Immunosuppression by Mycophenolate Mofetil Mitigates Intrarenal Angiotensinogen Augmentation in Angiotensin II-Dependent Hypertension
Abstract
:1. Introduction
2. Results
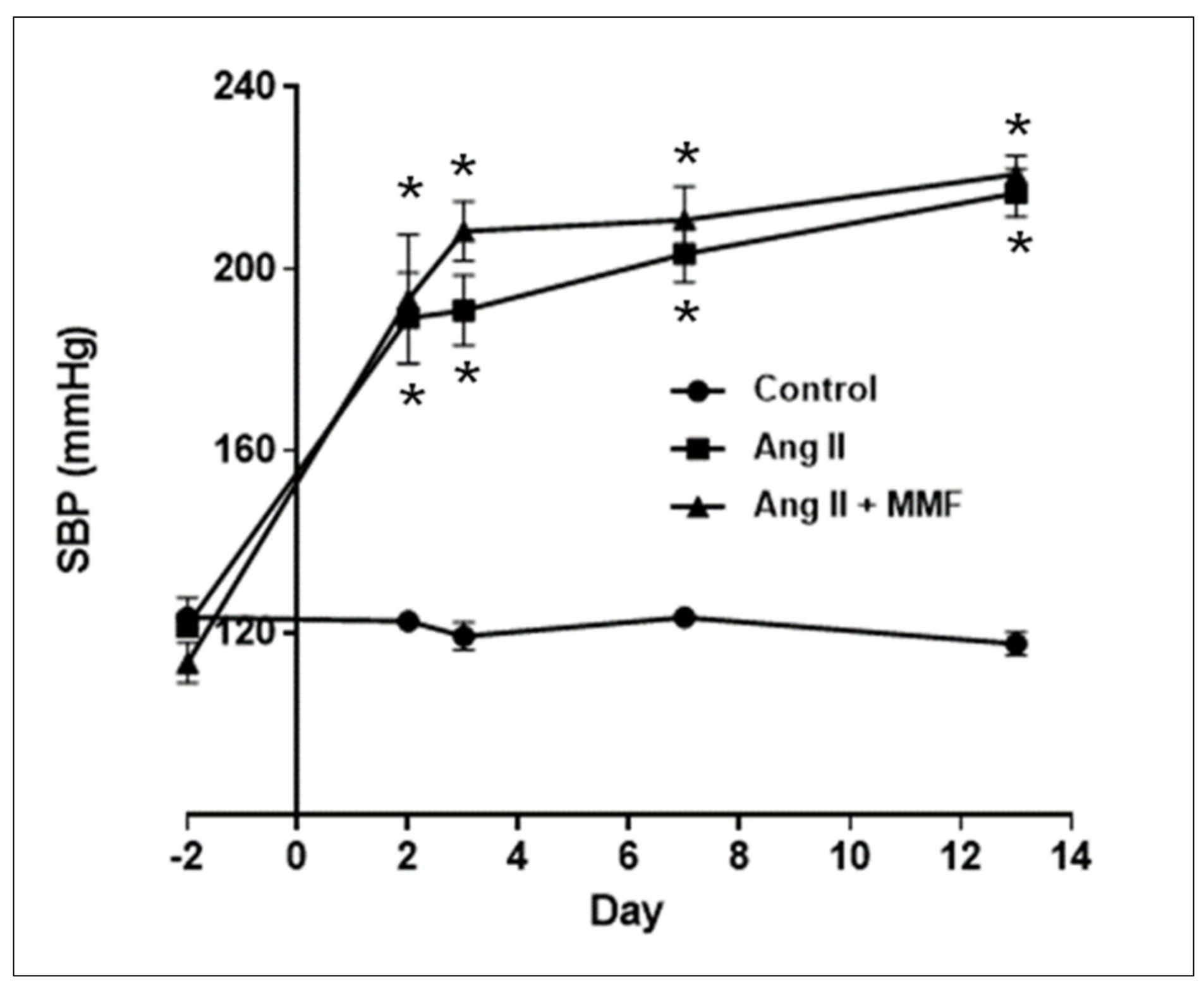
2.1. Effects of MMF on Blood Pressure
2.2. Effects of MMF on GFR and Mean Arterial Pressure (MAP)
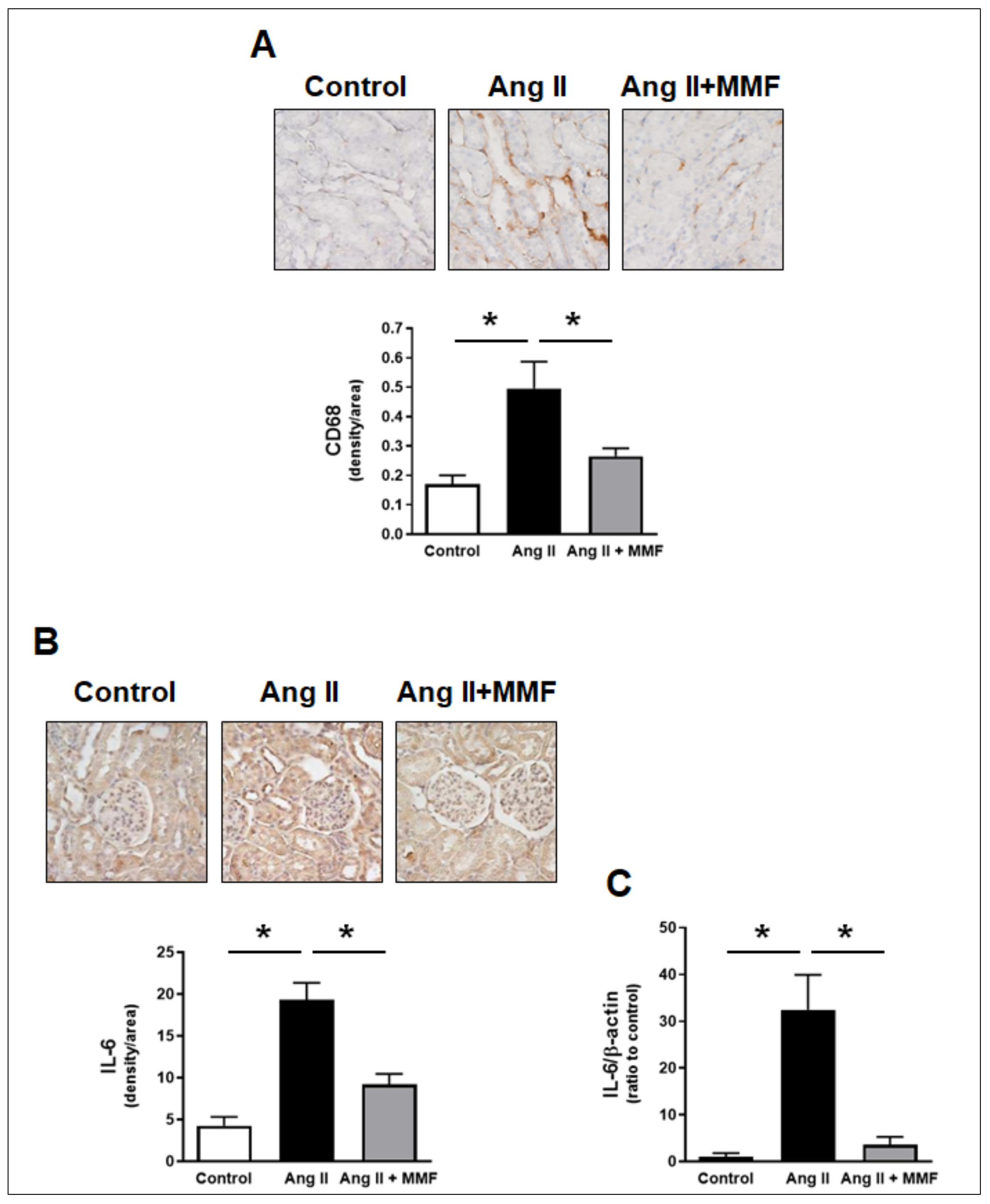
2.3. Effects of MMF on Intrarenal Immune Cells and IL-6 Levels
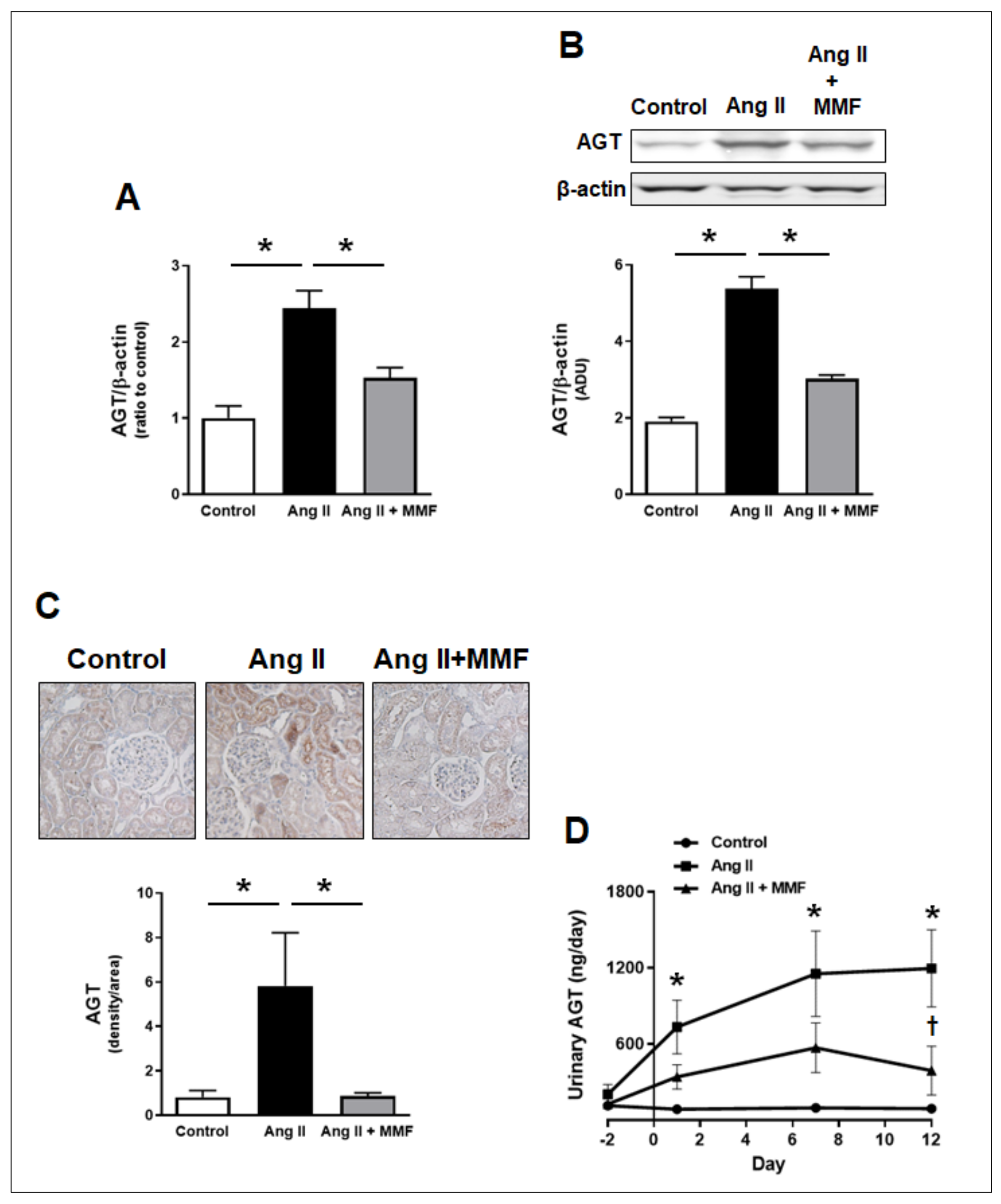
2.4. Effects of MMF on Intrarenal and Urinary AGT Levels
2.5. Effects of MMF on Hypertensive Kidney Injury
3. Discussion
4. Materials and Methods
4.1. Animals and Sample Collection
4.2. Induction of Hypertension
4.3. Blood Pressure Measurements
4.4. Urine Assays
4.5. Clearance Experiments
4.6. Real-Time RT-PCR
4.7. Droplet Digital PCR
4.8. Western Blot Analysis
4.9. Histological Analysis
4.10. Immunohistological Staining
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamasaki, N.; Kitaoka, H.; Matsumura, Y.; Furuno, T.; Nishinaga, M.; Doi, Y. Heart failure in the elderly. Intern. Med. 2003, 42, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Navar, L.G.; Prieto, M.C.; Satou, R.; Kobori, H. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr. Opin. Pharm. 2011, 11, 180–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobori, H.; Nangaku, M.; Navar, L.G.; Nishiyama, A. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev. 2007, 59, 251–287. [Google Scholar] [CrossRef] [PubMed]
- Kobori, H.; Harrison-Bernard, L.M.; Navar, L.G. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J. Am. Soc. Nephrol. 2001, 12, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Villalobos, R.A.; Seth, D.M.; Satou, R.; Horton, H.; Ohashi, N.; Miyata, K.; Katsurada, A.; Tran, D.V.; Kobori, H.; Navar, L.G. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am. J. Physiol. Renal Physiol. 2008, 295, F772–F779. [Google Scholar] [CrossRef] [Green Version]
