Evaluating the Role of Probiotics in the Prevention and Management of Age-Related Diseases
Abstract
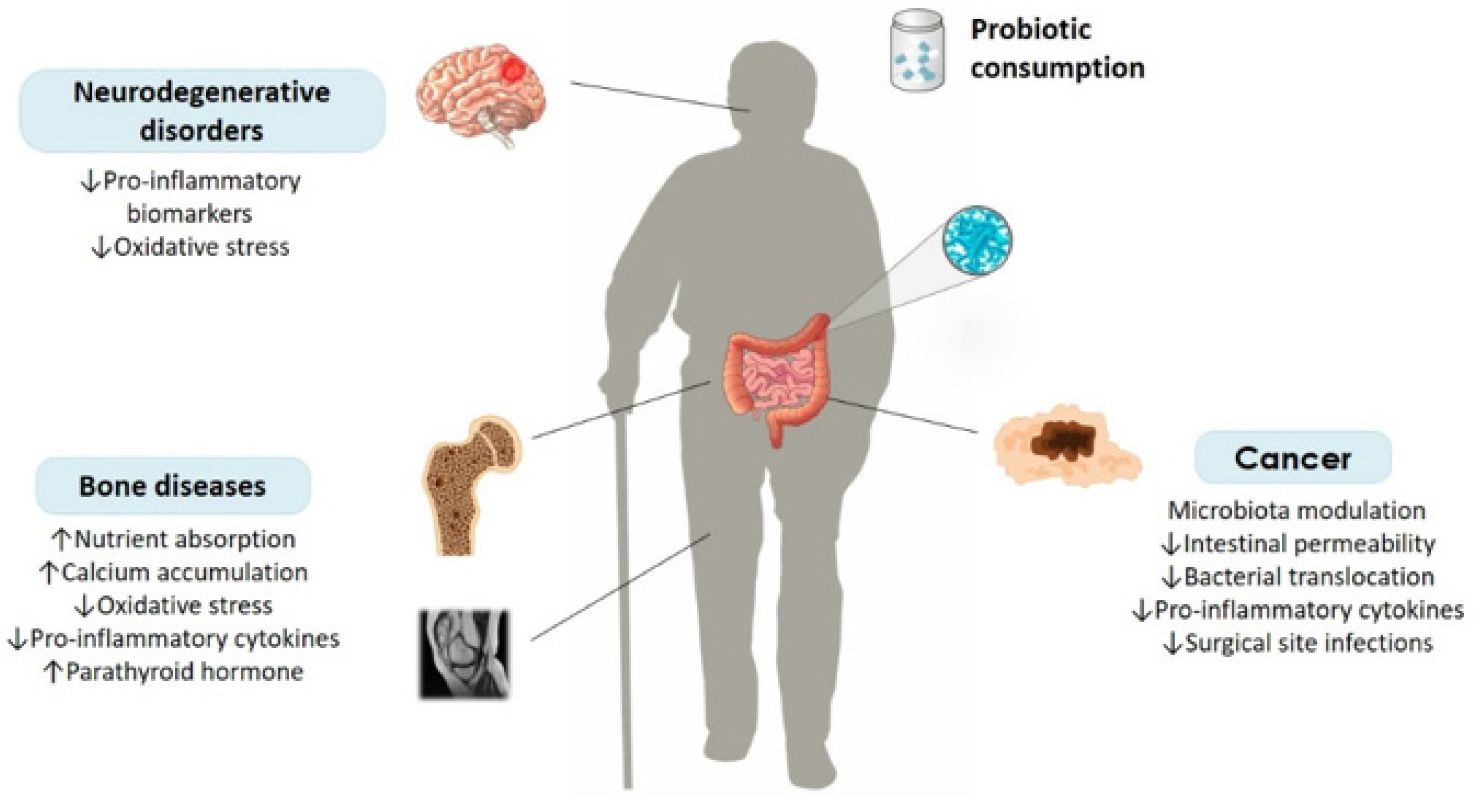
:1. Introduction
2. Age-Related Bone Diseases and Probiotics
2.1. Osteoporosis
2.2. Rheumatoid Arthritis
| Probiotic Strains | Participants | Age (Years, Mean ± SD) | Sex Ratio (M/F) | Type of Study | Intervention | Duration of Intervention | Key Molecular Findings | Clinical Outcomes | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Probiotic Group | Control Group | Probiotic Group | Control Group | Probiotic Group | Control Group | |||||||
| Primary Osteoporosis | ||||||||||||
| Bacillus subtilis C-3102 | 61 healthy post-meno-pausal Japanese women | 57.5 ± 4.3 | 57.8 ± 5.4 | All female | Randomized, double-blind, placebo-controlled study | 3 capsules, 3.4 × 109 CFU, once daily | Capsules containing dextrin | 24 weeks | ↑ BMD (total hip) ↓ TRACP-5b ↓ uNTx ↑ Bifidobacterium ↓ Relative abundance of Fusobacterium | ↓ Bone resorption | [31] | |
| L. reuteri 6475 | 70 women with low BMD | 76.4 ± 1.0 | 76.3 ± 1.1 | All female | Randomized, double-blind, placebo-controlled trial | Stick packs containing 5 × 109 CFU, twice daily | Stick-packs containing maltodextrin powder | 12 months | ΝS | ↓ Loss of total vBMD and trabecular bone volume fraction | [32] | |
| L. paracasei DSM 13434, L. plantarum DSM 15312, L. plantarum DSM 15313 | 234 healthy women in the early post-menopausal phase | 59.1 ± 3.8 | 58.1 ± 4.3 | All female | Randomized, double-blind, placebo-controlled, multicenter trial | Capsules, 10¹⁰ CFU, once daily | Placebo capsules, content not mentioned | 12 months | ΝA | ↓ LS-BMD loss | [33] | |
| L. casei, L. acidophilus, L. rhamnosus, Bifidobacterium breve, Streptococcus thermophilus | 41 osteopenic post-menopausal women | 58.85 ± 0.68 | 57.29 ± 0.72 | All female | Randomized, double-blind, placebo-controlled study | Multispecies capsules, once daily | Capsules containing 500 mg of corn starch | 6 months | ↓ BALP ↓ Serum CTX ↓ PTH ↓ Serum TNF-α | NS | [34] | |
| Kefir | 40 osteoporotic patients | 64.08 ± 14.51 | 67.94 ± 8.37 | 7/17 | 7/8 | Randomized, double-blind, placebo-controlled study | 1600 mg kefir and 1500 mg CaCO3 daily | 1600 mg unfermented raw milk and 1500 mg CaCO3 daily | 6 months | ↑ Serum PTH ↓ Serum β-CTX ↑ OC | ↑ BMD | [35] |
| L. casei Shirota | 381 patients with acute distal radius fracture | 64.3 ± 4.1 | 65.1 ± 3.7 | 93/96 | 94/96 | Randomized, double-blind, placebo-controlled trial | Skimmed milk containing 6 × 10 9 CFU, twice daily | Skimmed milk | 6 months | ΝA | ↑ Healing process ↓ DASH score ↓ pain VAS ↓ CRPS score | [44] |
| Rheumatoid Arthritis | ||||||||||||
| Bacillus coagulans GBI-30 | 45 patients | 62.5 | 9/36 | Randomized, double-blind, placebo-controlled trial | Caplets, 2 × 10 9 CFU, once daily | Capsules containing micro-crystalline cellulose | 60 days | ↓ CRP | Improvement in: patient pain assessment score, pain scale, patient global assessment and self-assessed disability | [40] | ||
| L. acidophilus La-14, L. casei Lc-11, Lactococcus lactis Ll-23, B. lactis Bl-04, B. bifidum Bb-06 | 42 patients | 59 | 57 | 3/18 | 2/19 | Randomized, double-blind, placebo-controlled trial | Sachet with freeze-dried bacterial strains, 109 CFU/g of each strain, once daily | Capsules containing maltodextrin | 2 months | ↓ WBC ↓ TNF-α ↓ IL-6 ↓ NOx ↑ SH ↑ TRAP | ΝS | [41] |
| L. acidophilus, L. casei, Bifidobacterium bifidum | 60 patients | 52.2 ± 12.2 | 50.6 ± 13.1 | 5/25 | 4/26 | Randomized, double-blind, placebo-controlled trial | Capsules, viable and freeze-dried strains 2 × 109 CFU/g of each strain, once daily | Capsules containing starch | 8 weeks | ↓ Serum insulin ↓ hs-CRP ↓ HOMA-B ↑ Plasma GSH Improved VAS | Improved DAS-28 | [42] |
| L. rhamnosus GR-1, L. reuteri RC-14 | 29 patients | 63.8 ± 7.5 | 59.1 ± 9.1 | 1/14 | 1/13 | Randomized, double-blind, placebo-controlled trial | Capsules, 2 × 109 CFU, twice daily | Capsules containing inactive ingredients | 3 months | Suppressed pro-inflammatory cytokine production | Improvement of HAQ score, No clinical improvement | [43] |
3. Age-Related Neurodegenerative Disorders and Probiotics
3.1. Alzheimer’s Disease
3.2. Parkinson’s Disease
4. Cancer in the Elderly and Probiotics
| Probiotic Strains | Participants | Age (Years, Mean ± SD) | Sex Ratio (M/F) | Type of Study | Intervention | Duration of Intervention | Key Molecular Findings | Clinical Outcomes | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Probiotic Group | Control Group | Probiotic Group | Control Group | Probiotic Group | Control Group | |||||||
