Sexual Dimorphism in Adipose-Hypothalamic Crosstalk and the Contribution of Aryl Hydrocarbon Receptor to Regulate Energy Homeostasis
Abstract
1. Introduction
2. Adipose–Hypothalamic Axis
2.1. Sexual Dimorphism in POMC Regulation of Energy Balance and Adiposity
2.2. Sexual Dimorphism in AgRP/NPY Regulation of Energy Balance and Adiposity
3. Hypothalamic–Pituitary–Adipose Axis
3.1. Hypothalamic–Pituitary–Gonadal (HPG) Axis
3.1.1. Estrogen Regulation of Adipose Tissue for Involvement in Sex Difference Energy Homeostasis
3.1.2. Androgen Regulation of Adipose Tissue Sex Differences in the Involvement of Regulating Energy Homeostasis
3.2. Hypothalamic–Pituitary–Adrenal (HPA) Axis
3.3. Hypothalamic–Pituitary–Somatotropic (HPS) Axis
4. Xenobiotics Modulating Aryl Hydrocarbon Receptor to Regulate Energy Homeostasis
4.1. AhR in Early Sexual and Neuroendocrine Development
4.2. AhR Contribution to Energy Metabolism and Obesity
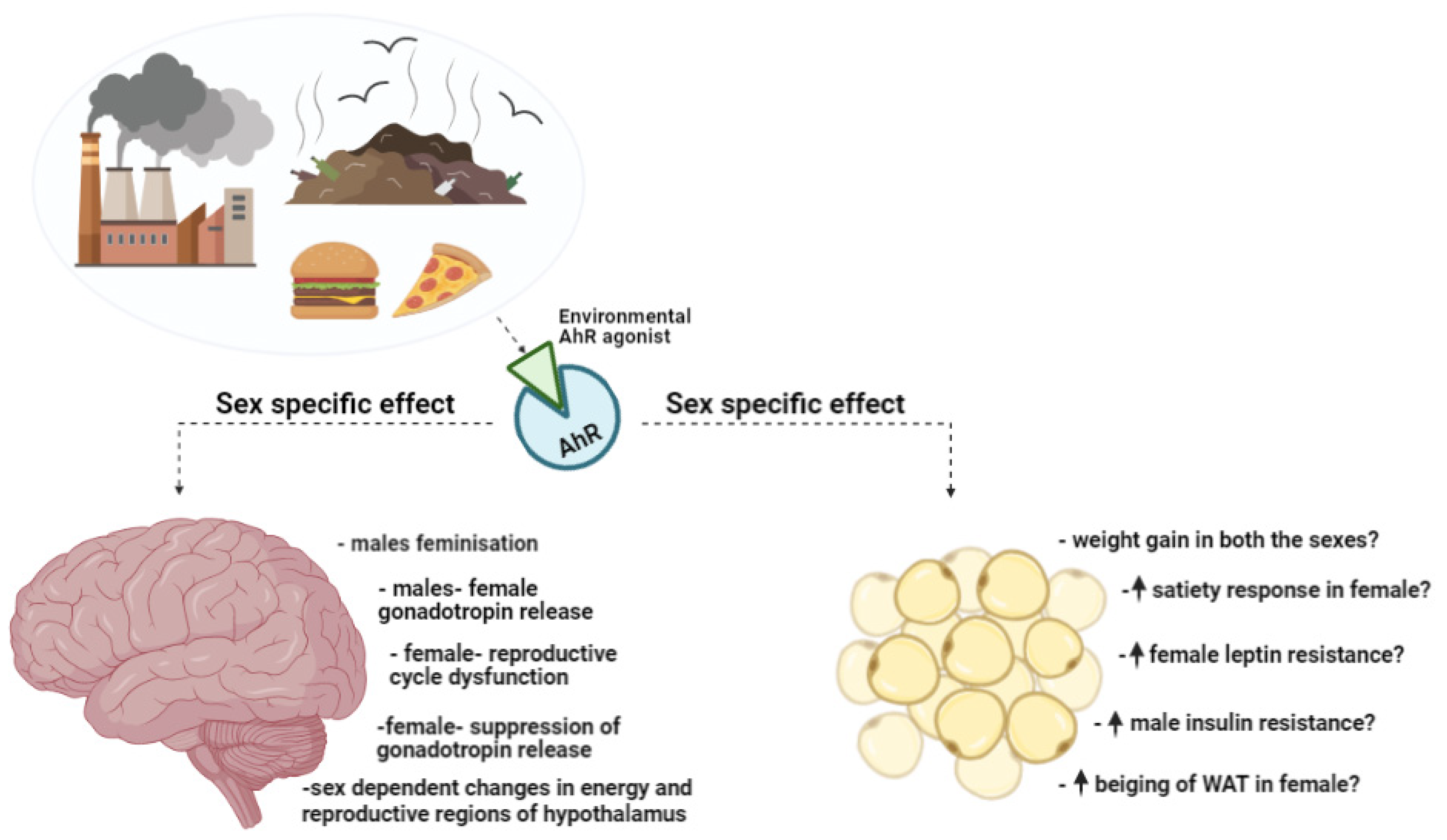
4.3. Sex-Specific AhR Modulation of Energy Balance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandoval, D.; Cota, D.; Seeley, R.J. The Integrative Role of CNS Fuel-Sensing Mechanisms in Energy Balance and Glucose Regulation. Annu. Rev. Physiol. 2008, 70, 513–535. [Google Scholar] [CrossRef]
- Shi, H.; Strader, A.D.; Woods, S.C.; Seeley, R.J. Sexually dimorphic responses to fat loss after caloric restriction or surgical lipectomy. Am. J. Physiol. Metab. 2007, 293, E316–E326. [Google Scholar] [CrossRef][Green Version]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues—The biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. The sexual dimorphism of obesity. Mol. Cell. Endocrinol. 2015, 402, 113–119. [Google Scholar] [CrossRef]
- Bi, P.; Shan, T.; Liu, W.; Yue, F.; Yang, X.; Liang, X.-R.; Wang, J.; Li, J.; Carlesso, N.; Liu, X.; et al. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nat. Med. 2014, 20, 911–918. [Google Scholar] [CrossRef]
- Cao, L.; Choi, E.Y.; Liu, X.; Martin, A.; Wang, C.; Xu, X.; During, M.J. White to Brown Fat Phenotypic Switch Induced by Genetic and Environmental Activation of a Hypothalamic-Adipocyte Axis. Cell Metab. 2011, 14, 324–338. [Google Scholar] [CrossRef]
- Rodriguez-Cuenca, S.; Pujol, E.; Justo, R.; Frontera, M.; Oliver, J.; Gianotti, M.; Roca, P.J.J. Sex-dependent Thermogenesis, Differences in Mitochondrial Morphology and Function, and Adrenergic Response in Brown Adipose Tissue. J. Biol. Chem. 2002, 277, 42958–42963. [Google Scholar] [CrossRef]
- Valle, A.; Català-Niell, A.; Colom, B.; Garcia-Palmer, F.J.; Oliver, J.O.; Roca, P. Sex-related differences in energy balance in response to caloric restriction. Am. J. Physiol. Metab. 2005, 289, E15–E22. [Google Scholar] [CrossRef][Green Version]
- Cardon, L.R.; Carmelli, D.; Fabsitz, R.R.; Reed, T. Genetic and environmental correlations between obesity and body fat distribution in adult male twins. Hum. Biol. 1994, 66, 465–479. [Google Scholar]
- Stunkard, A.J.; Sørensen, T.I.; Hanis, C.; Teasdale, T.W.; Chakraborty, R.; Schull, W.J.; Schulsinger, F. An Adoption Study of Human Obesity. N. Engl. J. Med. 1986, 314, 193–198. [Google Scholar] [CrossRef]
- Baillie-Hamilton, P.F. Chemical Toxins: A Hypothesis to Explain the Global Obesity Epidemic. J. Altern. Complement. Med. 2002, 8, 185–192. [Google Scholar] [CrossRef]
- Wright, S.M.; Aronne, L.J. Causes of obesity. Abdom. Radiol. 2012, 37, 730–732. [Google Scholar] [CrossRef]
- Fierens, S.; Mairesse, H.; Heilier, J.-F.; De Burbure, C.; Focant, J.-F.; Eppe, G.; De Pauw, E.; Bernard, A. Dioxin/polychlorinated biphenyl body burden, diabetes and endometriosis: Findings in a population-based study in Belgium. Biomarkers 2003, 8, 529–534. [Google Scholar] [CrossRef]
- Henriksen, G.L.; Ketchum, N.S.; Michalek, J.E.; Swaby, J.A. Serum Dioxin and Diabetes Mellitus in Veterans of Operation Ranch Hand. Epidemiology 1997, 8, 252–258. [Google Scholar] [CrossRef]
- Lee, D.-H.; Lee, I.-K.; Song, K.; Steffes, M.; Toscano, W.; Baker, B.A.; Jacobs, D.R. A Strong Dose-Response Relation Between Serum Concentrations of Persistent Organic Pollutants and Diabetes: Results from the National Health and Examination Survey 1999–2002. Diabetes Care 2006, 29, 1638–1644. [Google Scholar] [CrossRef]
- Magliano, D.J.; Rancière, F.; Slama, R.; Roussel, R.; Kiviranta, H.; Coumoul, X.; Balkau, B.; Botton, J. Exposure to persistent organic pollutants and the risk of type 2 diabetes: A case-cohort study. Diabetes Metab. 2021, 47, 101234. [Google Scholar] [CrossRef]
- Turyk, M.; Anderson, H.A.; Knobeloch, L.; Imm, P.; Persky, V.W. Prevalence of diabetes and body burdens of polychlorinated biphenyls, polybrominated diphenyl ethers, and p,p′-diphenyldichloroethene in Great Lakes sport fish consumers. Chemosphere 2009, 75, 674–679. [Google Scholar] [CrossRef]
- Wang, S.-L.; Tsai, P.-C.; Yang, C.-Y.; Guo, Y.L. Increased Risk of Diabetes and Polychlorinated Biphenyls and Dioxins. Diabetes Care 2008, 31, 1574–1579. [Google Scholar] [CrossRef]
- McMillan, B.J.; Bradfield, C.A. The Aryl hydrocarbon receptor is activated by modified low-density lipoprotein. Proc. Natl. Acad. Sci. USA 2007, 104, 1412–1417. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum Immunoreactive-Leptin Concentrations in Normal-Weight and Obese Humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef]
- Cone, R.D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005, 8, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Bertagna, X. Proopiomelanocortin-derived peptides. Endocrinol. Metab. Clin. N. Am. 1994, 23, 467–485. [Google Scholar] [CrossRef]
- Morrison, E.; Castro, M.G. Post-Translational Processing of Proopiomelanocortin in the Pituitary and in the Brain. Crit. Rev. Neurobiol. 1997, 11, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, K.G.; Robbins, L.S.; Mortrud, M.T.; Cone, R.D. The Cloning of a Family of Genes That Encode the Melanocortin Receptors. Science 1992, 257, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Valverde, P.; Healy, E.; Jackson, I.J.; Rees, J.L.; Thody, A.J. Variants of the melanocyte–stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat. Genet. 1995, 11, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.J.; Riedy, C.A.; Smith, K.A.B.; Benoit, S.C.; Woods, S.C. Differential Sensitivity to Central Leptin and Insulin in Male and Female Rats. Diabetes 2003, 52, 682–687. [Google Scholar] [CrossRef]
- Garaulet, M.; Perex-Llamas, F.; Fuente, T.; Zamora, S.; Tebar, F.J. Anthropometric, computed tomography and fat cell data in an obese population: Relationship with insulin, leptin, tumor necrosis factor-alpha, sex hormone-binding globulin and sex hormones. Eur. J. Endocrinol. 2000, 143, 657–666. [Google Scholar] [CrossRef]
- Gottschling-Zeller, H.; Birgel, M.; Scriba, D.; Blum, W.F.; Hauner, H. Depot-specific release of leptin from subcutaneous and omental adipocytes in suspension culture: Effect of tumor necrosis factor-alpha and transforming growth factor-beta1. Eur. J. Endocrinol. 1999, 141, 436–442. [Google Scholar] [CrossRef][Green Version]
- Havel, P.J.; Kasim-Karakas, S.; Dubuc, G.R.; Muller, W.; Phinney, S.D. Gender differences in plasma leptin concentrations. Nat. Med. 1996, 2, 949–950. [Google Scholar] [CrossRef]
- Luukkaa, V.; Pesonen, U.; Huhtaniemi, I.; Lehtonen, A.; Tilvis, R.; Tuomilehto, J.; Koulu, M.; Huupponen, R. Inverse Correlation between Serum Testosterone and Leptin in Men1. J. Clin. Endocrinol. Metab. 1998, 83, 3243–3246. [Google Scholar] [CrossRef][Green Version]
- Haffner, S.M.; Miettinen, H.; Karhapää, P.; Mykkänen, L.; Laakso, M. Leptin Concentrations, Sex Hormones, and Cortisol in Nondiabetic Men. J. Clin. Endocrinol. Metab. 1997, 82, 1807–1809. [Google Scholar] [CrossRef] [PubMed]
- Margetic, S.; Gazzola, C.; Pegg, G.G.; Hill, R.A. Leptin: A review of its peripheral actions and interactions. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1407–1433. [Google Scholar] [CrossRef] [PubMed]
- Van Harmelen, V.; Reynisdottir, S.; Eriksson, P.; Thörne, A.; Hoffstedt, J.; Lönnqvist, F.; Arner, P. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 1998, 47, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Bennett, F.I.; McFarlane-Anderson, N.; Wilks, R.; Luke, A.; Cooper, R.S.; Forrester, T.E. Leptin concentration in women is influenced by regional distribution of adipose tissue. Am. J. Clin. Nutr. 1997, 66, 1340–1344. [Google Scholar] [CrossRef][Green Version]
- Nohara, K.; Zhang, Y.; Waraich, R.S.; Laque, A.; Tiano, J.P.; Tong, J.; Münzberg, H.; Mauvais-Jarvis, F. Early-Life Exposure to Testosterone Programs the Hypothalamic Melanocortin System. Endocrinology 2011, 152, 1661–1669. [Google Scholar] [CrossRef]
- Wang, C.; He, Y.; Xu, P.; Yang, Y.; Saito, K.; Xia, Y.; Yan, X.; Hinton, A., Jr.; Yan, C.; Ding, H.; et al. TAp63 contributes to sexual dimorphism in POMC neuron functions and energy homeostasis. Nat. Commun. 2018, 9, 1544. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Y. Mechanisms for sex differences in energy homeostasis. J. Mol. Endocrinol. 2019, 62, R129–R143. [Google Scholar] [CrossRef]
