Clinical Tear Fluid Proteomics—A Novel Tool in Glaucoma Research
Abstract
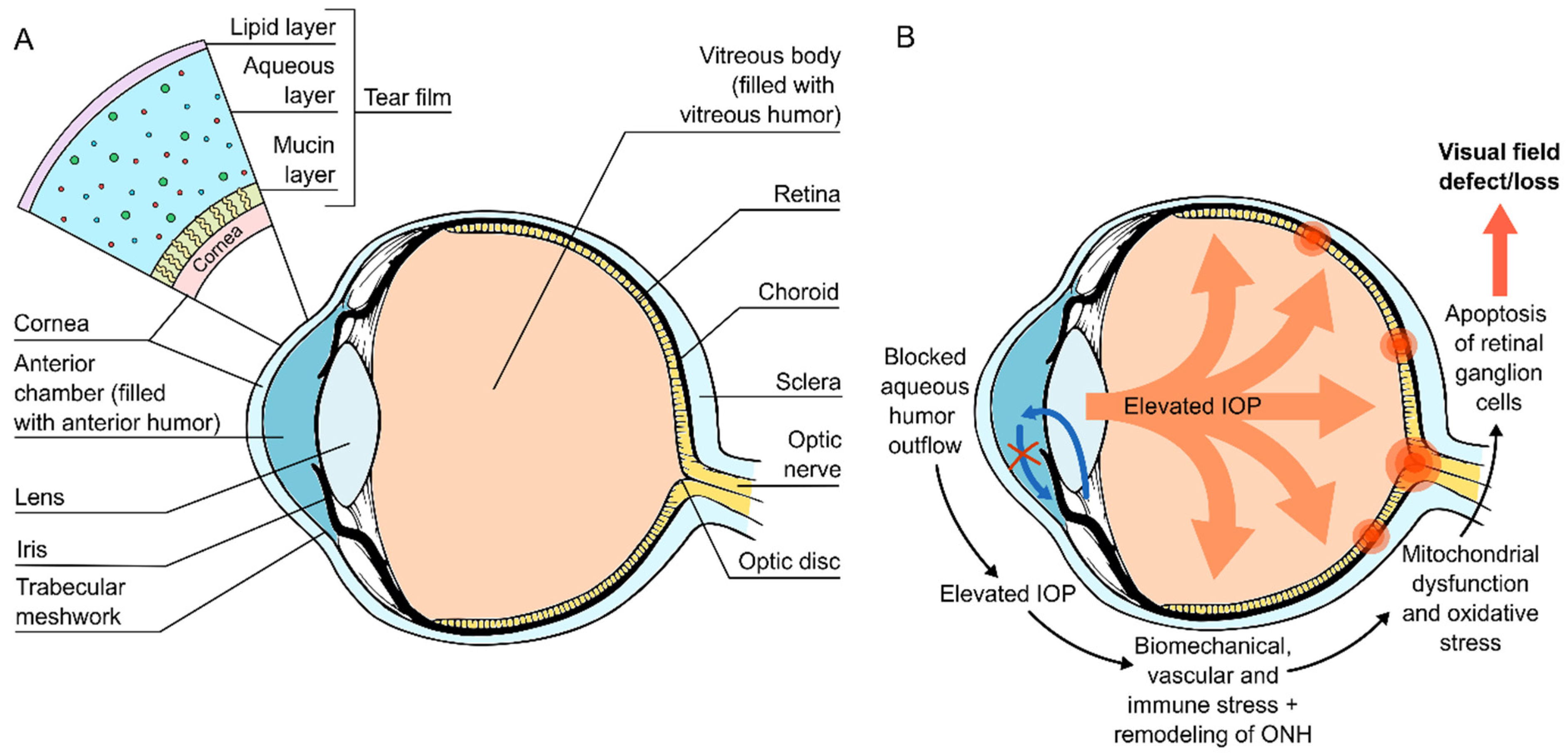
:1. Introduction
2. Tear Fluid Analytics
3. Glaucomatous Changes and Their Reflection on the Tear Fluid Proteomics
4. Tear Fluid Proteomics in the Evaluation of Therapeutic Outcomes in Glaucoma
4.1. Topical Medication
4.2. Glaucoma Surgery
5. New Approaches and the Future Directions of Tear Proteomics in Glaucoma Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, J.Y.W.; Sze, Y.H.; Bian, J.F.; Lam, T.C. Critical Role of Mass Spectrometry Proteomics in Tear Biomarker Discovery for Multifactorial Ocular Diseases (Review). Int. J. Mol. Med. 2021, 47, 83. [Google Scholar] [CrossRef]
- Dor, M.; Eperon, S.; Lalive, P.H.; Guex-Crosier, Y.; Hamedani, M.; Salvisberg, C.; Turck, N. Investigation of the Global Protein Content from Healthy Human Tears. Exp. Eye Res. 2019, 179, 64–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Zhao, S.Z.; Koh, S.K.; Chen, L.; Vaz, C.; Tanavde, V.; Li, X.R.; Beuerman, R.W. In-Depth Analysis of the Human Tear Proteome. J. Proteom. 2012, 75, 3877–3885. [Google Scholar] [CrossRef] [PubMed]
- Azbukina, N.V.; Chistyakov, D.V.; Goriainov, S.V.; Kotelin, V.I.; Fedoseeva, E.V.; Petrov, S.Y.; Sergeeva, M.G.; Iomdina, E.N.; Zernii, E.Y. Targeted Lipidomic Analysis of Aqueous Humor Reveals Signaling Lipid-Mediated Pathways in Primary Open-Angle Glaucoma. Biology 2021, 10, 658. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Beuerman, R.W. Tear Analysis in Ocular Surface Diseases. Prog. Retin. Eye Res. 2012, 31, 527–550. [Google Scholar] [CrossRef]
- Willcox, M.D.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Beuerman, R.W.; Chan, C.M.; Zhao, S.Z.; Li, X.R.; Yang, H.; Tong, L.; Liu, S.; Stern, M.E.; Tan, D. Identification of Tear Fluid Biomarkers in Dry Eye Syndrome Using ITRAQ Quantitative Proteomics. J. Proteome Res. 2009, 8, 4889–4905. [Google Scholar] [CrossRef]
- Ambaw, Y.A.; Timbadia, D.P.; Raida, M.; Torta, F.; Wenk, M.R.; Tong, L. Profile of Tear Lipid Mediator as a Biomarker of Inflammation for Meibomian Gland Dysfunction and Ocular Surface Diseases: Standard Operating Procedures. Ocul. Surf. 2020; in press. [Google Scholar] [CrossRef]
- Green-Church, K.B.; Butovich, I.; Willcox, M.; Borchman, D.; Paulsen, F.; Barabino, S.; Glasgow, B.J. The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Tear Film Lipids and Lipid–Protein Interactions in Health and Disease. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1979–1993. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, A. Allergy and Allergic Mediators in Tears. Exp. Eye Res. 2013, 117, 106–117. [Google Scholar] [CrossRef]