| Colorectal Cancer | ||||||||||||
| E. faecalis T110, Clostridium butyricum TO-A, Bacillus mesentericus TO-A | 156 CRC patients scheduled for surgery | 68.0 ± 13.8 | 69.1 ± 11.3 | 47/28 | 44/37 | Rando-mized clinical trial | Multispecies tablets, 6 times daily | No placebo | 3–15 days before surgery | ↑ Adenosine triphosphate ↑ Bifidobacterium abundance | ↓ Superficial incisional SSIs ↑ Immune responses | [96] |
| L. acidophilus LA-5, L. plantarum, B. lactis BB-12, S. boulardii | 164 CRC patients scheduled for elective, open, colonic resection | 65.9 ± 11.5 | 66.4 ± 11.9 | 57/27 | 58/22 | Rando-mized, double-blind, placebo-controlled trial | Multispecies capsules, twice daily | Placebo capsules containing powdered glucose polymer | 16 days; 1 day before major colorectal surgery and 15 days post-operatively | Expression of SOCS3 → positively related with expression of TNF-α and circulating IL-6 | ↓ Major postoperative complications | [97] |
| L. plantarum, L. acidophilus, B. longum | 156 CRC patients scheduled for radical colectomy | 66.06 ± 11.02 | 62.28 ± 12.41 | 38/37 | 40/35 | Randomized, double-blind, placebo-controlled trial | Multispecies capsules, total daily dose of 2.6 × 1014 CFU | Placebo capsules containing maltodextrin | 16 days; 6 days before and 10 days after surgery | ↓ Postoperative serum zonulin Inhibition of the p38 MAPK signaling pathway | ↓ Bacterial translocation ↓ Intestinal permeability ↓ Pyrexia ↓ Duration of antibiotic therapy | [98] |
| L. acidophilus BCMCTM12130, L. casei BCMCTM12313, L. lactis BCMCTM12451, B. bifidum BCMCTM02290, B. longum BCMCTM02120, B. infantis BCMCTM02129 | 40 CRC patients scheduled for surgery | 64.3 ± 14.5 | 68.4 ± 11.9 | 11/9 | 13/7 | Rando-mized, double-blind, placebo-con-trolled trial | Sachets, 3 × 1010 CFU, twice daily | Placebo capsules, content not mentioned | 7 days before surgery | ΝA | Faster return of normal gut function Faster recovery Shorter duration of hospital stay | [99] |
| B. longum, L. acidophilus, and E. faecalis | 60 CRC patients scheduled for confined colorectal cancer resection operation | 63.90 ± 12.25 | 62.17 ± 11.06 | 15/15 | 12/18 | Rando-mized, double-blind, placebo-con-trolled trial | Probiotic powder, 107 CFU/g of each strain, 2 g daily | Placebo powder containing maltodextrin and sucrose | 12 days; 5 days before and 7 days after CRC resection operation | NS | ↓ Incidence of diarrhea Faster recovery of bowel function | [100] |
| B. breve HA-129, B. bifidum HA-132, B. longum HA-135, L. rhamnosus HA-111, L. acidophilus HA-122, L. casei HA-108, L. plantarum HA-119, S. thermopilus HA-110, L. brevis HA-112, B. infantis HA-116 | 46 CRC patients starting new line of chemotherapy | 62 | 64 | 14/9 | 12/11 | Rando-mized, double blind, placebo con-trolled pilot study | Capsules, 1010 CFU, 3 times daily | Placebo capsules, only inactive ingredients | 12 weeks | NA | ↓ Incidence of severe diarrhea of grade 3 or 4 ↓ Overall incidence of diarrhea ↓ Incidence of enterocolitis | [101] |
| B. infantis, L. acidophilus, E. faecalis, B. cereus | 100 CRC patients undergoing chemo-therapy | 62.1 ± 10.9 | 60.1 ± 9.9 | 35/15 | 33/17 | Rando-mized clinical trial | 4 tablets (con-centration not reported), 3 times daily | No placebo | 4 weeks | NS | Alleviated functional constipation during chemotherapy | [102] |
| L. acidophilus BCMC® 12130, L. lactis BCMC® 12451, L. casei BCMC® 12313, B. longum BCMC® 02120, B. bifidum BCMC® 02290, B. infantis BCMC® 02129 | 52 CRC patients scheduled for surgery | 67.33 ± 9.4 | 66.5 ± 8.5 | 19/8 | 15/10 | Rando-mized, double-blind, placebo-controlled trial | Mixture, 3 × 1010 CFU, twice daily | Placebo capsules -content not mentioned | 6 months starting 4 weeks after surgery | ↓ TNF-α ↓ IL-6 ↓ IL-10 ↓ IL-12 ↓ IL-17A ↓ IL-17C ↓ IL-22 | Safety of probiotic consumption | [103] |
| B. longum, L. acidophilus, E. faecalis | 60 CRC patients scheduled for radical colorectal resection | 67.5 | 61.5 | 10/20 | 14/16 | Randomized, double-blind, placebo-controlled trial | 3 capsules, 108 CFU/g, 3 times daily | Placebo capsules containing malto-dextrin | 3 days before surgery | ↑ Bifidobacterium ↑ Escherichia ↓ Endotoxins ↓ D-lactic acids ↓ IL-6 ↓ CRP ↑ IgG ↑ sIgA | ↓ Occurrence of infectious complications | [104] |
| B. animalis subsp. lactis HY8002, L. casei HY278, L. plantarum HY7712 | 60 CRC patients scheduled for anterior resection | 60.10 | 61.03 | 19/10 | 13/18 | Randomized, double-blind, multicenter, exploratory placebo-controlled trial | Probiotic powder, twice daily | Placebo powder of prebiotics and sugars | 4 weeks, starting at one week before surgery | Compositional changes in gut microbiota ↓ Serum zonulin | NS | [105] |