- Kobori, H.; Ozawa, Y.; Satou, R.; Katsurada, A.; Miyata, K.; Ohashi, N.; Hase, N.; Suzaki, Y.; Sigmund, C.D.; Navar, L.G. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am. J. Physiol. Renal Physiol. 2007, 293, F938–F945. [Google Scholar] [CrossRef] [Green Version]
- Satou, R.; Gonzalez-Villalobos, R.A.; Miyata, K.; Ohashi, N.; Katsurada, A.; Navar, L.G.; Kobori, H. Costimulation with angiotensin II and interleukin 6 augments angiotensinogen expression in cultured human renal proximal tubular cells. Am. J. Physiol. Renal Physiol. 2008, 295, F283–F289. [Google Scholar] [CrossRef] [Green Version]
- Satou, R.; Gonzalez-Villalobos, R.A.; Miyata, K.; Ohashi, N.; Urushihara, M.; Acres, O.W.; Navar, L.G.; Kobori, H. IL-6 augments angiotensinogen in primary cultured renal proximal tubular cells. Mol. Cell Endocrinol. 2009, 311, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Harrison, D.G.; Guzik, T.J.; Lob, H.E.; Madhur, M.S.; Marvar, P.J.; Thabet, S.R.; Vinh, A.; Weyand, C.M. Inflammation, immunity, and hypertension. Hypertension 2011, 57, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Iturbe, B.; Pons, H.; Johnson, R.J. Role of the Immune System in Hypertension. Physiol. Rev. 2017, 97, 1127–1164. [Google Scholar] [CrossRef]
- Rudemiller, N.P.; Crowley, S.D. Interactions between the Immune and the Renin-Angiotensin Systems in Hypertension. Hypertension 2016, 68, 289–296. [Google Scholar] [CrossRef]
- Nataraj, C.; Oliverio, M.I.; Mannon, R.B.; Mannon, P.J.; Audoly, L.P.; Amuchastegui, C.S.; Ruiz, P.; Smithies, O.; Coffman, T.M. Angiotensin II regulates cellular immune responses through a calcineurin-dependent pathway. J. Clin. Investig. 1999, 104, 1693–1701. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Ruiz-Ortega, M.; Gomez-Guerrero, C.; Tomino, Y.; Egido, J. Angiotensin II, the immune system and renal diseases: Another road for RAS? Nephrol. Dial. Transplant. 2003, 18, 1423–1426. [Google Scholar] [CrossRef] [Green Version]