- Samuel, P.; Khan, M.A.; Nag, S.; Inagami, T.; Hussain, T. Angiotensin AT2 Receptor Contributes towards Gender Bias in Weight Gain. PLoS ONE 2013, 8, e48425. [Google Scholar] [CrossRef]
- Seminara, S.B.; Messager, S.; Chatzidaki, E.E.; Thresher, R.R.; Acierno, J.S., Jr.; Shagoury, J.K.; Bo-Abbas, Y.; Kuohung, W.; Schwinof, K.M.; Hendrick, A.G.; et al. The GPR54 gene as a regulator of puberty. Obstet. Gynecol. Surv. 2003, 349, 1614–1627. [Google Scholar] [CrossRef]
- de Tassigny, X.D.; Fagg, L.A.; Dixon, J.P.C.; Day, K.; Leitch, H.G.; Hendrick, A.G.; Zahn, D.; Franceschini, I.; Caraty, A.; Carlton, M.B.L.; et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc. Natl. Acad. Sci. USA 2007, 104, 10714–10719. [Google Scholar] [CrossRef]
- Xu, A.W.; Ste-Marie, L.; Kaelin, C.B.; Barsh, G.S. Inactivation of Signal Transducer and Activator of Transcription 3 in Proopiomelanocortin (Pomc) Neurons Causes Decreased Pomc Expression, Mild Obesity, and Defects in Compensatory Refeeding. Endocrinology 2007, 148, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.K.; Doslikova, B.; D’Agostino, G.; Greenwald-Yarnell, M.; Georgescu, T.; Chianese, R.; de Morentin, P.B.M.; Ogunnowo-Bada, E.; Cansell, C.; Valencia-Torres, L.; et al. Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Mol. Metab. 2016, 5, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Bamshad, M.; Song, C.K.; Bartness, T.J. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1999, 276, R1569–R1578. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, N.J.; Stock, M.J. Effect of diet and fenfluramine on thermogenesis in the rat: Possible involvement of serotonergic mechanisms. Int. J. Obes. 1987, 11, 319–324. [Google Scholar]
- Lowell, M.; Flier, M.; PhD, B.B. Brown adipose tissue, β3-adrenergic receptors, and obesity. Annu. Rev. Med. 1997, 48, 307–316. [Google Scholar] [CrossRef]
- Tecott, L.H.; Sun, L.M.; Akana, S.F.; Strack, A.M.; Lowenstein, D.H.; Dallman, M.F.; Julius, D. Eating disorder and epilepsy in mice lacking 5-HT2C serotonin receptors. Nature 1995, 374, 542–546. [Google Scholar] [CrossRef]
- Nonogaki, K.; Strack, A.M.; Dallman, M.F.; Tecott, L.H. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat. Med. 1998, 4, 1152–1156. [Google Scholar] [CrossRef]
- Xu, Y.; Jones, J.E.; Kohno, D.; Williams, K.W.; Lee, C.E.; Choi, M.J.; Anderson, J.G.; Heisler, L.K.; Zigman, J.M.; Lowell, B.B.; et al. 5-HT2CRs Expressed by Pro-Opiomelanocortin Neurons Regulate Energy Homeostasis. Neuron 2008, 60, 582–589. [Google Scholar] [CrossRef]
- Berglund, E.D.; Liu, C.; Sohn, J.-W.; Liu, T.; Kim, M.H.; Lee, C.E.; Vianna, C.R.; Williams, K.; Xu, Y.; Elmquist, J.K. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J. Clin. Investig. 2013, 123, 5061–5070. [Google Scholar] [CrossRef]
- Gao, Y.; Yao, T.; Deng, Z.; Sohn, J.-W.; Sun, J.; Huang, Y.; Kong, X.; Yu, K.-J.; Wang, R.-T.; Chen, H.; et al. TrpC5 Mediates Acute Leptin and Serotonin Effects via Pomc Neurons. Cell Rep. 2017, 18, 583–592. [Google Scholar] [CrossRef]
- Qiu, J.; Fang, Y.; Rønnekleiv, O.K.; Kelly, M.J. Leptin Excites Proopiomelanocortin Neurons via Activation of TRPC Channels. J. Neurosci. 2010, 30, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhang, C.; Borgquist, A.; Nestor, C.C.; Smith, A.W.; Bosch, M.A.; Ku, S.; Wagner, E.J.; Rønnekleiv, O.K.; Kelly, M.J. Insulin Excites Anorexigenic Proopiomelanocortin Neurons via Activation of Canonical Transient Receptor Potential Channels. Cell Metab. 2014, 19, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Bosch, M.A.; Meza, C.; Navarro, U.-V.; Nestor, C.; Wagner, E.J.; Rønnekleiv, O.K.; Kelly, M.J. Estradiol Protects Proopiomelanocortin Neurons Against Insulin Resistance. Endocrinology 2017, 159, 647–664. [Google Scholar] [CrossRef]
- Broberger, C.; Johansen, J.; Johansson, C.; Schalling, M.; Hokfelt, T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl. Acad. Sci. USA 1998, 95, 15043–15048. [Google Scholar] [CrossRef]
- Langhans, W.; Geary, N. Overview of the Physiological Control of Eating. Forum Nutr. 2009, 63, 9–53. [Google Scholar] [CrossRef] [PubMed]
- Asarian, L.; Geary, N. Sex differences in the physiology of eating. Am. J. Physiol. Integr. Comp. Physiol. 2013, 305, R1215–R1267. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, D.; Betley, J.N.; Su, H.H.; Sternson, S.M. Deconstruction of a neural circuit for hunger. Nature 2012, 488, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Sternson, S.M. Hypothalamic Survival Circuits: Blueprints for Purposive Behaviors. Neuron 2013, 77, 810–824. [Google Scholar] [CrossRef]
- Goodin, S.Z.; Keichler, A.R.; Smith, M.; Wendt, D.; Strader, A.D. Effect of gonadectomy on AgRP-induced weight gain in rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, R1747–R1753. [Google Scholar] [CrossRef]
- Urban, J.H.; Bauer-Dantoin, A.C.; Levine, J.E. Neuropeptide Y gene expression in the arcuate nucleus: Sexual dimorphism and modulation by testosterone. Endocrinology 1993, 132, 139–145. [Google Scholar] [CrossRef]
- Sahu, A.; Kalra, S.P.; Crowley, W.R.; Kalra, P.S. Testosterone Raises Neuropeptide-Y Concentration in Selected Hypothalamic Sites and in Vitro Release from the Medial Basal Hypothalamus of Castrated Male Rats. Endocrinology 1989, 124, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S.S.; Belsham, D.D. Estrogen inhibits NPY secretion through membrane-associated estrogen receptor (ER)-α in clonal, immortalized hypothalamic neurons. Int. J. Obes. 2010, 35, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Rebouças, E.C.C.; Leal, S.; Sá, S.I. Regulation of NPY and α-MSH expression by estradiol in the arcuate nucleus of Wistar female rats: A stereological study. Neurol. Res. 2016, 38, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Santollo, J.; Eckel, L.A. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav. Brain Res. 2008, 191, 173–177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruohonen, S.T.; Pesonen, U.; Moritz, N.; Kaipio, K.; Röyttä, M.; Koulu, M.; Savontaus, E. Transgenic Mice Overexpressing Neuropeptide Y in Noradrenergic Neurons. Diabetes 2008, 57, 1517–1525. [Google Scholar] [CrossRef]
- Schäffler, A.; Binart, N.; Schölmerich, J.; Büchler, C. Hypothesis paper Brain talks with fat—Evidence for a hypothalamic–pituitary–adipose axis? Neuropeptides 2005, 39, 363–367. [Google Scholar] [CrossRef]
- Schäffler, A.; Schölmerich, J.; Buechler, C. The role of ‘adipotropins’ and the clinical importance of a potential hypothalamic–pituitary–adipose axis. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 374–383. [Google Scholar] [CrossRef]
- Corbier, P.; Edwards, D.A.; Roffi, J. The neonatal testosterone surge: A comparative study. Arch. Int. Physiol. Biochim. Biophys. 1992, 100, 127–131. [Google Scholar] [CrossRef]
- Arnold, A.P.; Gorski, R.A. Gonadal Steroid Induction of Structural Sex Differences in the Central Nervous System. Annu. Rev. Neurosci. 1984, 7, 413–442. [Google Scholar] [CrossRef]
- Simerly, R.B. Wired for reproduction: Organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 2002, 25, 507–536. [Google Scholar] [CrossRef]
- Negri-Cesi, P.; Colciago, A.; Pravettoni, A.; Casati, L.; Conti, L.; Celotti, F. Sexual differentiation of the rodent hypothalamus: Hormonal and environmental influences. J. Steroid Biochem. Mol. Biol. 2008, 109, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The Role of Estrogens in Control of Energy Balance and Glucose Homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.A.; Budish, R.A.; Kashyap, S.; Lindsey, S.H. GPER-novel membrane estrogen receptor. Clin. Sci. 2016, 130, 1005. [Google Scholar] [CrossRef]
- Marchetti, P.M.; Barth, J.H. Clinical biochemistry of dihydrotestosterone. Ann. Clin. Biochem. 2013, 50, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-X.; Wong, C.-I.; Sar, M.; Wilson, E.M. The androgen receptor: An overview. In Proceedings of the 1992 Laurentian Hormone Conference, San Diego, CA, USA, 9 February 1994; pp. 249–274. [Google Scholar]
- Rodriguez-Cuenca, S.; Monjo, M.; Frontera, M.; Gianotti, M.; Proenza, A.M.; Roca, P. Sex Steroid Receptor Expression Profile in Brown Adipose Tissue. Effects of Hormonal Status. Cell. Physiol. Biochem. 2007, 20, 877–886. [Google Scholar] [CrossRef]
- Wade, G.N.; Gray, J.M. Cytoplasmic 17β-[3H] Estradiol Binding in Rat Adipose Tissues. Endocrinology 1978, 103, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.B.; Børglum, J.D.; Eriksen, E.F.; Richelsen, B. Nuclear estradiol binding in rat adipocytes. Regional variations and regulatory influences of hormones. Biochim. Biophys. Acta 1991, 1093, 80–86. [Google Scholar] [CrossRef]
- Wade, G.N.; Gray, J.M. Gonadal effects on food intake and adiposity: A metabolic hypothesis. Physiol. Behav. 1979, 22, 583–593. [Google Scholar] [CrossRef]
- Gorres, B.K.; Bomhoff, G.L.; Gupte, A.A.; Geiger, P.C. Altered estrogen receptor expression in skeletal muscle and adipose tissue of female rats fed a high-fat diet. J. Appl. Physiol. 2011, 110, 1046–1053. [Google Scholar] [CrossRef]