| B. bifidum | 294 CRC patients scheduled for elective colon cancer operation | 67 ± 9 | 66 ± 12 | 49/51 | 51/44 | Prospective randomized trial | 3 tablets, 1010 CFU, 3 times daily | No placebo | 17 days total; 7 days before surgery, 10 days after surgery | NS | NS | [106] |
| Pelvic Cancer | ||||||||||||
| L. acidophilus LAC-361, B. longum BB536 | 229 pelvic cancer patients receiving radio-therapy treatments | 61.7 | 60.6 | 97/43 | 56/33 | Randomized, double blind, placebo controlled study | Capsule, 1,3 × 109 CFU, twice daily (standard dose) or 10 × 109 CFU, 3 times daily (high dose) | Placebo tablets -content not mentioned | During the radiation therapy treatments | NS | ↓ Radiation induced grade 2–3-4 diarrhea | [110] |
| Lung Cancer | ||||||||||||
| Clostridium butyricum | 41 patients with lung cancer undergoing chemo-therapy | 57 ± 8.75 | 54 ± 8.35 | 15/5 | 15/6 | Randomized, double blind, placebo controlled study | 3 tablets (420 mg/tablet), 3 times daily | Placebo tablets -content not mentioned | 3 weeks | ↓ NLR ↓ PLR ↑ LMR at week 3 ↑ Clostridium and Lactobacillus genera | ↓ Chemotherapy-induced diarrhea Alleviated inflammatory response Maintained gut homeostasis | [111] |
5. Probiotics and Aging; Pitfalls and Future Perspectives
5.1. Other Diseases of Aging
5.2. Deciphering the Mechanisms of Probiotic Action in Aging
5.3. Safety of Probiotic Consumption in the Elderly
5.4. Refining Probiotic Research in the Elderly
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 27 January 2022).
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology. 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Austad, S.N. The Geroscience Hypothesis: Is It Possible to Change the Rate of Aging? In Advances in Geroscience; Sierra, F., Kohanski, R., Eds.; Springer: New York, NY, USA, 2016; pp. 1–36. [Google Scholar]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fane, M.; Weeraratna, A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Jaul, E.; Barron, J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front. Public Health 2017, 5, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueira, I.; Fernandes, A.; Mladenovic Djordjevic, A.; Lopez-Contreras, A.; Henriques, C.M.; Selman, C.; Ferreiro, E.; Gonos, E.S.; Trejo, J.L.; Misra, J.; et al. Interventions for age-related diseases: Shifting the paradigm. Mech. Ageing Dev. 2016, 160, 69–92. [Google Scholar] [CrossRef]
- Bosco, N.; Noti, M. The aging gut microbiome and its impact on host immunity. Genes Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef]
- Kim, M.; Benayoun, B.A. The microbiome: An emerging key player in aging and longevity. Transl. Med. Aging 2020, 4, 103–116. [Google Scholar] [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.M.; Dequenne, I.; de Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metchnikoff, E. The Prolongation of Life; Mitchell, P.C., Ed.; G. P. Putnam’s Sons: New York, NY, USA, 1910; p. 96. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiousi, D.E.; Chorianopoulos, N.; Tassou, C.C.; Galanis, A. The Clash of Microbiomes: From the Food Matrix to the Host Gut. Microorganisms 2022, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Demontiero, O.; Vidal, C.; Duque, G. Aging and bone loss: New insights for the clinician. Ther. Adv. Musculoskelet. Dis. 2012, 4, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Sims, N.A.; Gooi, J.H. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin. Cell Dev. Biol. 2008, 19, 444–451. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [Green Version]
- Bullock, J.; Rizvi, S.A.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2018, 27, 501–507. [Google Scholar] [CrossRef]
- Föger-Samwald, U.; Dovjak, P.; Azizi-Semrad, U.; Kerschan-Schindl, K.; Pietschmann, P. Osteoporosis: Pathophysiology and therapeutic options. EXCLI J. 2020, 19, 1017–1037. [Google Scholar] [CrossRef]
- Hyassat, D.; Alyan, T.; Jaddou, H.; Ajlouni, K.M. Prevalence and Risk Factors of Osteoporosis Among Jordanian Postmenopausal Women Attending the National Center for Diabetes, Endocrinology and Genetics in Jordan. Biores. Open Access. 2017, 6, 85–93. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Epidemiology of Osteoporosis and Fragility Fractures. Available online: https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fractures (accessed on 27 January 2022).
