijms23147674