- Ribas, V.; Nguyen, M.T.A.; Henstridge, D.C.; Nguyen, A.-K.; Beaven, S.W.; Watt, M.J.; Hevener, A.L. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERα-deficient mice. Am. J. Physiol. Metab. 2010, 298, E304–E319. [Google Scholar] [CrossRef]
- Ohlsson, C.; Hellberg, N.; Parini, P.; Vidal, O.; Bohlooly, M.; Rudling, M.; Lindberg, M.K.; Warner, M.; Angelin, B.; Gustafsson, J. Obesity and Disturbed Lipoprotein Profile in Estrogen Receptor-α-Deficient Male Mice. Biochem. Biophys. Res. Commun. 2000, 278, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Foryst-Ludwig, A.; Clemenz, M.; Hohmann, S.; Hartge, M.; Sprang, C.; Frost, N.; Krikov, M.; Bhanot, S.; Barros, R.; Morani, A.; et al. Metabolic Actions of Estrogen Receptor Beta (ERβ) are Mediated by a Negative Cross-Talk with PPARγ. PLoS Genet. 2008, 4, e1000108. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cuenca, S.; Monjo, M.; Proenza, A.M.; Roca, P. Depot differences in steroid receptor expression in adipose tissue: Possible role of the local steroid milieu. Am. J. Physiol. Metab. 2005, 288, E200–E207. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.P.; Boyd, J.; Frank, G.R.; Takahashi, H.; Cohen, R.M.; Specker, B.; Williams, T.C.; Lubahn, D.B.; Korach, K.S. Estrogen Resistance Caused by a Mutation in the Estrogen-Receptor Gene in a Man. N. Engl. J. Med. 1994, 331, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 12729–12734. [Google Scholar] [CrossRef]
- Pedersen, S.B.; Børglum, J.D.; Møller-Pedersen, T.; Richelsen, B. Effects of in vivo estrogen treatment on adipose tissue metabolism and nuclear estrogen receptor binding in isolated rat adipocytes. Mol. Cell. Endocrinol. 1992, 85, 13–19. [Google Scholar] [CrossRef]
- Hirsch, J.; Han, P.W. Cellularity of rat adipose tissue: Effects of growth, starvation, and obesity. J. Lipid Res. 1969, 10, 77–82. [Google Scholar] [CrossRef]
- Chung, J.; Nguyen, A.-K.; Henstridge, D.C.; Holmes, A.G.; Chan, M.H.S.; Mesa, J.L.; Lancaster, G.I.; Southgate, R.J.; Bruce, C.R.; Duffy, S.J.; et al. HSP72 protects against obesity-induced insulin resistance. Proc. Natl. Acad. Sci. USA 2008, 105, 1739–1744. [Google Scholar] [CrossRef]
- Gorres, B.K.; Bomhoff, G.L.; Morris, J.K.; Geiger, P.C. In vivo stimulation of oestrogen receptor α increases insulin-stimulated skeletal muscle glucose uptake. J. Physiol. 2011, 589, 2041–2054. [Google Scholar] [CrossRef]
- Rüegg, J.; Cai, W.; Karimi, M.; Kiss, N.B.; Swedenborg, E.; Larsson, C.; Ekström, T.J.; Pongratz, I. Epigenetic Regulation of Glucose Transporter 4 by Estrogen Receptor β. Mol. Endocrinol. 2011, 25, 2017–2028. [Google Scholar] [CrossRef]
- Tara, M.; Souza, S.C.; Aronovitz, M.; Obin, M.S.; Fried, S.K.; Greenberg, A.S. Estrogen regulation of adiposity and fuel partitioning: Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J. Biol. Chem. 2005, 280, 35983–35991. [Google Scholar] [CrossRef]
- Lundholm, L.; Zang, H.; Hirschberg, A.L.; Gustafsson, J.-A.; Arner, P.; Dahlman-Wright, K. Key lipogenic gene expression can be decreased by estrogen in human adipose tissue. Fertil. Steril. 2008, 90, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Homma, H.; Kurachi, H.; Nishio, Y.; Takeda, T.; Yamamoto, T.; Adachi, K.; Morishige, K.-I.; Ohmichi, M.; Murata, Y.J. Estrogen suppresses transcription of lipoprotein lipase gene: Existence of a unique estrogen response element on the lipoprotein lipase promoter. J. Biol. Chem. 2000, 275, 11404–11411. [Google Scholar] [CrossRef]
- Urabe, M.; Yamamoto, T.; Kashiwagi, T.; Okubo, T.; Tsuchiya, H.; Iwasa, K.; Kikuchi, N.; Yokota, K.; Hosokawa, K.; Honjo, H.J.E.J. Effect of estrogen replacement therapy on hepatic triglyceride lipase, lipoprotein liase and lipids including apolipoprotein e in climacteric and elderly women. Endocr. J. 1996, 43, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Richard, D. Effects of ovarian hormones on energy balance and brown adipose tissue thermogenesis. Am. J. Physiol. Integr. Comp. Physiol. 1986, 250, R245–R249. [Google Scholar] [CrossRef]
- Guyard, B.; Fricker, J.; Brigant, L.; Betoulle, D.; Apfelbaum, M. Effects of ovarian steroids on energy balance in rats fed a highly palatable diet. Metabolism 1991, 40, 529–533. [Google Scholar] [CrossRef]
- Laudenslager, M.L.; Wilkinson, C.W.; Carlisle, H.J.; Hammel, H.T. Energy balance in ovariectomized rats with and without estrogen replacement. Am. J. Physiol. Integr. Comp. Physiol. 1980, 238, R400–R405. [Google Scholar] [CrossRef]
- Quarta, C.; Mazza, R.; Pasquali, R.; Pagotto, U. Role of sex hormones in modulation of brown adipose tissue activity. J. Mol. Endocrinol. 2012, 49, R1–R7. [Google Scholar] [CrossRef]
- Monjo, M.; Rodríguez, A.M.; Palou, A.; Roca, P. Direct Effects of Testosterone, 17β-Estradiol, and Progesterone on Adrenergic Regulation in Cultured Brown Adipocytes: Potential Mechanism for Gender-Dependent Thermogenesis. Endocrinology 2003, 144, 4923–4930. [Google Scholar] [CrossRef]
- Rodríguez-Cuenca, S.; Monjo, M.; Gianotti, M.; Proenza, A.M.; Roca, P. Expression of mitochondrial biogenesis-signaling factors in brown adipocytes is influenced specifically by 17β-estradiol, testosterone, and progesterone. Am. J. Physiol. Metab. 2007, 292, E340–E346. [Google Scholar] [CrossRef]
- Zhang, W.; Schmull, S.; Du, M.; Liu, J.; Lu, Z.; Zhu, H.; Xue, S.; Lian, F. Estrogen Receptor α and β in Mouse: Adipose-Derived Stem Cell Proliferation, Migration, and Brown Adipogenesis In Vitro. Cell. Physiol. Biochem. 2016, 38, 2285–2299. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Yoshida, T.; Wakabayashi, Y.; Nishioka, H.; Kondo, M. Reduced Brown Adipose Tissue Thermogenesis of Obese Rats After Ovariectomy. Endocrinol. Jpn. 1988, 35, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.B.; Bruun, J.; Kristensena, K.; Richelsen, B. Regulation of UCP1, UCP2, and UCP3 mRNA Expression in Brown Adipose Tissue, White Adipose Tissue, and Skeletal Muscle in Rats by Estrogen. Biochem. Biophys. Res. Commun. 2001, 288, 191–197. [Google Scholar] [CrossRef] [PubMed]
- de Morentin, P.B.M.; González-García, I.; Martins, L.; Lage, R.; Fernández-Mallo, D.; Martínez-Sánchez, N.; Ruíz-Pino, F.; Liu, J.; Morgan, D.A.; Pinilla, L.; et al. Estradiol Regulates Brown Adipose Tissue Thermogenesis via Hypothalamic AMPK. Cell Metab. 2014, 20, 41–53. [Google Scholar] [CrossRef]
- Kim, N.R.; David, K.; Corbeels, K.; Khalil, R.; Antonio, L.; Schollaert, D.; Deboel, L.; Ohlsson, C.; Gustafsson, J.; Vangoitsenhoven, R.; et al. Testosterone Reduces Body Fat in Male Mice by Stimulation of Physical Activity Via Extrahypothalamic ERα Signaling. Endocrinology 2021, 162, bqab045. [Google Scholar] [CrossRef] [PubMed]
- Birzniece, V.; Schooling, C.M.; Himmelstein, D.U.; Woolhandler, S.; Schellhammer, P.F.; Finkelstein, J.S.; Yu, E.W.; Burnett-Bowie, S.-A.M.; Malnick, S.; Somin, M.; et al. Gonadal Steroids and Body Composition, Strength, and Sexual Function in Men. N. Engl. J. Med. 2013, 369, 2455–2457. [Google Scholar] [CrossRef]
- Dieudonné, M.N.; Pecquery, R.; Boumediene, A.; Leneveu, M.C.; Giudicelli, Y. Androgen receptors in human preadipocytes and adipocytes: Regional specificities and regulation by sex steroids. Am. J. Physiol. Physiol. 1998, 274, C1645–C1652. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F. Developmental androgenization programs metabolic dysfunction in adult mice. Adipocyte 2014, 3, 151–154. [Google Scholar] [CrossRef]
- Alexanderson, C.; Eriksson, E.; Stener-Victorin, E.; Lystig, T.; Gabrielsson, B.; Lönn, M.; Holmäng, A. Postnatal Testosterone Exposure Results in Insulin Resistance, Enlarged Mesenteric Adipocytes, and an Atherogenic Lipid Profile in Adult Female Rats: Comparisons with Estradiol and Dihydrotestosterone. Endocrinology 2007, 148, 5369–5376. [Google Scholar] [CrossRef]
- Barnes, R.B.; Rosenfield, R.L.; Ehrmann, D.A.; Cara, J.F.; Cuttler, L.; Levitsky, L.L.; Rosenthal, I.M. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: Evidence for perinatal masculinization of neuroendocrine function in women. J. Clin. Endocrinol. Metab. 1994, 79, 1328–1333. [Google Scholar] [CrossRef]
- Eisner, J.R.; Dumesic, D.A.; Kemnitz, J.W.; Colman, R.J.; Abbott, D.H. Increased Adiposity in Female Rhesus Monkeys Exposed to Androgen Excess During Early Gestation. Obes. Res. 2003, 11, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Niklasson, M.; Eriksson, E.; Björntorp, P.; Holmäng, A. Imprinting of female offspring with testosterone results in insulin resistance and changes in body fat distribution at adult age in rats. J. Clin. Investig. 1998, 101, 74–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nohara, K.; Waraich, R.S.; Liu, S.; Ferron, M.; Waget, A.; Meyers, M.S.; Karsenty, G.; Burcelin, R.; Mauvais-Jarvis, F. Developmental androgen excess programs sympathetic tone and adipose tissue dysfunction and predisposes to a cardiometabolic syndrome in female mice. Am. J. Physiol. Metab. 2013, 304, E1321–E1330. [Google Scholar] [CrossRef] [PubMed]