- Wang, J.; Wang, Y.; Gao, W.; Wang, B.; Zhao, H.; Zeng, Y.; Ji, Y.; Hao, D. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ 2017, 5, e3450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, F.L.; Rios-Arce, N.D.; Schepper, J.D.; Parameswaran, N.; McCabe, L.R. The Potential of Probiotics as a Therapy for Osteoporosis. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Akkawi, I.; Zmerly, H. Osteoporosis: Current Concepts. Joints 2018, 6, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaheer, S.; LeBoff, M.S. Osteoporosis: Prevention and Treatment. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279073/ (accessed on 27 January 2022).
- Augustine, M.; Horwitz, M.J. Parathyroid hormone and parathyroid hormone-related protein analogs as therapies for osteoporosis. Curr. Osteoporos. Rep. 2013, 11, 400–406. [Google Scholar] [CrossRef] [Green Version]
- Weaver, C.M.; Alexander, D.D.; Boushey, C.J.; Dawson-Hughes, B.; Lappe, J.M.; LeBoff, M.S.; Liu, S.; Looker, A.C.; Wallace, T.C.; Wang, D.D. Calcium plus vitamin D supplementation and risk of fractures: An updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016, 27, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Takimoto, T.; Hatanaka, M.; Hoshino, T.; Takara, T.; Tanaka, K.; Shimizu, A.; Morita, H.; Nakamura, T. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: A randomized, placebo-controlled, double-blind clinical trial. Biosci. Microbiota Food Health 2018, 37, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, A.G.; Sundh, D.; Bäckhed, F.; Lorentzon, M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: A randomized, placebo-controlled, double-blind, clinical trial. J. Intern. Med. 2018, 284, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Jansson, P.A.; Curiac, D.; Lazou Ahrén, I.; Hansson, F.; Martinsson Niskanen, T.; Sjögren, K.; Ohlsson, C. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019, 1, e154–e162. [Google Scholar] [CrossRef]
- Jafarnejad, S.; Djafarian, K.; Fazeli, M.R.; Yekaninejad, M.S.; Rostamian, A.; Keshavarz, S.A. Effects of a Multispecies Probiotic Supplement on Bone Health in Osteopenic Postmenopausal Women: A Randomized, Double-blind, Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 497–506. [Google Scholar] [CrossRef]
- Tu, M.Y.; Chen, H.L.; Tung, Y.T.; Kao, C.C.; Hu, F.C.; Chen, C.M. Short-Term Effects of Kefir-Fermented Milk Consumption on Bone Mineral Density and Bone Metabolism in a Randomized Clinical Trial of Osteoporotic Patients. PLoS ONE 2015, 10, e0144231. [Google Scholar] [CrossRef]
- Ke, Y.; Dai, X.; Xu, D.; Liang, J.; Yu, Y.; Cao, H.; Chen, W.; Lin, J. Features and Outcomes of Elderly Rheumatoid Arthritis: Does the Age of Onset Matter? A Comparative Study from a Single Center in China. Rheumatol Ther. 2021, 8, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Vaghef-Mehrabany, E.; Alipour, B.; Homayouni-Rad, A.; Sharif, S.K.; Asghari-Jafarabadi, M.; Zavvari, S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 2014, 30, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, X.; Huang, E.; Wang, Q.; Wen, C.; Yang, G.; Lu, L.; Cui, D. Inflammasome and Its Therapeutic Targeting in Rheumatoid Arthritis. Front. Immunol. 2022, 12, 816839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Mandel, D.R.; Eichas, K.; Holmes, J. Bacillus coagulans: A viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC Complement. Altern. Med. 2010, 10, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannarella, L.A.T.; Mari, N.L.; Alcântara, C.C.; Iryioda, T.M.V.; Costa, N.T.; Oliveira, S.R.; Lozovoy, M.A.B.; Reiche, E.M.V.; Dichi, I.; Simão, A.N.C. Mixture of probiotics reduces inflammatory biomarkers and improves the oxidative/nitrosative profile in people with rheumatoid arthritis. Nutrition 2021, 89, 111282. [Google Scholar] [CrossRef] [PubMed]
- Zamani, B.; Golkar, H.R.; Farshbaf, S.; Emadi-Baygi, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akhavan, R.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled trial. Int. J. Rheum. Dis. 2016, 19, 869–879. [Google Scholar] [CrossRef]
- Pineda Mde, L.; Thompson, S.F.; Summers, K.; de Leon, F.; Pope, J.; Reid, G. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med. Sci. Monit. 2011, 17, CR347–CR354. [Google Scholar] [CrossRef] [Green Version]
- Lei, M.; Hua, L.M.; Wang, D.W. The effect of probiotic treatment on elderly patients with distal radius fracture: A prospective double-blind, placebo-controlled randomised clinical trial. Benef. Microbes 2016, 7, 631–637. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Kovacs, G.G. Molecular pathology of neurodegenerative diseases: Principles and practice. J. Clin. Pathol. 2019, 72, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Pritzkow, S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; O, W.; Li, W.; Jiang, Z.G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cetinkalp, S.; Simsir, I.Y.; Ertek, S. Insulin resistance in brain and possible therapeutic approaches. Curr. Vasc. Pharmacol. 2014, 12, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Roy Sarkar, S.; Banerjee, S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 2019, 328, 98–104. [Google Scholar] [CrossRef]
- Lopez, O.L.; Kuller, L.H. Epidemiology of aging and associated cognitive disorders: Prevalence and incidence of Alzheimer’s disease and other dementias. Handb. Clin. Neurol. 2019, 167, 139–148. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef] [Green Version]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Kellar, D.; Craft, S. Brain insulin resistance in Alzheimer’s disease and related disorders: Mechanisms and therapeutic approaches. Lancet Neurol. 2020, 19, 758–766. [Google Scholar] [CrossRef]
- DArgenio, V.; Sarnataro, D. Probiotics, prebiotics and their role in Alzheimer’s disease. Neural Regen. Res. 2021, 16, 1768–1769. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamtaji, O.R.; Heidari-Soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 2019, 38, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Ton, A.M.M.; Campagnaro, B.P.; Alves, G.A.; Campagnaro, B.P.; Alves, G.A.; Aires, R.; Coco, L.Z.; Arpini, C.M.; Guerra e Oliverira, T.; Campos-Toimil, M.; et al. Oxidative Stress and Dementia in Alzheimer’s Patients: Effects of Synbiotic Supplementation. Oxid. Med. Cell Longev. 2020, 2020, 2638703. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Park, S.; Paik, J.W.; Chae, S.W.; Kim, D.H.; Jeong, D.G.; Ha, E.; Kim, M.; Hong, G.; Park, S.H.; et al. Efficacy and Safety of Lactobacillus Plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, Y.; Kinoshita, T.; Matsumoto, A.; Yoshino, K.; Saito, I.; Xiao, J.Z. Bifidobacterium Breve A1 Supplementation Improved Cognitive Decline in Older Adults with Mild Cognitive Impairment: An Open-Label, Single-Arm Study. J Prev. Alzheimers Dis. 2018, 6, 70–75. [Google Scholar] [CrossRef]