- Hahn, A.W.; Jonas, U.; Buhler, F.R.; Resink, T.J. Activation of human peripheral monocytes by angiotensin II. FEBS Lett. 1994, 347, 178–180. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Du, J.; Hu, Z.; Han, G.; Delafontaine, P.; Garcia, G.; Mitch, W.E. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J. Am. Soc. Nephrol. 2009, 20, 604–612. [Google Scholar] [CrossRef]
- Wei, Z.; Spizzo, I.; Diep, H.; Drummond, G.R.; Widdop, R.E.; Vinh, A. Differential phenotypes of tissue-infiltrating T cells during angiotensin II-induced hypertension in mice. PLoS ONE 2014, 9, e114895. [Google Scholar] [CrossRef]
- Qi, G.; Jia, L.; Li, Y.; Bian, Y.; Cheng, J.; Li, H.; Xiao, C.; Du, J. Angiotensin II infusion-induced inflammation, monocytic fibroblast precursor infiltration, and cardiac fibrosis are pressure dependent. Cardiovasc. Toxicol. 2011, 11, 157–167. [Google Scholar] [CrossRef]
- Ozawa, Y.; Kobori, H.; Suzaki, Y.; Navar, L.G. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am. J. Physiol. Renal Physiol. 2007, 292, F330–F339. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Ruperez, M.; Lorenzo, O.; Esteban, V.; Blanco, J.; Mezzano, S.; Egido, J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int. 2002, 62, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wen, Y.; Lv, L.L.; Liu, H.; Tang, R.N.; Ma, K.L.; Liu, B.C. Involvement of endoplasmic reticulum stress in angiotensin II-induced NLRP3 inflammasome activation in human renal proximal tubular cells in vitro. Acta Pharmacol. Sin. 2015, 36, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Liu, Y.; Tang, T.; Lv, L.; Liu, H.; Ma, K.; Liu, B. NLRP3 inflammasome activation is involved in Ang II-induced kidney damage via mitochondrial dysfunction. Oncotarget 2016, 7, 54290–54302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Bai, M.; Ding, G.; Zhang, Y.; Huang, S.; Jia, Z.; Zhang, A. Angiotensin II Stimulates the NLRP3 Inflammasome to Induce Podocyte Injury and Mitochondrial Dysfunction. Kidney Dis. 2018, 4, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xiong, X.Q.; Zang, Y.H.; Tong, Y.; Zhou, B.; Chen, Q.; Li, Y.H.; Gao, X.Y.; Kang, Y.M.; Zhu, G.Q. BCL6 attenuates renal inflammation via negative