- Zhan, X.; Li, J.; Guo, Y.; Golubnitschaja, O. Mass Spectrometry Analysis of Human Tear Fluid Biomarkers Specific for Ocular and Systemic Diseases in the Context of 3P Medicine. EPMA J. 2021, 12, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, J.; Li, Y.; Jiang, B. Prevalence of Primary Open Angle Glaucoma in the Last 20 Years: A Meta-Analysis and Systematic Review. Sci. Rep. 2021, 11, 13762. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The Number of People with Glaucoma Worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinreb, R.N.; Khaw, P.T. Primary Open-Angle Glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Stone, E.M.; Fingert, J.H.; Alward, W.L.M.; Nguyen, T.D.; Polansky, J.R.; Sunden, S.L.F.; Nishimura, D.; Clark, A.F.; Nystuen, A.; Nichols, B.E.; et al. Identification of a Gene That Causes Primary Open Angle Glaucoma. Science 1997, 275, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Liuska, P.J.; Lemmelä, S.; Havulinna, A.S.; Kaarniranta, K.; Uusitalo, H.; Laivuori, H.; Kiiskinen, T.; Daly, M.J.; Palotie, A.; Turunen, J.A.; et al. Association of the MYOC p.(Gln368Ter) Variant with Glaucoma in a Finnish Population. JAMA Ophthalmol. 2021, 139, 762–768. [Google Scholar] [CrossRef]
- Gharahkhani, P.; Jorgenson, E.; Hysi, P.; Khawaja, A.P.; Pendergrass, S.; Han, X.; Ong, J.S.; Hewitt, A.W.; Segrè, A.V.; Rouhana, J.M.; et al. Genome-Wide Meta-Analysis Identifies 127 Open-Angle Glaucoma Loci with Consistent Effect across Ancestries. Nat. Commun. 2021, 12, 1258. [Google Scholar] [CrossRef]
- Beykin, G.; Goldberg, J.L. Molecular Biomarkers for Glaucoma. Curr. Ophthalmol. Rep. 2019, 7, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Vega Cueto, A.; Álvarez, L.; García, M.; Álvarez-Barrios, A.; Artime, E.; Fernández-Vega Cueto, L.; Coca-Prados, M.; González-Iglesias, H. Candidate Glaucoma Biomarkers: From Proteins to Metabolites, and the Pitfalls to Clinical Applications. Biology 2021, 10, 763. [Google Scholar] [CrossRef]
- Khanna, R.K.; Catanese, S.; Emond, P.; Corcia, P.; Blasco, H.; Pisella, P.-J. Metabolomics and Lipidomics Approaches in Human Tears: A Systematic Review. Surv. Ophthalmol. 2022, 67, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Nättinen, J.; Aapola, U.; Jylhä, A.; Vaajanen, A.; Uusitalo, H. Comparison of Capillary and Schirmer Strip Tear Fluid Sampling Methods Using SWATH-MS Proteomics Approach. Transl. Vis. Sci. Technol. 2020, 9, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choy, C.K.M.; Cho, P.; Chung, W.-Y.; Benzie, I.F.F. Water-Soluble Antioxidants in Human Tears: Effect of the Collection Method. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3130–3134. [Google Scholar]