- Agahi, A.; Hamidi, G.A.; Daneshvar, R.; Hamdieh, M.; Soheili, M.; Alinaghipour, A.; Esmaeili Taba, S.M.; Salami, M. Does Severity of Alzheimer’s Disease Contribute to Its Responsiveness to Modifying Gut Microbiota? A Double-Blind Clinical Trial. Front. Neurol. 2018, 9, 662. [Google Scholar] [CrossRef] [Green Version]
- Jankovic, J.; Tan, E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- Gupta, A.; March, L. Treating osteoporosis. Aust. Prescr. 2016, 39, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, G.A.P.; Silva, J.L. Alpha-synuclein stepwise aggregation reveals features of an early onset mutation in Parkinson’s disease. Commun. Biol. 2019, 2, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, C.T. Dysfunction of the Microbiota-Gut-Brain Axis in Neurodegenerative Disease: The Promise of Therapeutic Modulation with Prebiotics, Medicinal Herbs, Probiotics, and Synbiotics. J. Evid. Based Integr. Med. 2020, 25, 2515690X20957225. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhao, D.; Ali Shah, S.Z.; Wu, W.; Lai, M.; Zhang, X.; Li, J.; Guan, Z.; Zhao, H.; Li, W.; et al. The Role of the Gut Microbiota in the Pathogenesis of Parkinson’s Disease. Front. Neurol. 2019, 10, 1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheperjans, F.; Aho, V.; Pereira, P.A.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P.; et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 2017, 32, 739–749. [Google Scholar] [CrossRef]
- Hopfner, F.; Künstner, A.; Müller, S.H.; Künzel, S.; Zeuner, K.E.; Margraf, N.G.; Deuschl, G.; Baines, J.F.; Kuhlenbäumer, G. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res. 2017, 1667, 41–45. [Google Scholar] [CrossRef]
- Barichella, M.; Pacchetti, C.; Bolliri, C.; Cassani, E.; Iorio, L.; Pusani, C.; Pinelli, G.; Privitera, G.; Cesari, I.; Faierman, S.A. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology 2016, 87, 1274–1280. [Google Scholar] [CrossRef]
- Georgescu, D.; Ancusa, O.E.; Georgescu, L.A.; Ionita, I.; Reisz, D. Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: Is there hope? Clin. Interv. Aging 2016, 11, 1601–1608. [Google Scholar] [CrossRef] [Green Version]
- Tan, A.H.; Lim, S.Y.; Chong, K.K.; Manap, M.A.A.A.; Hor, J.W.; Lim, J.L.; Low, S.C.; Chong, C.W.; Mahadeva, S.; Lang, A.E. Probiotics for Constipation in Parkinson Disease: A Randomized Placebo-Controlled Study. Neurology 2021, 96, e772–e782. [Google Scholar] [CrossRef]
- Ibrahim, A.; Ali, R.A.R.; Manaf, M.R.A.; Ahmad, N.; Tajurruddin, F.W.; Qin, W.Z.; Desa, S.H.M.; Ibrahim, N.M. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: A randomised controlled trial. PLoS ONE 2020, 15, e0244680. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Taghizadeh, M.; Daneshvar Kakhaki, R.; Kouchaki, E.; Bahmani, F.; Borzabadi, S.; Oryan, S.; Mafi, A.; Asemi, Z. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kuhara, T.; Oki, M.; Xiao, J.Z. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: A randomised, double-blind, placebo-controlled trial. Benef. Microbes 2019, 10, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Katsumata, N.; Bernier, F.; Ohno, K.; Yamauchi, Y.; Odamaki, T.; Yoshikawa, K.; Ito, K.; Kaneko, T. Probiotic Bifidobacterium breve in Improving Cognitive Functions of Older Adults with Suspected Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimers Dis. 2020, 77, 139–147. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef] [Green Version]
- Jung, G.; Hernández-Illán, E.; Moreira, L.; Balaguer, F.; Goel, A. Epigenetics of colorectal cancer: Biomarker and therapeutic potential. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 111–130. [Google Scholar] [CrossRef]
- Irrazábal, T.; Belcheva, A.; Girardin, S.E.; Martin, A.; Philpott, D.J. The multifaceted role of the intestinal microbiota in colon cancer. Mol. Cell 2014, 54, 309–320. [Google Scholar] [CrossRef] [Green Version]
- West, N.R.; McCuaig, S.; Franchini, F.; Powrie, F. Emerging cytokine networks in colorectal cancer. Nat. Rev. Immunol. 2015, 15, 615–629. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Pak, H.; Maghsoudi, L.H.; Soltanian, A.; Gholami, F. Surgical complications in colorectal cancer patients. Ann. Med. Surg. 2020, 55, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Koliarakis, I.; Athanasakis, E.; Sgantzos, M.; Mariolis-Sapsakos, T.; Xynos, E.; Chrysos, E.; Souglakos, J.; Tsiaoussis, J. Intestinal Microbiota in Colorectal Cancer Surgery. Cancers 2020, 12, 3011. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Murakami, M.; Nakao, K.; Asahara, T.; Nomoto, K.; Tsunoda, A. Randomized clinical trial of the influence of mechanical bowel preparation on faecal microflora in patients undergoing colonic cancer resection. Br. J. Surg. 2010, 97, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Drago, L. Probiotics and Colon Cancer. Microorganisms 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, V.B.; Schmidt, T.M. Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J. Clin. Microbiol. 2004, 42, 1203–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.S.; Ryan, E.J.; Doherty, G.A. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: The role of inflammation. World J. Gastroenterol. 2014, 20, 3751–3761. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Bornstein, J.C.; Nurgali, K. Colorectal Cancer Chemotherapy: The Evolution of Treatment and New Approaches. Curr. Med. Chem. 2017, 24, 1537–1557. [Google Scholar] [CrossRef]
- Zwielehner, J.; Lassl, C.; Hippe, B.; Pointner, A.; Switzeny, O.J.; Remely, M.; Kitzweger, E.; Ruckser, R.; Haslberger, A.G. Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS ONE 2011, 6, e28654. [Google Scholar] [CrossRef] [Green Version]