- Gentry, R.T.; Wade, G.N. Androgenic control of food intake and body weight in male rats. J. Comp. Physiol. Psychol. 1976, 90, 18–25. [Google Scholar] [CrossRef]
- Blouin, K.; Boivin, A.; Tchernof, A. Androgens and body fat distribution. J. Steroid Biochem. Mol. Biol. 2007, 108, 272–280. [Google Scholar] [CrossRef]
- Tsai, E.C.; Matsumoto, A.M.; Fujimoto, W.Y.; Boyko, E.J. Association of Bioavailable, Free, and Total Testosterone with Insulin Resistance. Diabetes Care 2004, 27, 861–868. [Google Scholar] [CrossRef]
- Woodhouse, L.J.; Gupta, N.; Bhasin, M.; Singh, A.B.; Ross, R.; Phillips, J.; Bhasin, S. Dose-Dependent Effects of Testosterone on Regional Adipose Tissue Distribution in Healthy Young Men. J. Clin. Endocrinol. Metab. 2004, 89, 718–726. [Google Scholar] [CrossRef]
- Xu, X.; Pergola, G.; Björntorp, P. Testosterone increases lipolysis and the number of β-adrenoceptors in male rat adipocytes. Endocrinology 1991, 128, 379–382. [Google Scholar] [CrossRef]
- Dicker, A.; Rydén, M.; Näslund, E.; Muehlen, I.E.; Wirén, M.; Lafontan, M.; Arner, P. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia 2004, 47, 420–428. [Google Scholar] [CrossRef]
- Handelsman, D.J.; Hirschberg, A.L.; Bermon, S. Circulating Testosterone as the Hormonal Basis of Sex Differences in Athletic Performance. Endocr. Rev. 2018, 39, 803–829. [Google Scholar] [CrossRef]
- Varlamov, O.; Chu, M.P.; McGee, W.K.; Cameron, J.L.; O’Rourke, R.; Meyer, K.A.; Bishop, C.; Stouffer, R.L.; Roberts, C. Ovarian Cycle-Specific Regulation of Adipose Tissue Lipid Storage by Testosterone in Female Nonhuman Primates. Endocrinology 2013, 154, 4126–4135. [Google Scholar] [CrossRef]
- Varlamov, O.; White, A.E.; Carroll, J.M.; Bethea, C.L.; Reddy, A.; Slayden, O.; O’Rourke, R.; Roberts, C. Androgen Effects on Adipose Tissue Architecture and Function in Nonhuman Primates. Endocrinology 2012, 153, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.M.; Monjo, M.; Roca, P.; Palou, A. Opposite actions of testosterone and progesterone on UCP1 mRNA expression in cultured brown adipocytes. Cell. Mol. Life Sci. 2002, 59, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, O.; Noda, T.; Morita, A.; Morita, M.; Ohtsuki, H.; Sugiyama, M.; Funaba, M. Castration induced browning in subcutaneous white adipose tissue in male mice. Biochem. Biophys. Res. Commun. 2016, 478, 1746–1750. [Google Scholar] [CrossRef]
- Gasparini, S.J.; Swarbrick, M.M.; Kim, S.; Thai, L.J.; Henneicke, H.; Cavanagh, L.L.; Tu, J.; Weber, M.-C.; Zhou, H.; Seibel, M.J. Androgens sensitise mice to glucocorticoid-induced insulin resistance and fat accumulation. Diabetologia 2019, 62, 1463–1477. [Google Scholar] [CrossRef]
- Fan, W.; Yanase, T.; Nomura, M.; Okabe, T.; Goto, K.; Sato, T.; Kawano, H.; Kato, S.; Nawata, H. Androgen Receptor Null Male Mice Develop Late-Onset Obesity Caused by Decreased Energy Expenditure and Lipolytic Activity but Show Normal Insulin Sensitivity with High Adiponectin Secretion. Diabetes 2005, 54, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Movérare-Skrtic, S.; Venken, K.; Andersson, N.; Lindberg, M.K.; Svensson, J.; Swanson, C.; Vanderschueren, D.; Oscarsson, J.; Gustafsson, J.Å.; Ohlsson, C. Dihydrotestosterone Treatment Results in Obesity and Altered Lipid Metabolism in Orchidectomized Mice. Obesity 2006, 14, 662–672. [Google Scholar] [CrossRef]
- Nohara, K.; Laque, A.; Allard, C.; Münzberg, H.; Mauvais-Jarvis, F. Central mechanisms of adiposity in adult female mice with androgen excess. Obesity 2014, 22, 1477–1484. [Google Scholar] [CrossRef]
- Spaanderman, D.C.E.; Nixon, M.; Buurstede, J.C.; Sips, H.C.; Schilperoort, M.; Kuipers, E.N.; Backer, E.A.; Kooijman, S.; Rensen, P.C.N.; Homer, N.Z.M.; et al. Androgens modulate glucocorticoid receptor activity in adipose tissue and liver. J. Endocrinol. 2018, 240, 52–63. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.; Álvarez-Blasco, F.; Botella-Carretero, J.I.; Luque-Ramírez, M. The striking similarities in the metabolic associations of female androgen excess and male androgen deficiency. Hum. Reprod. 2014, 29, 2083–2091. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Armario, A. The hypothalamic-pituitary-adrenal axis: What can it tell us about stressors? CNS Neurol. Disord. Drug Targets 2006, 5, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, S.C.; Menzaghi, F.; Pich, E.M.; Hauger, R.L.; Koob, G.F. Corticotropin-releasing factor in the paraventricular nucleus modulates feeding induced by neuropeptide Y. Brain Res. 1993, 611, 18–24. [Google Scholar] [CrossRef]
- Arase, K.; York, D.A.; Shimizu, H.; Shargill, N.; Bray, G.A. Effects of corticotropin-releasing factor on food intake and brown adipose tissue thermogenesis in rats. Am. J. Physiol. Metab. 1988, 255, E255–E259. [Google Scholar] [CrossRef]
- Egawa, M.; Yoshimatsu, H.; Bray, G. Effect of corticotropin releasing hormone and neuropeptide Y on electrophysiological activity of sympathetic nerves to interscapular brown adipose tissue. Neuroscience 1990, 34, 771–775. [Google Scholar] [CrossRef]
- Rothwell, N.J. Central effects of CRF on metabolism and energy balance. Neurosci. Biobehav. Rev. 1990, 14, 263–271. [Google Scholar] [CrossRef]
- Kvetnansky, R.; Sabban, E.L.; Palkovits, M. Catecholaminergic Systems in Stress: Structural and Molecular Genetic Approaches. Physiol. Rev. 2009, 89, 535–606. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- De Vriendt, T.; Moreno, L.; De Henauw, S. Chronic stress and obesity in adolescents: Scientific evidence and methodological issues for epidemiological research. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 511–519. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Santana, P.; Akana, S.F.; Hanson, E.S.; Strack, A.M.; Sebastian, R.J.; Dallman, M.F. Aldosterone and dexamethasone both stimulate energy acquisition whereas only the glucocorticoid alters energy storage. Endocrinology 1995, 136, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Dallman, M.F.; La Fleur, S.E.; Pecoraro, N.C.; Gomez, F.; Houshyar, H.; Akana, S.F. Minireview: Glucocorticoids—Food Intake, Abdominal Obesity, and Wealthy Nations in 2004. Endocrinology 2004, 145, 2633–2638. [Google Scholar] [CrossRef] [PubMed]
- Rabasa, C.; Dickson, S.L. Impact of stress on metabolism and energy balance. Curr. Opin. Behav. Sci. 2016, 9, 71–77. [Google Scholar] [CrossRef]
- Adam, T.C.; Epel, E.S. Stress, eating and the reward system. Physiol. Behav. 2007, 91, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Arima, H.; Watanabe, M.; Goto, M.; Banno, R.; Sato, I.; Ozaki, N.; Nagasaki, H.; Oiso, Y. Glucocorticoids Increase Neuropeptide Y and Agouti-Related Peptide Gene Expression via Adenosine Monophosphate-Activated Protein Kinase Signaling in the Arcuate Nucleus of Rats. Endocrinology 2008, 149, 4544–4553. [Google Scholar] [CrossRef][Green Version]
- Pecoraro, N.; Reyes, F.; Gomez, F.; Bhargava, A.; Dallman, M.F. Chronic Stress Promotes Palatable Feeding, which Reduces Signs of Stress: Feedforward and Feedback Effects of Chronic Stress. Endocrinology 2004, 145, 3754–3762. [Google Scholar] [CrossRef]
- Foster, M.T.; Warne, J.P.; Ginsberg, A.B.; Horneman, H.F.; Pecoraro, N.C.; Akana, S.F.; Dallman, M.F. Palatable Foods, Stress, and Energy Stores Sculpt Corticotropin-Releasing Factor, Adrenocorticotropin, and Corticosterone Concentrations after Restraint. Endocrinology 2008, 150, 2325–2333. [Google Scholar] [CrossRef]
- Nieuwenhuizen, A.G.; Rutters, F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol. Behav. 2008, 94, 169–177. [Google Scholar] [CrossRef]
- Zakrzewska, K.E.; Cusin, I.; Sainsbury, A.; Rohner-Jeanrenaud, F.; Jeanrenaud, B. Glucocorticoids as Counterregulatory Hormones of Leptin: Toward an Understanding of Leptin Resistance. Diabetes 1997, 46, 717–719. [Google Scholar] [CrossRef]
- Zakrzewska, K.E.; Cusin, I.; Stricker-Krongrad, A.; Boss, O.; Ricquier, D.; Jeanrenaud, B.; Rohner-Jeanrenaud, F. Induction of obesity and hyperleptinemia by central glucocorticoid infusion in the rat. Diabetes 1999, 48, 365–370. [Google Scholar] [CrossRef]
- Jéquier, E. Leptin Signaling, Adiposity, and Energy Balance. Ann. N. Y. Acad. Sci. 2006, 967, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Asensio, C.; Muzzin, P.; Rohner-Jeanrenaud, F. Role of glucocorticoids in the physiopathology of excessive fat deposition and insulin resistance. Int. J. Obes. 2004, 28, S45–S52. [Google Scholar] [CrossRef]
- Rebuffé-Scrive, M.; Brönnegård, M.; Nilsson, A.; Eldh, J.; Gustafsson, J.; Björntorp, P. Steroid Hormone Receptors in Human Adipose Tissues. J. Clin. Endocrinol. Metab. 1990, 71, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Mårin, P.; Darin, N.; Amemiya, T.; Andersson, B.; Jern, S.; Björntorp, P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism 1992, 41, 882–886. [Google Scholar] [CrossRef]