regulation of NLRP3 transcription. Cell Death Dis. 2017, 8, e3156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fan, C.; Cai, D.; Zhang, Y.; Zuo, R.; Zhu, L.; Cao, Y.; Zhang, J.; Liu, C.; Chen, Y.; et al. Contribution of TGF-Beta-Mediated NLRP3-HMGB1 Activation to Tubulointerstitial Fibrosis in Rat with Angiotensin II-Induced Chronic Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 1. [Google Scholar] [CrossRef]
- Bautista-Perez, R.; Perez-Mendez, O.; Cano-Martinez, A.; Pacheco, U.; Santamaria, J.; Rodriguez-Iturbe, F.R.B.; Navar, L.G.; Franco, M. The Role of P2X7 Purinergic Receptors in the Renal Inflammation Associated with Angiotensin II-induced Hypertension. Int. J. Mol. Sci. 2020, 21, 4041. [Google Scholar] [CrossRef]
- Satou, R.; Miyata, K.; Gonzalez-Villalobos, R.A.; Ingelfinger, J.R.; Navar, L.G.; Kobori, H. Interferon-gamma biphasically regulates angiotensinogen expression via a JAK-STAT pathway and suppressor of cytokine signaling 1 (SOCS1) in renal proximal tubular cells. FASEB J. 2012, 26, 1821–1830. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, R.; Penrose, H.; Miyata, K.; Satou, R. Macrophage-derived IL-6 contributes to ANG II-mediated angiotensinogen stimulation in renal proximal tubular cells. Am. J. Physiol. Renal Physiol. 2016, 310, F1000–F1007. [Google Scholar] [CrossRef] [Green Version]
- Bravo, Y.; Quiroz, Y.; Ferrebuz, A.; Vaziri, N.D.; Rodriguez-Iturbe, B. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am. J. Physiol. Renal Physiol. 2007, 293, F616–F623. [Google Scholar] [CrossRef] [Green Version]
- Ingelfinger, J.R.; Zuo, W.M.; Fon, E.A.; Ellison, K.E.; Dzau, V.J. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J. Clin. Investig. 1990, 85, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Terada, Y.; Tomita, K.; Nonoguchi, H.; Marumo, F. PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int. 1993, 43, 1251–1259. [Google Scholar] [CrossRef] [Green Version]