- Amitay, E.L.; Carr, P.R.; Gies, A.; Laetsch, D.C.; Brenner, H. Probiotic/Synbiotic Treatment and Postoperative Complications in Colorectal Cancer Patients: Systematic Review and Meta-analysis of Randomized Controlled Trials. Clin. Transl. Gastroenterol. 2020, 11, e00268. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Mańkowska-Wierzbicka, D.; Mardas, M.; Stelmach-Mardas, M. Role of Probiotics in Modulating Human Gut Microbiota Populations and Activities in Patients with Colorectal Cancer-A Systematic Review of Clinical Trials. Nutrients 2021, 13, 1160. [Google Scholar] [CrossRef]
- Aisu, N.; Tanimura, S.; Yamashita, Y.; Yamashita, K.; Maki, K.; Yoshida, Y.; Sasaki, T.; Takeno, S.; Hoshino, S. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Exp. Ther. Med. 2015, 10, 966–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A Four-Probiotics Regimen Reduces Postoperative Complications After Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. World J. Surg. 2015, 39, 2776–2783. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Huang, M.J.; Zhang, X.W.; Wang, L.; Huang, N.Q.; Peng, H.; Lan, P.; Peng, J.S.; Yang, Z.; Xia, Y.; et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: A double-center and double-blind randomized clinical trial. Am. J. Clin. Nutr. 2013, 97, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.K.; Said, S.; Rajandram, R.; Wang, Z.; Roslani, A.C.; Chin, K.F. Pre-surgical Administration of Microbial Cell Preparation in Colorectal Cancer Patients: A Randomized Controlled Trial. World J. Surg. 2016, 40, 1985–1992. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Chen, H.; Hong, L.; Feng, J.; Yang, J.; Yang, Z.; Shi, C.; Wu, W.; Gao, R.; et al. The effect of perioperative probiotics treatment for colorectal cancer: Short-term outcomes of a randomized controlled trial. Oncotarget 2016, 7, 8432–8440. [Google Scholar] [CrossRef] [Green Version]
- Mego, M.; Chovanec, J.; Vochyanova-Andrezalova, I.; Konkolovsky, P.; Mikulova, M.; Reckova, M.; Miskovska, V.; Bystricky, B.; Beniak, J.; Medvecova, L.; et al. Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo-controlled pilot study. Complement. Ther. Med. 2015, 23, 356–362. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.E. Efficacy of Bifidobacterium tetragenous viable bacteria tablets for cancer patients with functional constipation. Asian Pac. J. Cancer Prev. 2014, 15, 10241–10244. [Google Scholar] [CrossRef] [Green Version]
- Zaharuddin, L.; Mokhtar, N.M.; Muhammad Nawawi, K.N.; Raja Ali, R.A. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.W.; Du, P.; Gao, J.; Yang, B.R.; Fang, W.J.; Ying, C.M. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am. J. Med. Sci. 2012, 343, 199–205. [Google Scholar] [CrossRef]
- Park, I.J.; Lee, J.H.; Kye, B.H.; Oh, H.K.; Cho, Y.B.; Kim, Y.T.; Kim, J.Y.; Sung, N.Y.; Kang, S.B.; Seo, J.M.; et al. Effects of PrObiotics on the Symptoms and Surgical ouTComes after Anterior REsection of Colon Cancer (POSTCARE): A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Med. 2020, 9, 2181. [Google Scholar] [CrossRef]
- Sadahiro, S.; Suzuki, T.; Tanaka, A.; Okada, K.; Kamata, H.; Ozaki, T.; Koga, Y. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: Prospective randomized trial. Surgery 2014, 155, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, C.; Taylor, L. Treatment-related diarrhea in patients with cancer. Clin. J. Oncol. Nurs. 2012, 16, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Li, S.T.; Shu, Y.; Zhan, H.Q. Probiotics for prevention of radiation-induced diarrhea: A meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0178870. [Google Scholar] [CrossRef] [Green Version]
- Demers, M.; Dagnault, A.; Desjardins, J. A randomized double-blind controlled trial: Impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin. Nutr. 2014, 33, 761–767. [Google Scholar] [CrossRef]
- Tian, Y.; Li, M.; Song, W.; Jiang, R.; Li, Y.Q. Effects of probiotics on chemotherapy in patients with lung cancer. Oncol. Lett. 2019, 17, 2836–2848. [Google Scholar] [CrossRef] [Green Version]
- Helman, A.; Klochendler, A.; Azazmeh, N.; Gabai, Y.; Horwitz, E.; Anzi, S.; Swisa, A.; Condiotti, R.; Granit, R.Z.; Nevo, Y.; et al. p16(Ink4a)-induced senescence of pancreatic beta cells enhances insulin secretion. Nat. Med. 2016, 22, 412–420. [Google Scholar] [CrossRef] [Green Version]
- The Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.W.; Gu, Y.L.; Mao, X.Q.; Zhang, L.; Pei, Y.F. Effects of probiotics on type II diabetes mellitus: A meta-analysis. J. Transl. Med. 2020, 18, 30. [Google Scholar] [CrossRef] [Green Version]
- Dixon, A.; Robertson, K.; Yung, A.; Que, M.; Randall, H.; Wellalagodage, D.; Cox, T.; Robertson, D.; Chi, C.; Sun, J. Efficacy of Probiotics in Patients of Cardiovascular Disease Risk: A Systematic Review and Meta-analysis. Curr. Hypertens. Rep. 2020, 22, 74. [Google Scholar] [CrossRef]
- Holtze, S.; Gorshkova, E.; Braude, S.; Cellerino, A.; Dammann, P.; Hildebrandt, T.B.; Hoeflich, A.; Hoffmann, S.; Koch, P.; Terzibasi Tozzini, E.; et al. Alternative Animal Models of Aging Research. Front. Mol. Biosci. 2021, 8, 660959. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A. Old and new models for the study of human ageing. Nat. Rev. Mol. Cell Biol. 2020, 21, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.J.; Scheibye-Knudsen, M.; Longo, D.L.; de Cabo, R. Animal models of aging research: Implications for human aging and age-related diseases. Annu. Rev. Anim. Biosci. 2015, 3, 283–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellantuono, I.; Potter, P.K. Modelling ageing and age-related disease. Drug Discov. Today Dis. Models 2016, 20, 27–32. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Cheng, L.H.; Liu, Y.W.; Jeng, O.J.; Lee, Y.K. Gerobiotics: Probiotics targeting fundamental aging processes. Biosci. Microbiota Food Health 2021, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.C.; Pak, A.W. A catalog of biases in questionnaires. Prev. Chronic Dis. 2005, 2, A13. [Google Scholar] [PubMed]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The Genus Enterococcus: Between Probiotic Potential and Safety Concerns-An Update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). Guidelines for the Evaluation of Probiotics in Food. 2002. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 27 January 2022).