- Rosmond, R.; Dallman, M.F.; Björntorp, P. Stress-Related Cortisol Secretion in Men: Relationships with Abdominal Obesity and Endocrine, Metabolic and Hemodynamic Abnormalities. J. Clin. Endocrinol. Metab. 1998, 83, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; McEwen, B.; Seeman, T.; Matthews, K.; Castellazzo, G.; Brownell, K.D.; Bell, J.; Ickovics, J.R. Stress and Body Shape: Stress-Induced Cortisol Secretion Is Consistently Greater Among Women with Central Fat. Psychosom. Med. 2000, 62, 623–632. [Google Scholar] [CrossRef]
- Barry, D.; Pietrzak, R.H.; Petry, N.M. Gender Differences in Associations Between Body Mass Index and DSM-IV Mood and Anxiety Disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Ann. Epidemiol. 2008, 18, 458–466. [Google Scholar] [CrossRef]
- Spencer, S.J.; Tilbrook, A. Neonatal overfeeding alters adult anxiety and stress responsiveness. Psychoneuroendocrinology 2009, 34, 1133–1143. [Google Scholar] [CrossRef]
- Lundgren, M.; Burén, J.; Lindgren, P.; Myrnäs, T.; Ruge, T.; Eriksson, J.W. Sex- and Depot-specific Lipolysis Regulation in Human Adipocytes: Interplay between Adrenergic Stimulation and Glucocorticoids. Horm. Metab. Res. 2008, 40, 854–860. [Google Scholar] [CrossRef]
- Veilleux, A.; Rheéaume, C.; Daris, M.; Luu-The, V.; Tchernof, A. Omental Adipose Tissue Type 1 11β-Hydroxysteroid Dehydrogenase Oxoreductase Activity, Body Fat Distribution, and Metabolic Alterations in Women. J. Clin. Endocrinol. Metab. 2009, 94, 3550–3557. [Google Scholar] [CrossRef]
- Albiston, A.; Smith, R.; Krozowski, Z. Sex- and tissue- specific regulation of 11β-hydroxysteroid dehydrogenase mRNA. Mol. Cell. Endocrinol. 1995, 109, 183–188. [Google Scholar] [CrossRef]
- Weaver, J.U.; Taylor, N.F.; Monson, J.P.; Wood, P.J.; Kelly, W.F. Sexual dimorphism in 11 β hydroxysteroid dehydrogenase activity and its relation to fat distribution and insulin sensitivity; a study in hypopituitary subjects. Clin. Endocrinol. 1998, 49, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Toogood, A.; Taylor, N.F.; Shalet, S.M.; Monson, J.P. Sexual dimorphism of cortisol metabolism is maintained in elderly subjects and is not oestrogen dependent. Clin. Endocrinol. 2000, 52, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Adler, E.S.; Hollis, J.H.; Clarke, I.J.; Grattan, D.R.; Oldfield, B.J. Neurochemical Characterization and Sexual Dimorphism of Projections from the Brain to Abdominal and Subcutaneous White Adipose Tissue in the Rat. J. Neurosci. 2012, 32, 15913–15921. [Google Scholar] [CrossRef]
- Ramis, J.M.; Salinas, R.; García-Sanz, J.M.; Moreiro, J.; Proenza, A.M.; Lladó, I. Depot- and Gender-related Differences in the Lipolytic Pathway of Adipose Tissue from Severely Obese Patients. Cell. Physiol. Biochem. 2006, 17, 173–180. [Google Scholar] [CrossRef]
- Arvaniti, K.; Ricquier, D.; Champigny, O.; Richard, D. Leptin and Corticosterone Have Opposite Effects on Food Intake and the Expression of UCP1 mRNA in Brown Adipose Tissue oflepob/lepobMice. Endocrinology 1998, 139, 4000–4003. [Google Scholar] [CrossRef][Green Version]
- Kaikaew, K.; Steenbergen, J.; Van Dijk, T.H.; Grefhorst, A.; Visser, J.A. Sex Difference in Corticosterone-Induced Insulin Resistance in Mice. Endocrinology 2019, 160, 2367–2387. [Google Scholar] [CrossRef]
- Beukel, J.C.V.D.; Boon, M.R.; Steenbergen, J.; Rensen, P.C.N.; Meijer, O.C.; Themmen, A.P.N.; Grefhorst, A. Cold Exposure Partially Corrects Disturbances in Lipid Metabolism in a Male Mouse Model of Glucocorticoid Excess. Endocrinology 2015, 156, 4115–4128. [Google Scholar] [CrossRef]
- Beukel, J.C.; Grefhorst, A.; Quarta, C.; Steenbergen, J.; Mastroberardino, P.G.; Lombès, M.; Delhanty, P.J.; Mazza, R.; Pagotto, U.; Lely, A.J.; et al. Direct activating effects of adrenocorticotropic hormone (ACTH) on brown adipose tissue are attenuated by corticosterone. FASEB J. 2014, 28, 4857–4867. [Google Scholar] [CrossRef]
- Mousovich-Neto, F.; Matos, M.S.; Costa, A.C.R.; de Melo Reis, R.A.; Atella, G.C.; Miranda-Alves, L.; Carvalho, D.P.; Ketzer, L.A.; Correa da Costa, V.M.J.E.P. Brown adipose tissue remodelling induced by corticosterone in male Wistar rats. Exp. Physiol. 2019, 104, 514–528. [Google Scholar] [CrossRef]
- Chapman, K.; Holmes, M.; Seckl, J. 11β-Hydroxysteroid Dehydrogenases: Intracellular Gate-Keepers of Tissue Glucocorticoid Action. Physiol. Rev. 2013, 93, 1139–1206. [Google Scholar] [CrossRef]
- Doig, C.; Fletcher, R.S.; Morgan, S.A.; McCabe, E.L.; Larner, D.P.; Tomlinson, J.; Stewart, P.M.; Philp, A.; Lavery, G.G. 11β-HSD1 Modulates the Set Point of Brown Adipose Tissue Response to Glucocorticoids in Male Mice. Endocrinology 2017, 158, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- Kroon, J.; Koorneef, L.; Heuvel, J.K.V.D.; Verzijl, C.R.C.; van de Velde, N.M.; Mol, I.M.; Sips, H.C.M.; Hunt, H.; Rensen, P.C.N.; Meijer, O. Selective Glucocorticoid Receptor Antagonist CORT125281 Activates Brown Adipose Tissue and Alters Lipid Distribution in Male Mice. Endocrinology 2017, 159, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Moriscot, A.; Rabelo, R.; Bianco, A.C. Corticosterone inhibits uncoupling protein gene expression in brown adipose tissue. Am. J. Physiol. Metab. 1993, 265, E81–E87. [Google Scholar] [CrossRef] [PubMed]
- Koorneef, L.L.; Kroon, J.; Viho, E.M.G.; Wahl, L.F.; Heckmans, K.M.L.; Dorst, M.M.A.R.V.; Hoekstra, M.; Houtman, R.; Hunt, H.; Meijer, O.C. The selective glucocorticoid receptor antagonist CORT125281 has tissue-specific activity. J. Endocrinol. 2020, 246, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Glantschnig, C.; Mattijssen, F.; Vogl, E.S.; Khan, A.A.; Garcia, M.R.; Fischer, K.; Müller, T.; Uhlenhaut, H.; Nawroth, P.; Scheideler, M.; et al. The glucocorticoid receptor in brown adipocytes is dispensable for control of energy homeostasis. EMBO Rep. 2019, 20, e48552. [Google Scholar] [CrossRef]
- Hartman, M.L. Physiological regulators of growth hormone secretion. In Growth Hormone in Adults; Cambridge University Press: Cambridge, UK, 2000; pp. 3–53. [Google Scholar]
- Giustina, A.; Veldhuis, J.D. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr. Rev. 1998, 19, 717–797. [Google Scholar] [CrossRef]
- Ballesteros, M.; Leung, K.-C.; Ross, R.J.M.; Iismaa, T.P.; Ho, K.K.Y. Distribution and Abundance of Messenger Ribonucleic Acid for Growth Hormone Receptor Isoforms in Human Tissues. J. Clin. Endocrinol. Metab. 2000, 85, 2865–2871. [Google Scholar] [CrossRef]
- Jørgensen, J.O.; Møller, L.; Krag, M.; Billestrup, N.; Christiansen, J.S. Effects of Growth Hormone on Glucose and Fat Metabolism in Human Subjects. Endocrinol. Metab. Clin. N. Am. 2007, 36, 75–87. [Google Scholar] [CrossRef]
- Nam, S.Y.; Lobie, P.E. The mechanism of effect of growth hormone on preadipocyte and adipocyte function. Obes. Rev. 2000, 1, 73–86. [Google Scholar] [CrossRef]
- Flint, D.J.; Binart, N.; Kopchick, J.; Kelly, P. Effects of growth hormone and prolactin on adipose tissue development and function. Pituitary 2003, 6, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Wabitsch, M.; Braun, S.; Hauner, H.; Heinze, E.; Ilondo, M.M.; Shymko, R.; De Meyts, P.; Teller, W.M. Mitogenic and Antiadipogenic Properties of Human Growth Hormone in Differentiating Human Adipocyte Precursor Cells in Primary Culture. Pediatr. Res. 1996, 40, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Flint, D.J.; Binart, N.; Boumard, S.; Kopchick, J.J.; Kelly, P. Developmental aspects of adipose tissue in GH receptor and prolactin receptor gene disrupted mice: Site-specific effects upon proliferation, differentiation and hormone sensitivity. J. Endocrinol. 2006, 191, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Iranmanesh, A.; Lizarralde, G.; Veldhuis, J.D. Age and Relative Adiposity Are Specific Negative Determinants of the Frequency and Amplitude of Growth Hormone (GH) Secretory Bursts and the Half-Life of Endogenous GH in Healthy Men. J. Clin. Endocrinol. Metab. 1991, 73, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Erman, A.; Veilleux, A.; Tchernof, A.; Goodyer, C.G. Human growth hormone receptor (GHR) expression in obesity: I. GHR mRNA expression in omental and subcutaneous adipose tissues of obese women. Int. J. Obes. 2011, 35, 1511–1519. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Iranmanesh, A.; Ho, K.K.Y.; Waters, M.J.; Johnson, M.L.; Lizarralde, G. Dual Defects in Pulsatile Growth Hormone Secretion and Clearance Subserve the Hyposomatotropism of Obesity in Man. J. Clin. Endocrinol. Metab. 1991, 72, 51–59. [Google Scholar] [CrossRef]
- Williams, T.; Berelowitz, M.; Joffe, S.N.; Thorner, M.O.; Rivier, J.; Vale, W.; Frohman, L.A. Impaired Growth Hormone Responses to Growth Hormone–Releasing Factor in Obesity. N. Engl. J. Med. 1984, 311, 1403–1407. [Google Scholar] [CrossRef]
- Vahl, N.; Jorgensen, J.O.; Skjaerbaek, C.; Veldhuis, J.D.; Orskov, H.; Christiansen, J.S. Abdominal adiposity rather than age and sex predicts mass and regularity of GH secretion in healthy adults. Am. J. Physiol. Content 1997, 272, E1108–E1116. [Google Scholar] [CrossRef]