- Posa, A.; Bräuer, L.; Schicht, M.; Garreis, F.; Beileke, S.; Paulsen, F. Schirmer Strip vs. Capillary Tube Method: Non-Invasive Methods of Obtaining Proteins from Tear Fluid. Ann. Anat. 2013, 195, 137–142. [Google Scholar] [CrossRef]
- Nättinen, J.; Aapola, U.; Nukareddy, P.; Uusitalo, H. Looking Deeper into Ocular Surface Health: An Introduction to Clinical Tear Proteomics Analysis. Acta Ophthalmol. 2021, 100, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Ponzini, E.; Santambrogio, C.; De Palma, A.; Mauri, P.; Tavazzi, S.; Grandori, R. Mass Spectrometry-Based Tear Proteomics for Noninvasive Biomarker Discovery. Mass Spectrom. Rev. 2021; in press. [Google Scholar] [CrossRef]
- Nättinen, J.; Jylhä, A.; Aapola, U.; Enríquez-de-Salamanca, A.; Pinto-Fraga, J.; López-Miguel, A.; González-García, M.J.; Stern, M.E.; Calonge, M.; Zhou, L.; et al. Topical Fluorometholone Treatment and Desiccating Stress Change Inflammatory Protein Expression in Tears. Ocul. Surf. 2018, 16, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roda, M.; Corazza, I.; Bacchi Reggiani, M.L.; Pellegrini, M.; Taroni, L.; Giannaccare, G.; Versura, P. Dry Eye Disease and Tear Cytokine Levels—A Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3111. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Yeon, Y.; Lee, W.J.; Shin, Y.U.; Cho, H.; Sung, Y.-K.; Kim, D.R.; Lim, H.W.; Kang, M.H. Comparison of MicroRNA Expression in Tears of Normal Subjects and Sjögren Syndrome Patients. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4889–4895. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, A.; Nakajima, T.; Azuma, M. Tear MiRNA Expression Analysis Reveals MiR-203 as a Potential Regulator of Corneal Epithelial Cells. BMC Ophthalmol. 2021, 21, 377. [Google Scholar] [CrossRef]
- Tamkovich, S.; Grigor’eva, A.; Eremina, A.; Tupikin, A.; Kabilov, M.; Chernykh, V.; Vlassov, V.; Ryabchikova, E. What Information Can Be Obtained from the Tears of a Patient with Primary Open Angle Glaucoma? Clin. Chim. Acta 2019, 495, 529–537. [Google Scholar] [CrossRef]
- Wu, J.; Xu, M.; Liu, W.; Huang, Y.; Wang, R.; Chen, W.; Feng, L.; Liu, N.; Sun, X.; Zhou, M.; et al. Glaucoma Characterization by Machine Learning of Tear Metabolic Fingerprinting. Small Methods 2022, 6, 2200264. [Google Scholar] [CrossRef] [PubMed]
- Nättinen, J.; Jylhä, A.; Aapola, U.; Mäkinen, P.; Beuerman, R.; Pietilä, J.; Vaajanen, A.; Uusitalo, H. Age-Associated Changes in Human Tear Proteome. Clin. Proteom. 2019, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The Molecular Basis of Retinal Ganglion Cell Death in Glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G. The Immune Response in Glaucoma: A Perspective on the Roles of Oxidative Stress. Exp. Eye Res. 2011, 93, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Chrysostomou, V.; Trounce, I.A.; Crowston, J.G. Mechanisms of Retinal Ganglion Cell Injury in Aging and Glaucoma. Ophthalmic Res. 2010, 44, 173–178. [Google Scholar] [CrossRef]