- van den Nieuwboer, M.; Claassen, E. Dealing with the remaining controversies of probiotic safety. Benef. Microbes 2019, 10, 605–616. [Google Scholar] [CrossRef]
- Pace, F.; Macchini, F.; Massimo Castagna, V. Safety of probiotics in humans: A dark side revealed? Dig. Liver Dis. 2020, 52, 981–985. [Google Scholar] [CrossRef]
- Tapiovaara, L.; Lehtoranta, L.; Poussa, T.; Mäkivuokko, H.; Korpela, R.; Pitkäranta, A. Absence of adverse events in healthy individuals using probiotics—Analysis of six randomised studies by one study group. Benef. Microbes 2016, 7, 161–169. [Google Scholar] [CrossRef]
- Wachholz, P.A.; Nunes, V.D.S.; Polachini do Valle, A.; Jacinto, A.F.; Villas-Boas, P.J.F. Effectiveness of probiotics on the occurrence of infections in older people: Systematic review and meta-analysis. Age Ageing 2018, 47, 527–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, L.; Yu, G.; Yang, K.; Hao, W.; Chen, H. The Improving Effect and Safety of Probiotic Supplements on Patients with Osteoporosis and Osteopenia: A Systematic Review and Meta-Analysis of 10 Randomized Controlled Trials. Evid. Based Complement. Alternat. Med. 2021, 2021, 9924410. [Google Scholar] [CrossRef] [PubMed]
- Langella, P.; Guarner, F.; Martín, R. Editorial: Next-Generation Probiotics: From Commensal Bacteria to Novel Drugs and Food Supplements. Front. Microb. 2019, 10, 1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [Green Version]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef]
- Akatsu, H. Exploring the Effect of Probiotics, Prebiotics, and Postbiotics in Strengthening Immune Activity in the Elderly. Vaccines 2021, 9, 136. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Ahadi, S.; Zhou, W.; Schüssler-Fiorenza Rose, S.M.; Sailani, M.R.; Contrepois, K.; Avina, M.; Ashland, M.; Brunet, A.; Snyder, M. Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nat. Med. 2020, 26, 83–90. [Google Scholar] [CrossRef]
- Dönertaş, H.M.; Fabian, D.K.; Valenzuela, M.F.; Partridge, L.; Thornton, J.M. Common genetic associations between age-related diseases. Nat. Aging 2021, 1, 400–412. [Google Scholar] [CrossRef]
- Aleman, F.D.D.; Valenzano, D.R. Microbiome evolution during host aging. PLoS Pathog. 2019, 15, e1007727. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Jeffery, I.B. Gut microbiota and aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef] [PubMed]
- Ale, E.C.; Binetti, A.G. Role of Probiotics, Prebiotics, and Synbiotics in the Elderly: Insights into Their Applications. Front. Microbiol. 2021, 12, 631254. [Google Scholar] [CrossRef] [PubMed]
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Giacosa, A.; Faliva, M.A.; Perna, S.; Allieri, F.; Castellazzi, A.M. Review on microbiota and effectiveness of probiotics use in older. World J. Clin. Cases 2015, 3, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z.; Federici, S.; et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018, 174, 1406–1423. [Google Scholar] [CrossRef] [Green Version]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.; et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018, 174, 1388–1405. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, A.N.; Bergh, C.; Kruger, K.; Sűsserová, M.; Allen, J.; Améen, S.; Tingö, L. The Effect of Probiotics on Health Outcomes in the Elderly: A Systematic Review of Randomized, Placebo-Controlled Studies. Microorganisms 2021, 9, 1344. [Google Scholar] [CrossRef]
- Eloe-Fadrosh, E.A.; Brady, A.; Crabtree, J.; Drabek, E.F.; Ma, B.; Mahurkar, A.; Ravel, J.; Haverkamp, M.; Fiorino, A.M.; Botelho, C.; et al. Functional dynamics of the gut microbiome in elderly people during probiotic consumption. mBio 2015, 6, e00231-15. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Kiousi, D.E.; Karapetsas, A.; Karolidou, K.; Panayiotidis, M.I.; Pappa, A.; Galanis, A. Probiotics in Extraintestinal Diseases: Current Trends and New Directions. Nutrients 2019, 11, 788. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.R.; de Cássia Orlandi Sardi, J.; de Souza Pitangui, N.; Roque, S.M.; da Silva, A.C.B.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Kiousi, D.E.; Rathosi, M.; Tsifintaris, M.; Chondrou, P.; Galanis, A. Pro-biomics: Omics Technologies to Unravel the Role of Probiotics in Health and Disease. Adv. Nutr. 2021, 12, 1802–1820. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cheung, P.C. Comparative proteome analysis of Bifidobacterium longum subsp. infantis grown on β-glucans from different sources and a model for their utilization. J. Agric. Food Chem. 2013, 61, 4360–4370. [Google Scholar]
- Yuan, J.; Wang, B.; Sun, Z.; Bo, X.; Yuan, X.; He, X.; Zhao, H.; Du, X.; Wang, F.; Jiang, Z.; et al. Analysis of host-inducing proteome changes in Βifidobacterium longum NCC2705 grown in vivo. J. Proteome Res. 2008, 7, 375–385. [Google Scholar] [CrossRef]
| Probiotic Strains | Participants | Age (Years, Mean ± SD) | Sex Ratio (M/F) | Type of Study | Intervention | Duration | Key Molecular Findings | Clinical Outcomes | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Probiotic Group | Control Group | Probiotic Group | Control Group | Probiotic Group | Control Group | |||||||
| Alzheimer’s Disease | ||||||||||||
| L. acidophilus, L. casei, B. bifidum, L. fermentum | 60 patients | 77.67 ± 2.62 | 82.00 ± 1.69 | 6/24 | 6/24 | Rando-mized, double-blind, and controlled clinical trial | Probiotic milk, 200 mL/day (2 × 109 CFU/g of each strain) | Milk, 200 mL/day | 12 weeks | ↓ hs-CRP ↓ HOMH-IR ↓ HOMA-B ↑ QUICKI ↓ TG level ↓ VLDL ↓ MDA | Improvement in MMSE score | [58] |