- Kobori, H.; Navar, L.G. Urinary Angiotensinogen as a Novel Biomarker of Intrarenal Renin-Angiotensin System in Chronic Kidney Disease. Int. Rev. Thromb. 2011, 6, 108–116. [Google Scholar]
- Shao, W.; Miyata, K.; Katsurada, A.; Satou, R.; Seth, D.M.; Rosales, C.B.; Prieto, M.C.; Mitchell, K.D.; Navar, L.G. Increased angiotensinogen expression, urinary angiotensinogen excretion, and tissue injury in nonclipped kidneys of two-kidney, one-clip hypertensive rats. Am. J. Physiol. Renal Physiol. 2016, 311, F278–F290. [Google Scholar] [CrossRef] [Green Version]
- Pingili, A.K.; Davidge, K.N.; Thirunavukkarasu, S.; Khan, N.S.; Katsurada, A.; Majid, D.S.A.; Gonzalez, F.J.; Navar, L.G.; Malik, K.U. 2-Methoxyestradiol Reduces Angiotensin II-Induced Hypertension and Renal Dysfunction in Ovariectomized Female and Intact Male Mice. Hypertension 2017, 69, 1104–1112. [Google Scholar] [CrossRef]
- Herrera, J.; Ferrebuz, A.; MacGregor, E.G.; Rodriguez-Iturbe, B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J. Am. Soc. Nephrol. 2006, 17, S218–S225. [Google Scholar] [CrossRef] [Green Version]
- Moes, A.D.; Severs, D.; Verdonk, K.; van der Lubbe, N.; Zietse, R.; Danser, A.H.J.; Hoorn, E.J. Mycophenolate Mofetil Attenuates DOCA-Salt Hypertension: Effects on Vascular Tone. Front. Physiol. 2018, 9, 578. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Iturbe, B.; Quiroz, Y.; Nava, M.; Bonet, L.; Chavez, M.; Herrera-Acosta, J.; Johnson, R.J.; Pons, H.A. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am. J. Physiol. Renal Physiol. 2002, 282, F191–F201. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Chen, X.L.; Wang, F.; Liang, X.; Sun, Y.X.; Wang, Y.J. Role of angiotensin II type 1 receptor in angiotensin II-induced cytokine production in macrophages. J. Interferon. Cytokine Res. 2011, 31, 351–361. [Google Scholar] [CrossRef]
- Liu, F.; Dai, S.; Feng, D.; Qin, Z.; Peng, X.; Sakamuri, S.; Ren, M.; Huang, L.; Cheng, M.; Mohammad, K.E.; et al. Distinct fate, dynamics and niches of renal macrophages of bone marrow or embryonic origins. Nat. Commun. 2020, 11, 2280. [Google Scholar] [CrossRef]