- Pieragostino, D.; Agnifili, L.; Fasanella, V.; D’Aguanno, S.; Mastropasqua, R.; Ilio, C.D.; Sacchetta, P.; Urbani, A.; Boccio, P.D. Shotgun Proteomics Reveals Specific Modulated Protein Patterns in Tears of Patients with Primary Open Angle Glaucoma Naïve to Therapy. Mol. BioSyst. 2013, 9, 1108–1116. [Google Scholar] [CrossRef]
- Rossi, C.; Cicalini, I.; Cufaro, M.C.; Agnifili, L.; Mastropasqua, L.; Lanuti, P.; Marchisio, M.; De Laurenzi, V.; Del Boccio, P.; Pieragostino, D. Multi-Omics Approach for Studying Tears in Treatment-Naïve Glaucoma Patients. Int. J. Mol. Sci. 2019, 20, 4029. [Google Scholar] [CrossRef] [Green Version]
- Assouti, M.; Vynios, D.H.; Anagnostides, S.T.; Papadopoulos, G.; Georgakopoulos, C.D.; Gartaganis, S.P. Collagen Type IX and HNK-1 Epitope in Tears of Patients with Pseudoexfoliation Syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2006, 1762, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Sahay, P.; Reddy, S.; Prusty, B.K.; Modak, R.; Rao, A. TGFβ1, MMPs and Cytokines Profiles in Ocular Surface: Possible Tear Biomarkers for Pseudoexfoliation. PLoS ONE 2021, 16, e0249759. [Google Scholar] [CrossRef]
- Gupta, D.; Wen, J.C.; Huebner, J.L.; Stinnett, S.; Kraus, V.B.; Tseng, H.C.; Walsh, M. Cytokine Biomarkers in Tear Film for Primary Open-Angle Glaucoma. Clin. Ophthalmol. 2017, 11, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Sahay, P.; Rao, A.; Padhy, D.; Sarangi, S.; Das, G.; Reddy, M.M.; Modak, R. Functional Activity of Matrix Metalloproteinases 2 and 9 in Tears of Patients with Glaucoma. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO106–BIO113. [Google Scholar] [CrossRef] [PubMed]
- Pieragostino, D.; Bucci, S.; Agnifili, L.; Fasanella, V.; D’Aguanno, S.; Mastropasqua, A.; Ciancaglini, M.; Mastropasqua, L.; Ilio, C.D.; Sacchetta, P.; et al. Differential Protein Expression in Tears of Patients with Primary Open Angle and Pseudoexfoliative Glaucoma. Mol. BioSyst. 2012, 8, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Ghaffariyeh, A.; Honarpisheh, N.; Shakiba, Y.; Puyan, S.; Chamacham, T.; Zahedi, F.; Zarrineghbal, M. Brain-Derived Neurotrophic Factor in Patients with Normal-Tension Glaucoma. Optometry 2009, 80, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Demirdöğen, B.C.; Koçan Akçin, C.; Özge, G.; Mumcuoğlu, T. Evaluation of Tear and Aqueous Humor Level, and Genetic Variants of Connective Tissue Growth Factor as Biomarkers for Early Detection of Pseudoexfoliation Syndrome/Glaucoma. Exp. Eye Res. 2019, 189, 107837. [Google Scholar] [CrossRef]