| L. acidophilus, B. bifidum, B. longum | 79 patients | 76.2 ± 8.1 | 78.5 ± 8.0 | NA | NA | Randomized, double-blind, controlled clinical study | 2 × 109 CFU of each strain plus selenium (200 mg/day), once daily | Placebo (packaging not reported) | 12 weeks | Probiotic plus selenium intake: ↓ hs-CRP ↓ HOMA-IR ↓ FPG ↑ TAC levels ↑ GSH levels ↓ serum insulin ↓ serum TG ↑ QUICKI (compared with only selenium and placebo) | Probiotic plus selenium intake: Improvement in MMSE score | [59] |
| Acetobacter aceti, L. delbrueckii delbrueckii, L. fermentum, L. fructivorans, Enterococcus faecium, Leuconostoc spp., L. kefiranofaciens, Candida famata, C. krusei | 13 patients | 78.5 ± 7 | - | 2/11 | - | Uncontrolled clinical trial | Pasteurized milk with 4% kefir grains | - | 90 days | ↓ TNF-α ↓ IL-8 ↓ IL12p70 ↓ IL-8/IL-10 ↓ IL-12/IL-10 ↓ serum levels of O2−, H2O2, and ONOO−/OH− ↑ serum NO | ↑ MMSE score | [60] |
| L. plantarum C29 | 100 individuals diagnosed with MCI | 68.0 ± 5.12 | 69.2 ± 7.00 | 20/30 | 14/36 | Rando-mized, double-blind, placebo-controlled clinical trial | DW2009 capsules, 800 mg/day (1.25 × 1010 CFU/g) | Placebo capsules containing cellulose | 12 weeks | ↑ Lactobacilli population | Improve cognitive performance ↑ Sserum BDNF | [61] |
| B. breve A1 | 19 elderly patients with MCI | 82.5 ± 5.3 | - | 1/18 | - | Open-label, single arm study | Capsules, >1 × 1010 CFU, twice daily | - | 24 weeks | ΝA | ↑ MMSE score Improved POMS2 and GSRS scores | [62] |
| L. acidophilus, L. casei, L. fermentum, B. bifidum | 48 patients | 79.70 ± 1.72 | 80.57 ± 1.79 | 7/18 | 10/13 | Rando-mized, double-blind, placebo-controlled clinical trial | Capsules, 3 × 109 CFU, once daily | Placebo capsules containing malto-dextrin | 12 weeks | NS | NS | [63] |
| B. breve A1 | 121 individuals with subjective memory complaints | 61.5 ± 6.83 | 61.6 ± 6.37 | 30/31 | 30/30 | Rando-mized, double-blind, placebo-controlled trial | Capsules, > 2 × 1010 CFU, twice daily | Placebo capsules containing corn starch | 12 weeks | NS | NS | [78] |
| B. breve A1 | 80 healthy older adults with MCI | 61.3 ± 7.7 | 60.9 ± 6.9 | 19/21 | 20/20 | Randomized, double-blind, placebo-controlled trial | Capsules, 2 × 1010 CFU, once daily | Placebo capsules containing maize starch | 16 weeks | ΝA | Improvement of cognitive function ↑ RBANS score ↑ JMCIS score | [79] |
| Parkinson’s Disease | ||||||||||||
| Streptococcus salivarius subsp thermophilus, E. faecium, L. rhamnosus GG, L. acidophilus, L. plantarum, L. paracasei, L. delbrueckii subsp bulgaricus, B. breve, B. animalis subsp lactis | 120 patients | 71.8 ± 7.7 | 69.5 ± 10.3 | 41/39 | 24/16 | Randomized, parallel group, double-blind, placebo-controlled study | Fermented milk, 250 × 109 CFU, daily | Pasteurized, fermented, fiber-free milk | 4 weeks | NA | Improved bowel habits ↑ CBMs | [73] |
| L. acidophilus, B. infantis | 40 patients | 69.80 ± 5.64 | 75.65 ± 9.66 | 10/10 | 7/13 | Randomized, parallel group study | 60 mg, twice daily | Trimebutine 200 mg 3× day | 12 weeks | NA | ↓ Abdominal pain ↓ Bloating | [74] |
| L. acidophilus, L. reuteri, L. gasseri, L. rhamnosus, B. bifidum, B. longum, Enterococcus faecalis, E. faecium | 72 patients | 70.9 ± 6.6 | 68.6 ± 6.7 | 20/14 | 28/10 | Randomized, single-center, double-blind, placebo-controlled study | Capsules, 109 CFU, once daily | Placebo capsules containing an inactive substance | 4 weeks | NS (fecal calprotectin levels) | ↑ SBM Improved stool consistency and QOL related to constipation | [75] |
| L. acidophilus BCMC® 12130, L. casei BCMC® 12313, L. lactis BCMC® 12451, B. infantis BCMC® 02129, B. longum BCMC® 02120 | 55 patients | 69.0 | 70.5 | 16/9 | 17/10 | Rando-mized, double-blind, placebo-controlled, study | Capsules, 3 × 1010 CFU, twice daily | Granulated milk containing lactose | 8 weeks | NA | ↑ BOF ↓ GTT | [76] |
| L. acidophilus, B. bifidum, L. reuteri, L. fermentum | 60 patients | 68.2 ± 7.8 | 67.7 ± 10.2 | ΝA | ΝA | Rando-mized, double-blinded, placebo-controlled trial | Capsules, 8 × 109 CFU/g, once daily | Placebo capsules | 12 weeks | ↓ Serum insulin, ↓ HOMA-IR, ↓ QUICKI, ↓ hs-CRP, ↓ MDA, ↑ GSH ↓ TG ↓ VLDL-cholesterol levels | ↓ MDS-UPDRS | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiousi, D.E.; Kouroutzidou, A.Z.; Neanidis, K.; Matthaios, D.; Pappa, A.; Galanis, A. Evaluating the Role of Probiotics in the Prevention and Management of Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 3628. https://doi.org/10.3390/ijms23073628
Kiousi DE, Kouroutzidou AZ, Neanidis K, Matthaios D, Pappa A, Galanis A. Evaluating the Role of Probiotics in the Prevention and Management of Age-Related Diseases. International Journal of Molecular Sciences. 2022; 23(7):3628. https://doi.org/10.3390/ijms23073628
Chicago/Turabian StyleKiousi, Despoina E., Antonia Z. Kouroutzidou, Konstantinos Neanidis, Dimitrios Matthaios, Aglaia Pappa, and Alex Galanis. 2022. "Evaluating the Role of Probiotics in the Prevention and Management of Age-Related Diseases" International Journal of Molecular Sciences 23, no. 7: 3628. https://doi.org/10.3390/ijms23073628
APA StyleKiousi, D. E., Kouroutzidou, A. Z., Neanidis, K., Matthaios, D., Pappa, A., & Galanis, A. (2022). Evaluating the Role of Probiotics in the Prevention and Management of Age-Related Diseases. International Journal of Molecular Sciences, 23(7), 3628. https://doi.org/10.3390/ijms23073628