- Puranik, A.S.; Leaf, I.A.; Jensen, M.A.; Hedayat, A.F.; Saad, A.; Kim, K.W.; Saadalla, A.M.; Woollard, J.R.; Kashyap, S.; Textor, S.C.; et al. Kidney-resident macrophages promote a proangiogenic environment in the normal and chronically ischemic mouse. Kidney Sci. Rep. 2018, 8, 13948. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, K.A.; Huang, J.; He, L.; Revell, D.Z.; Li, Z.; Hsu, J.S.; Fitzgibbon, W.R.; Hazard, E.S.; Hardiman, G.; Mrug, M.; et al. Interferon Regulatory Factor-5 in Resident Macrophage Promotes Polycystic Kidney Disease. Kidney360 2020, 1, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Abdo, S.; Shi, Y.; Otoukesh, A.; Ghosh, A.; Lo, C.S.; Chenier, I.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.L.; Chan, J.S. Catalase overexpression prevents nuclear factor erythroid 2-related factor 2 stimulation of renal angiotensinogen gene expression, hypertension, and kidney injury in diabetic mice. Diabetes 2014, 63, 3483–3496. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, T.J.; Fustier, P.; Wei, C.C.; Zhang, S.L.; Filep, J.G.; Tang, S.S.; Ingelfinger, J.R.; Fantus, I.G.; Hamet, P.; Chan, J.S. Reactive oxygen species blockade and action of insulin on expression of angiotensinogen gene in proximal tubular cells. J. Endocrinol. 2004, 183, 535–550. [Google Scholar] [CrossRef]
- Satou, R.; Cypress, M.W.; Woods, T.C.; Katsurada, A.; Dugas, C.M.; Fonseca, V.A.; Navar, L.G. Blockade of sodium-glucose cotransporter 2 suppresses high glucose-induced angiotensinogen augmentation in renal proximal tubular cells. Am. J. Physiol. Renal Physiol. 2020, 318, F67–F75. [Google Scholar] [CrossRef]
- Woods, T.C.; Satou, R.; Miyata, K.; Katsurada, A.; Dugas, C.M.; Klingenberg, N.C.; Fonseca, V.A.; Navar, L.G. Canagliflozin Prevents Intrarenal Angiotensinogen Augmentation and Mitigates Kidney Injury and Hypertension in Mouse Model of Type 2 Diabetes Mellitus. Am. J. Nephrol. 2019, 49, 331–342. [Google Scholar] [CrossRef]