- Meinhardt, U.J.; Ho, K.K.Y. Modulation of growth hormone action by sex steroids. Clin. Endocrinol. 2006, 65, 413–422. [Google Scholar] [CrossRef]
- Ho, K.Y.; Evans, W.S.; Blizzard, R.M.; Veldhuis, J.D.; Merriam, G.R.; Samojlik, E.; Furlanetto, R.; Rogol, A.D.; Kaiser, D.L.; Thorner, M.O. Effects of Sex and Age on the 24-Hour Profile of Growth Hormone Secretion in Man: Importance of Endogenous Estradiol Concentrations. J. Clin. Endocrinol. Metab. 1987, 64, 51–58. [Google Scholar] [CrossRef]
- Johansson, A.G. Gender difference in growth hormone response in adults. J. Endocrinol. Investig. 1999, 22, 58–60. [Google Scholar]
- Clasey, J.L.; Weltman, A.; Patrie, J.; Weltman, J.Y.; Pezzoli, S.; Bouchard, C.; Thorner, M.O.; Hartman, M.L. Abdominal Visceral Fat and Fasting Insulin Are Important Predictors of 24-Hour GH Release Independent of Age, Gender, and Other Physiological Factors. J. Clin. Endocrinol. Metab. 2001, 86, 3845–3852. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.H.; Hvidberg, A.; Hilsted, J.; Juul, A.; Main, K.M.; Gotfredsen, A.; Skakkebaek, N.E.; Skakkebae, N.E. Massive weight loss restores 24-hour growth hormone release profiles and serum insulin-like growth factor-I levels in obese subjects. J. Clin. Endocrinol. Metab. 1995, 80, 1407–1415. [Google Scholar] [CrossRef]
- Imaki, T.; Shibasaki, T.; Shizume, K.; Masuda, A.; Hotta, M.; Kiyosawa, Y.; Jibiki, K.; Demura, H.; Tsushima, T.; Ling, N. The Effect of Free Fatty Acids on Growth Hormone(GH)-Releasing Hormone-Mediated GH Secretion in Man. J. Clin. Endocrinol. Metab. 1985, 60, 290–293. [Google Scholar] [CrossRef]
- Yamashita, S.; Melmed, S. Effects of Insulin on Rat Anterior Pituitary Cells: Inhibition of Growth Hormone Secretion and mRNA Levels. Diabetes 1986, 35, 440–447. [Google Scholar] [CrossRef]
- Hartman, M.L.; Clayton, P.E.; Johnson, M.L.; Celniker, A.; Perlman, A.J.; Alberti, K.G.; Thorner, M.O. A low dose euglycemic infusion of recombinant human insulin-like growth factor I rapidly suppresses fasting-enhanced pulsatile growth hormone secretion in humans. J. Clin. Investig. 1993, 91, 2453–2462. [Google Scholar] [CrossRef]
- Vahl, N.; Jørgensen, J.O.; Jurik, A.G.; Christiansen, J.S. Abdominal adiposity and physical fitness are major determinants of the age associated decline in stimulated GH secretion in healthy adults. J. Clin. Endocrinol. Metab. 1996, 81, 2209–2215. [Google Scholar] [CrossRef]
- Weltman, A.; Weltman, J.Y.; Hartman, M.L.; Abbott, R.D.; Rogol, A.D.; Evans, W.S.; Veldhuis, J.D. Relationship between age, percentage body fat, fitness, and 24-hour growth hormone release in healthy young adults: Effects of gender. J. Clin. Endocrinol. Metab. 1994, 78, 543–548. [Google Scholar] [CrossRef]
- Juricek, L.; Coumoul, X. The Aryl Hydrocarbon Receptor and the Nervous System. Int. J. Mol. Sci. 2018, 19, 2504. [Google Scholar] [CrossRef]
- Jain, S.; Maltepe, E.; Lu, M.M.; Simon, C.; Bradfield, C.A. Expression of ARNT, ARNT2, HIF1α, HIF2α and Ah receptor mRNAs in the developing mouse. Mech. Dev. 1998, 73, 117–123. [Google Scholar] [CrossRef]
- Kimura, E.; Tohyama, C. Embryonic and Postnatal Expression of Aryl Hydrocarbon Receptor mRNA in Mouse Brain. Front. Neuroanat. 2017, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Nebert, D.W. Drug-metabolizing enzymes in ligand-modulated transcription. Biochem. Pharmacol. 1994, 47, 25–37. [Google Scholar] [CrossRef]
- Hankinson, O. The Aryl Hydrocarbon Receptor Complex. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 307–340. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, A. Oestrogen-receptors (ER) are likely to be promiscuous: Wider role for oestrogens and mimics. Med. Hypotheses 2005, 65, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Janošek, J.; Hilscherová, K.; Bláha, L.; Holoubek, I. Environmental xenobiotics and nuclear receptors—Interactions, effects and in vitro assessment. Toxicol. Vitr. 2006, 20, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Hombach-Klonisch, S.; Pocar, P.; Kietz, S.; Klonisch, T. Molecular Actions of Polyhalogenated Arylhydrocarbons (PAHs) in Female Reproduction. Curr. Med. Chem. 2005, 12, 599–616. [Google Scholar] [CrossRef]
- Beischlag, T.V.; Perdew, G.H. ERα-AHR-ARNT Protein-Protein Interactions Mediate Estradiol-dependent Transrepression of Dioxin-inducible Gene Transcription. J. Biol. Chem. 2005, 280, 21607–21611. [Google Scholar] [CrossRef]
- Wormke, M.; Stoner, M.; Saville, B.; Walker, K.; Abdelrahim, M.; Burghardt, R.; Safe, S. The Aryl Hydrocarbon Receptor Mediates Degradation of Estrogen Receptor α through Activation of Proteasomes. Mol. Cell. Biol. 2003, 23, 1843–1855. [Google Scholar] [CrossRef]
- Jacobs, M.; Dickins, M.; Lewis, D. Homology modelling of the nuclear receptors: Human oestrogen receptorβ (hERβ), the human pregnane-X-receptor (PXR), the Ah receptor (AhR) and the constitutive androstane receptor (CAR) ligand binding domains from the human oestrogen receptor α (hERα) crystal structure, and the human peroxisome proliferator activated receptor α (PPARα) ligand binding domain from the human PPARγ crystal structure. J. Steroid Biochem. Mol. Biol. 2003, 84, 117–132. [Google Scholar] [CrossRef]
- Jana, N.; Sarkar, S.; Ishizuka, M.; Yonemoto, J.; Tohyama, C.; Sone, H. Cross-Talk between 2,3,7,8-Tetrachlorodibenzo-p-dioxin and Testosterone Signal Transduction Pathways in LNCaP Prostate Cancer Cells. Biochem. Biophys. Res. Commun. 1999, 256, 462–468. [Google Scholar] [CrossRef]
- Morrow, D.; Qin, C.; Smith, R.; Safe, S. Aryl hydrocarbon receptor-mediated inhibition of LNCaP prostate cancer cell growth and hormone-induced transactivation. J. Steroid Biochem. Mol. Biol. 2004, 88, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; McDougal, A. Mechanism of action and development of selective aryl hydrocarbon receptor modulators for treatment of hormone-dependent cancers (Review). Int. J. Oncol. 2002, 20, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M.; Kaur, K.; Swanson, H. The Aryl Hydrocarbon Receptor Interacts with Estrogen Receptor Alpha and Orphan Receptors COUP-TFI and ERRα. Arch. Biochem. Biophys. 2000, 373, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Abbott, B. Review of the interaction between TCDD and glucocorticoids in embryonic palate. Toxicology 1995, 105, 365–373. [Google Scholar] [CrossRef]
- Yamada-Okabe, T.; Aono, T.; Sakai, H.; Kashima, Y.; Yamada-Okabe, H. 2,3,7,8-tetrachlorodibenzo-p-dioxin augments the modulation of gene expression mediated by the thyroid hormone receptor. Toxicol. Appl. Pharmacol. 2004, 194, 201–210. [Google Scholar] [CrossRef]
- Matthews, J.; Ahmed, S. AHR-and ER-mediated toxicology and chemoprevention. In Advances in Molecular Toxicology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 7, pp. 1–38. [Google Scholar]
- Gargano, J.-R.M.A.M.; Tichomirowa, M.D.; D’Innocenzo, D.A.V.J.; Esposito, E.B. Expression of aryl hydrocarbon receptor (AHR) and AHR-interacting protein in pituitary adenomas: Pathological and clinical implications. Endocr.-Relat. Cancer 2009, 16, 1029–1043. [Google Scholar]
- Jaffrain-Rea, M.-L.; Rotondi, S.; Turchi, A.; Occhi, G.; Barlier, A.; Peverelli, E.; Rostomyan, L.; Defilles, C.; Angelini, M.; Oliva, M.-A.; et al. Somatostatin analogues increase AIP expression in somatotropinomas, irrespective of Gsp mutations. Endocr.-Relat. Cancer 2013, 20, 753–766. [Google Scholar] [CrossRef]
- Cao, J.; Patisaul, H.B.; Petersen, S.L.J.M. Aryl hydrocarbon receptor activation in lactotropes and gonadotropes interferes with estradiol-dependent and-independent preprolactin, glycoprotein alpha and luteinizing hormone beta gene expression. Mol. Cell. Endocrinol. 2011, 333, 151–159. [Google Scholar] [CrossRef]
- Huang, P.; Ceccatelli, S.; Håkansson, H.; Grandison, L.; Rannug, A. Constitutive and TCDD-Induced Expression of Ah Receptor-Responsive Genes in the Pituitary. NeuroToxicology 2002, 23, 783–793. [Google Scholar] [CrossRef]
- Savage, J.J.; Yaden, B.C.; Kiratipranon, P.; Rhodes, S.J. Transcriptional control during mammalian anterior pituitary development. Gene 2003, 319, 1–19. [Google Scholar] [CrossRef]
- Lipkin, S.M.; Näär, A.M.; Kalla, K.A.; Sack, R.A.; Rosenfeld, M.G. Identification of a novel zinc finger protein binding a conserved element critical for Pit-1-dependent growth hormone gene expression. Genes Dev. 1993, 7, 1674–1687. [Google Scholar] [CrossRef] [PubMed]
- Aluru, N.; Vijayan, M.M. Brain transcriptomics in response to β-naphthoflavone treatment in rainbow trout: The role of aryl hydrocarbon receptor signaling. Aquat. Toxicol. 2008, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ojo, E.S.; Tischkau, S.A. The Role of AhR in the Hallmarks of Brain Aging: Friend and Foe. Cells 2021, 10, 2729. [Google Scholar] [CrossRef]