- Ortiz, R.M.; Mamalis, A.; Navar, L.G. Aldosterone Receptor Antagonism Reduces Urinary C-Reactive Protein Excretion in Angiotensin II-Infused, Hypertensive Rats. J. Am. Soc. Hypertens. 2009, 3, 184–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kanneganti, T.D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, M.; Tapia, E.; Bautista, R.; Pacheco, U.; Santamaria, J.; Quiroz, Y.; Johnson, R.J.; Rodriguez-Iturbe, B. Impaired pressure natriuresis resulting in salt-sensitive hypertension is caused by tubulointerstitial immune cell infiltration in the kidney. Am. J. Physiol. Renal Physiol. 2013, 304, F982–F990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, W.D.; Sackner, M.A. Simplification of the Anthrone Method for the Determination of Inulin in Clearance Studies. J. Lab. Clin. Med. 1963, 62, 351–356. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satou, R.; Franco, M.; Dugas, C.M.; Katsurada, A.; Navar, L.G. Immunosuppression by Mycophenolate Mofetil Mitigates Intrarenal Angiotensinogen Augmentation in Angiotensin II-Dependent Hypertension. Int. J. Mol. Sci. 2022, 23, 7680. https://doi.org/10.3390/ijms23147680
Satou R, Franco M, Dugas CM, Katsurada A, Navar LG. Immunosuppression by Mycophenolate Mofetil Mitigates Intrarenal Angiotensinogen Augmentation in Angiotensin II-Dependent Hypertension. International Journal of Molecular Sciences. 2022; 23(14):7680. https://doi.org/10.3390/ijms23147680
Chicago/Turabian StyleSatou, Ryousuke, Martha Franco, Courtney M. Dugas, Akemi Katsurada, and L. Gabriel Navar. 2022. "Immunosuppression by Mycophenolate Mofetil Mitigates Intrarenal Angiotensinogen Augmentation in Angiotensin II-Dependent Hypertension" International Journal of Molecular Sciences 23, no. 14: 7680. https://doi.org/10.3390/ijms23147680
APA StyleSatou, R., Franco, M., Dugas, C. M., Katsurada, A., & Navar, L. G. (2022). Immunosuppression by Mycophenolate Mofetil Mitigates Intrarenal Angiotensinogen Augmentation in Angiotensin II-Dependent Hypertension. International Journal of Molecular Sciences, 23(14), 7680. https://doi.org/10.3390/ijms23147680