- Takeda, T.; Taura, J.; Hattori, Y.; Ishii, Y.; Yamada, H. Dioxin-induced retardation of development through a reduction in the expression of pituitary hormones and possible involvement of an aryl hydrocarbon receptor in this defect: A comparative study using two strains of mice with different sensitivities to dioxin. Toxicol. Appl. Pharmacol. 2014, 278, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.C. Effects of TCDD on thyroid hormone homeostasis in the rat. Drug Chem. Toxicol. 2000, 23, 259–277. [Google Scholar] [CrossRef]
- Bestervelt, L.L.; Cai, Y.; Piper, D.W.; Nolan, C.J.; Pitt, J.A.; Piper, W.N. TCDD alters pituitary-adrenal function I: Adrenal responsiveness to exogenous ACTH. Neurotoxicology Teratol. 1993, 15, 365–370. [Google Scholar] [CrossRef][Green Version]
- Bestervelt, L. In Vitro2,3,7,8-Tetrachlorodibenzo-p-dioxin Interference with the Anterior Pituitary Hormone Adrenocorticortropin. Toxicol. Sci. 1998, 44, 107–115. [Google Scholar] [CrossRef]
- Shearman, L.; Zylka, M.; Reppert, S.; Weaver, D. Expression of basic helix-loop-helix/pas genes in the mouse suprachiasmatic nucleus. Neuroscience 1999, 89, 387–397. [Google Scholar] [CrossRef]
- Petersen, S.L.; Curran, M.A.; Marconi, S.A.; Carpenter, C.D.; Lubbers, L.S.; McAbee, M.D. Distribution of mRNAs encoding the arylhydrocarbon receptor, arylhydrocarbon receptor nuclear translocator, and arylhydrocarbon receptor nuclear translocator-2 in the rat brain and brainstem. J. Comp. Neurol. 2000, 427, 428–439. [Google Scholar] [CrossRef]
- Hosoya, T.; Oda, Y.; Takahashi, S.; Morita, M.; Kawauchi, S.; Ema, M.; Yamamoto, M.; Fujii-Kuriyama, Y. Defective development of secretory neurones in the hypothalamus of Arnt2-knockout mice. Genes Cells 2001, 6, 361–374. [Google Scholar] [CrossRef]
- Denison, M.S.; Nagy, S.R. Activation of the Aryl Hydrocarbon Receptor by Structurally Diverse Exogenous and Endogenous Chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334. [Google Scholar] [CrossRef] [PubMed]
- Barroso, A.; Mahler, J.V.; Fonseca-Castro, P.H.; Quintana, F.J. The aryl hydrocarbon receptor and the gut–brain axis. Cell. Mol. Immunol. 2021, 18, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Safe, S. Polychlorinated Biphenyls (PCBs), Dibenzo-p-Dioxins (PCDDs), Dibenzofurans (PCDFs), and Related Compounds: Environmental and Mechanistic Considerations which Support the Development of Toxic Equivalency Factors (TEFs). Crit. Rev. Toxicol. 1990, 21, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Myre, M.; Imbeault, P. Persistent organic pollutants meet adipose tissue hypoxia: Does cross-talk contribute to inflammation during obesity? Obes. Rev. 2013, 15, 19–28. [Google Scholar] [CrossRef]
- Pravettoni, A.; Colciago, A.; Negri-Cesi, P.; Villa, S.; Celotti, F. Ontogenetic development, sexual differentiation, and effects of Aroclor 1254 exposure on expression of the arylhydrocarbon receptor and of the arylhydrocarbon receptor nuclear translocator in the rat hypothalamus. Reprod. Toxicol. 2005, 20, 521–530. [Google Scholar] [CrossRef]
- Bjerke, D. Reproductive Toxicity of 2,3,7,8-Tetrachlorodibenzo-p-dioxin in Male Rats: Different Effects of in Utero Versus Lactational Exposure. Toxicol. Appl. Pharmacol. 1994, 127, 241–249. [Google Scholar] [CrossRef]
- Muntean, N.; Jermini, M.; Small, I.; Falzon, D.; Fürst, P.; Migliorati, G.; Scortichini, G.; Forti, A.F.; Anklam, E.; Von Holst, C.; et al. Assessment of dietary exposure to some persistent organic pollutants in the Republic of Karakalpakstan of Uzbekistan. Environ. Health Perspect. 2003, 111, 1306–1311. [Google Scholar] [CrossRef]
- Kreuzer, P.E.; Csanády, G.A.; Baur, C.; Kessler, W.; Päpke, O.; Greim, H.; Filser, J.G. 2,3,7,8-Tetrachlorodibenzo- p -dioxin (TCDD) and congeners in infants. A toxicokinetic model of human lifetime body burden by TCDD with special emphasis on its uptake by nutrition. Arch. Toxicol. 1997, 71, 383–400. [Google Scholar] [CrossRef]
- Lai, K.; Li, W.; Xu, Y.; Wong, M.; Wong, C.K. Dioxin-like components in human breast milk collected from Hong Kong and Guangzhou. Environ. Res. 2004, 96, 88–94. [Google Scholar] [CrossRef]
- Patandin, S.; Dagnelie, P.C.; Mulder, P.G.; De Coul, E.O.; Van Der Veen, J.E.; Weisglas-Kuperus, N.; Sauer, P.J. Dietary exposure to polychlorinated biphenyls and dioxins from infancy until adulthood: A comparison between breast-feeding, toddler, and long-term exposure. Environ. Health Perspect. 1999, 107, 45–51. [Google Scholar] [CrossRef]
- Gray, L., Jr.; Ostby, J.; Wolf, C.; Miller, D.; Kelce, W.; Gordon, C.; Birnbaum, L.J.O.C. Functional developmental toxicity of low doses of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin and a dioxin-like PCB (169) in Long Evans rats and Syrian hamsters: Reproductive, behavioral and thermoregulatory alterations. Organohalogen Compd. 1995, 25, 33–38. [Google Scholar]
- Mably, T.A.; Moore, R.; Goy, R.W.; Peterson, R.E. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin: 2. Effects on sexual behavior and the regulation of luteinizing hormone secretion in adulthood. Toxicol. Appl. Pharmacol. 1992, 114, 108–117. [Google Scholar] [CrossRef]
- Bjerke, D.; Brown, T.; Maclusky, N.; Hochberg, R.; Peterson, R. Partial Demasculinization and Feminization of Sex Behavior in Male Rats by in Utero and Lactational Exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin Is Not Associated with Alterations in Estrogen Receptor Binding or Volumes of Sexually Differentiated Brain. Toxicol. Appl. Pharmacol. 1994, 127, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.L.; Krishnan, S.; Hudgens, E.D. The Aryl Hydrocarbon Receptor Pathway and Sexual Differentiation of Neuroendocrine Functions. Endocrinology 2006, 147, s33–s42. [Google Scholar] [CrossRef]
- Abbott, B.; Schmid, J.E.; Pitt, J.A.; Buckalew, A.R.; Wood, C.R.; Held, G.A.; Diliberto, J.J. Adverse Reproductive Outcomes in the Transgenic Ah Receptor-Deficient Mouse. Toxicol. Appl. Pharmacol. 1999, 155, 62–70. [Google Scholar] [CrossRef]
- Gray, L.; Ostby, J. In Utero 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) Alters Reproductive Morphology and Function in Female Rat Offspring. Toxicol. Appl. Pharmacol. 1995, 133, 285–294. [Google Scholar] [CrossRef]
- Gorski, R.; Gordon, J.; Shryne, J.; Southam, A. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978, 148, 333–346. [Google Scholar] [CrossRef]
- Simerly, R.; Swanson, L.; Gorski, R. The distribution of monoaminergic cells and fibers in a periventricular preoptic nucleus involved in the control of gonadotropin release: Immunohistochemical evidence for a dopaminergic sexual dimorphism. Brain Res. 1985, 330, 55–64. [Google Scholar] [CrossRef]
- Shughrue, P.J.; Lane, M.V.; Merchenthaler, I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J. Comp. Neurol. 1997, 388, 507–525. [Google Scholar] [CrossRef]
- Petersen, S.L.; Barraclough, C.A. Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain. Brain Res. 1989, 484, 279–289. [Google Scholar] [CrossRef]
- Gray, P.; Brooks, P.J. Effect of lesion location within the medial preoptic-anterior hypothalamic continuum on maternal and male sexual behaviors in female rats. Behav. Neurosci. 1984, 98, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, H. Regulation of Estrogen ReceptorαExpression in the Hypothalamus by Sex Steroids: Implication in the Regulation of Energy Homeostasis. Int. J. Endocrinol. 2015, 2015, 949085. [Google Scholar] [CrossRef] [PubMed]
- Fetissov, S.O.; Huang, P.; Zhang, Q.; Mimura, J.; Fujii-Kuriyama, Y.; Rannug, A.; Hökfelt, T.; Ceccatelli, S. Expression of hypothalamic neuropeptides after acute TCDD treatment and distribution of Ah receptor repressor. Regul. Pept. 2004, 119, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Poland, A.P.; Smith, D.; Metier, G.; Possick, P. A Health Survey of Workers in a 2,4-D and 2,4,5-T Plant. Arch. Environ. Health Int. J. 1971, 22, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Shirakawa, H.; Tomita, S.; Ohsaki, Y.; Haketa, K.; Tooi, O.; Santo, N.; Tohkin, M.; Furukawa, Y.; Gonzalez, F.J.; et al. Low-dose dioxins alter gene expression related to cholesterol biosynthesis, lipogenesis, and glucose metabolism through the aryl hydrocarbon receptor-mediated pathway in mouse liver. Toxicol. Appl. Pharmacol. 2008, 229, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Boverhof, D.R.; Burgoon, L.D.; Tashiro, C.; Sharratt, B.; Chittim, B.; Harkema, J.R.; Mendrick, D.L.; Zacharewski, T.R. Comparative Toxicogenomic Analysis of the Hepatotoxic Effects of TCDD in Sprague Dawley Rats and C57BL/6 Mice. Toxicol. Sci. 2006, 94, 398–416. [Google Scholar] [CrossRef]
- Fletcher, N.; Wahlström, D.; Lundberg, R.; Nilsson, C.B.; Nilsson, K.C.; Stockling, K.; Hellmold, H.; Håkansson, H. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters the mRNA expression of critical genes associated with cholesterol metabolism, bile acid biosynthesis, and bile transport in rat liver: A microarray study. Toxicol. Appl. Pharmacol. 2005, 207, 1–24. [Google Scholar] [CrossRef]
- Kurachiab, M.; Ichihashimotoa, S.; Obataa, A.; Nagaia, S.; Nagahataa, T.; Inaderac, H.; Soned, H.; Tohyamad, C.; Kanekob, S.; Ichikobayashib, K.; et al. Identification of 2,3,7,8-Tetrachlorodibenzo-p-dioxin-Responsive Genes in Mouse Liver by Serial Analysis of Gene Expression. Biochem. Biophys. Res. Commun. 2002, 292, 368–377. [Google Scholar] [CrossRef][Green Version]
- Lee, J.H.; Wada, T.; Febbraio, M.; He, J.; Matsubara, T.; Lee, M.J.; Gonzalez, F.J.; Xie, W. A Novel Role for the Dioxin Receptor in Fatty Acid Metabolism and Hepatic Steatosis. Gastroenterology 2010, 139, 653–663. [Google Scholar] [CrossRef]
- Wang, C.; Xu, C.-X.; Krager, S.L.; Bottum, K.M.; Liao, D.-F.; Tischkau, S.A. Aryl Hydrocarbon Receptor Deficiency Enhances Insulin Sensitivity and Reduces PPAR-α Pathway Activity in Mice. Environ. Health Perspect. 2011, 119, 1739–1744. [Google Scholar] [CrossRef]
- Kerley-Hamilton, J.S.; Trask, H.W.; Ridley, C.J.; DuFour, E.; Ringelberg, C.S.; Nurinova, N.; Wong, D.; Moodie, K.L.; Shipman, S.L.; Moore, J.H.; et al. Obesity Is Mediated by Differential Aryl Hydrocarbon Receptor Signaling in Mice Fed a Western Diet. Environ. Health Perspect. 2012, 120, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-X.; Wang, C.; Zhang, Z.-M.; Jaeger, C.D.; Krager, S.L.; Bottum, K.M.; Liu, J.; Liao, D.-F.; Tischkau, S.A. Aryl hydrocarbon receptor deficiency protects mice from diet-induced adiposity and metabolic disorders through increased energy expenditure. Int. J. Obes. 2015, 39, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An Interaction between Kynurenine and the Aryl Hydrocarbon Receptor Can Generate Regulatory T Cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef] [PubMed]
- Manzella, C.; Singhal, M.; Alrefai, W.A.; Saksena, S.; Dudeja, P.K.; Gill, R.K. Serotonin is an endogenous regulator of intestinal CYP1A1 via AhR. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Song, J.; Gao, J.; Cheng, J.; Xie, H.; Zhang, L.; Wang, Y.-H.; Gao, Z.; Wang, Y.; Wang, X.; et al. Adipocyte-derived kynurenine promotes obesity and insulin resistance by activating the AhR/STAT3/IL-6 signaling. Nat. Commun. 2022, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Selgas, R.; Romero, S.; Díez, J.J. mechanisms in endocrinology: Biological role, clinical significance, and therapeutic possibilities of the recently discovered metabolic hormone fibroblastic growth factor 21. Eur. J. Endocrinol. 2012, 167, 301–309. [Google Scholar] [CrossRef]
- Girer, N.G.; Tomlinson, C.R.; Elferink, C.J. The Aryl Hydrocarbon Receptor in Energy Balance: The Road from Dioxin-Induced Wasting Syndrome to Combating Obesity with Ahr Ligands. Int. J. Mol. Sci. 2020, 22, 49. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef]
- Hondares, E.; Rosell, M.; Gonzalez, F.J.; Giralt, M.; Iglesias, R.; Villarroya, F. Hepatic FGF21 Expression Is Induced at Birth via PPARα in Response to Milk Intake and Contributes to Thermogenic Activation of Neonatal Brown Fat. Cell Metab. 2010, 11, 206–212. [Google Scholar] [CrossRef]
- Adams, A.C.; Coskun, T.; Cheng, C.C.; O’farrell, L.S.; DuBois, S.L.; Kharitonenkov, A. Fibroblast growth factor 21 is not required for the antidiabetic actions of the thiazoladinediones. Mol. Metab. 2013, 2, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Emanuelli, B.; Vienberg, S.G.; Smyth, G.; Cheng, C.; Stanford, K.I.; Arumugam, M.; Michael, M.D.; Adams, A.C.; Kharitonenkov, A.; Kahn, C.R. Interplay between FGF21 and insulin action in the liver regulates metabolism. J. Clin. Investig. 2014, 124, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Ramos, D.; Mehta, R.; Aguilar-Salinas, C.A. Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Front. Physiol. 2019, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Hondares, E.; Iglesias, R.; Giralt, A.; Gonzalez, F.J.; Giralt, M.; Mampel, T.; Villarroya, F. Thermogenic Activation Induces FGF21 Expression and Release in Brown Adipose Tissue. J. Biol. Chem. 2011, 286, 12983–12990. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Dutchak, P.A.; Katafuchi, T.; Bookout, A.L.; Choi, J.H.; Yu, R.T.; Mangelsdorf, D.J.; Kliewer, S.A. Fibroblast Growth Factor-21 Regulates PPARγ Activity and the Antidiabetic Actions of Thiazolidinediones. Cell 2012, 148, 556–567. [Google Scholar] [CrossRef]
- Puigserver, P.; Picó, C.; Stock, M.; Palou, A. Effect of selective β-adrenoceptor stimulation on UCP synthesis in primary cultures of brown adipocytes. Mol. Cell. Endocrinol. 1996, 117, 7–16. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Almeda-Valdes, P.; Gómez-Pérez, F.J.; Meza-Arana, C.E.; Cruz-Bautista, I.; Arellano-Campos, O.; Navarrete-López, M.; Aguilar-Salinas, C.A. Daily physical activity, fasting glucose, uric acid, and body mass index are independent factors associated with serum fibroblast growth factor 21 levels. Eur. J. Endocrinol. 2010, 163, 469–477. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Almeda-Valdes, P.; Meza-Arana, C.E.; Brito-Córdova, G.; Gómez-Pérez, F.J.; Mehta, R.; Oseguera-Moguel, J.; Aguilar-Salinas, C.A. Exercise Increases Serum Fibroblast Growth Factor 21 (FGF21) Levels. PLoS ONE 2012, 7, e38022. [Google Scholar] [CrossRef]
- Chartoumpekis, D.V.; Habeos, I.G.; Ziros, P.G.; Psyrogiannis, A.I.; Kyriazopoulou, V.E.; Papavassiliou, A.G. Brown Adipose Tissue Responds to Cold and Adrenergic Stimulation by Induction of FGF21. Mol. Med. 2011, 17, 736–740. [Google Scholar] [CrossRef]
- Giralt, M.; Gavaldà-Navarro, A.; Villarroya, F. Fibroblast growth factor-21, energy balance and obesity. Mol. Cell. Endocrinol. 2015, 418, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Girer, N.; Murray, I.A.; Omiecinski, C.J.; Perdew, G.H. Hepatic Aryl Hydrocarbon Receptor Attenuates Fibroblast Growth Factor 21 Expression. J. Biol. Chem. 2016, 291, 15378–15387. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Vispute, S.G.; Liu, J.; Cheng, C.; Kharitonenkov, A.; Klaassen, C.D. Fibroblast growth factor (Fgf) 21 is a novel target gene of the aryl hydrocarbon receptor (AhR). Toxicol. Appl. Pharmacol. 2014, 278, 65–71. [Google Scholar] [CrossRef]
- Girer, N.G.; Carter, D.; Bhattarai, N.; Mustafa, M.; Denner, L.; Porter, C.; Elferink, C.J. Inducible Loss of the Aryl Hydrocarbon Receptor Activates Perigonadal White Fat Respiration and Brown Fat Thermogenesis via Fibroblast Growth Factor 21. Int. J. Mol. Sci. 2019, 20, 950. [Google Scholar] [CrossRef]
- Lu, P.; Yan, J.; Liu, K.; Garbacz, W.G.; Wang, P.; Xu, M.; Ma, X.; Xie, W. Activation of aryl hydrocarbon receptor dissociates fatty liver from insulin resistance by inducing fibroblast growth factor 21. Hepatology 2015, 61, 1908–1919. [Google Scholar] [CrossRef]
- Yang, X.; Schadt, E.E.; Wang, S.; Wang, H.; Arnold, A.P.; Ingram-Drake, L.; Drake, T.A.; Lusis, A.J. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006, 16, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Sunaga, H.; Miyata, K.; Shirasaki, H.; Uchiyama, Y.; Shimba, S. Aryl Hydrocarbon Receptor Plays Protective Roles against High Fat Diet (HFD)-induced Hepatic Steatosis and the Subsequent Lipotoxicity via Direct Transcriptional Regulation of Socs3 Gene Expression. J. Biol. Chem. 2016, 291, 7004–7016. [Google Scholar] [CrossRef]
- Baker, N.A.; Shoemaker, R.; English, V.; Larian, N.; Sunkara, M.; Morris, A.J.; Walker, M.; Yiannikouris, F.; Cassis, L.A. Effects of Adipocyte Aryl Hydrocarbon Receptor Deficiency on PCB-Induced Disruption of Glucose Homeostasis in Lean and Obese Mice. Environ. Health Perspect. 2015, 123, 944–950. [Google Scholar] [CrossRef]
- Vettor, R.; De Pergola, G.; Pagano, C.; Englaro, P.; Laudadio, E.; Giorgino, F.; Blum, W.F.; Giorgino, R.; Federspil, G. Gender differences in serum leptin in obese people: Relationships with testosterone, body fat distribution and insulin sensitivity. Eur. J. Clin. Investig. 1997, 27, 1016–1024. [Google Scholar] [CrossRef]
- Lutz, S.Z.; Wagner, R.; Fritsche, L.; Peter, A.; Rettig, I.; Willmann, C.; Fehlert, E.; Martus, P.; Todenhöfer, T.; Stefan, N.; et al. Sex-Specific Associations of Testosterone with Metabolic Traits. Front. Endocrinol. 2019, 10, 90. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, N.; Tischkau, S.A. Sexual Dimorphism in Adipose-Hypothalamic Crosstalk and the Contribution of Aryl Hydrocarbon Receptor to Regulate Energy Homeostasis. Int. J. Mol. Sci. 2022, 23, 7679. https://doi.org/10.3390/ijms23147679
Haque N, Tischkau SA. Sexual Dimorphism in Adipose-Hypothalamic Crosstalk and the Contribution of Aryl Hydrocarbon Receptor to Regulate Energy Homeostasis. International Journal of Molecular Sciences. 2022; 23(14):7679. https://doi.org/10.3390/ijms23147679
Chicago/Turabian StyleHaque, Nazmul, and Shelley A. Tischkau. 2022. "Sexual Dimorphism in Adipose-Hypothalamic Crosstalk and the Contribution of Aryl Hydrocarbon Receptor to Regulate Energy Homeostasis" International Journal of Molecular Sciences 23, no. 14: 7679. https://doi.org/10.3390/ijms23147679
APA StyleHaque, N., & Tischkau, S. A. (2022). Sexual Dimorphism in Adipose-Hypothalamic Crosstalk and the Contribution of Aryl Hydrocarbon Receptor to Regulate Energy Homeostasis. International Journal of Molecular Sciences, 23(14), 7679. https://doi.org/10.3390/ijms23147679