- Shpak, A.A.; Guekht, A.B.; Druzhkova, T.A.; Kozlova, K.I.; Gulyaeva, N.V. Ciliary Neurotrophic Factor in Patients with Primary Open-Angle Glaucoma and Age-Related Cataract. Mol. Vis. 2017, 23, 799–809. [Google Scholar]
- Benitez-Del-Castillo, J.; Cantu-Dibildox, J.; Sanz-González, S.M.; Zanón-Moreno, V.; Pinazo-Duran, M.D. Cytokine Expression in Tears of Patients with Glaucoma or Dry Eye Disease: A Prospective, Observational Cohort Study. Eur. J. Ophthalmol. 2019, 29, 437–443. [Google Scholar] [CrossRef]
- Galbis-Estrada, C.; Pinazo-Durán, M.D.; Cantú-Dibildox, J.; Marco-Ramírez, C.; Díaz-Llópis, M.; Benítez-del-Castillo, J. Patients Undergoing Long-Term Treatment with Antihypertensive Eye Drops Responded Positively with Respect to Their Ocular Surface Disorder to Oral Supplementation with Antioxidants and Essential Fatty Acids. Clin. Interv. Aging 2013, 8, 711–719. [Google Scholar] [CrossRef] [Green Version]
- Benitez-del-Castillo Sánchez, J.; Morillo-Rojas, M.D.; Galbis-Estrada, C.; Pinazo-Duran, M.D. Determination of Inmune Response and Inflammation Mediators in Tears: Changes in Dry Eye and Glaucoma as Compared to Healthy Controls. Arch. Soc. Esp. Oftalmol. 2017, 92, 210–217. [Google Scholar] [CrossRef]
- Kim, D.W.; Seo, J.H.; Lim, S.-H. Evaluation of Ocular Surface Disease in Elderly Patients with Glaucoma: Expression of Matrix Metalloproteinase-9 in Tears. Eye 2021, 35, 892–900. [Google Scholar] [CrossRef]
- Srinivasan, S.; Thangavelu, M.; Zhang, L.; Green, K.B.; Nichols, K.K. ITRAQ Quantitative Proteomics in the Analysis of Tears in Dry Eye Patients. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5052–5059. [Google Scholar] [CrossRef] [Green Version]
- Roedl, J.B.; Bleich, S.; Schlötzer-Schrehardt, U.; von Ahsen, N.; Kornhuber, J.; Naumann, G.O.H.; Kruse, F.E.; Jünemann, A.G.M. Increased Homocysteine Levels in Tear Fluid of Patients with Primary Open-Angle Glaucoma. Ophthalmic Res. 2008, 40, 249–256. [Google Scholar] [CrossRef]
- Roedl, J.B.; Bleich, S.; Reulbach, U.; Rejdak, R.; Kornhuber, J.; Kruse, F.E.; Schlötzer-Schrehardt, U.; Jünemann, A.G. Homocysteine in Tear Fluid of Patients with Pseudoexfoliation Glaucoma. J. Glaucoma 2007, 16, 234–239. [Google Scholar] [CrossRef]
- Han, F.-F.; Fu, X.-X. Vitamin Intake and Glaucoma Risk: A Systematic Review and Meta-Analysis. J. Fr. Ophtalmol. 2022, 45, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Huynh, B.; Shah, P.; Sii, F.; Hunter, D.; Carnt, N.; White, A. Low Systemic Vitamin D as a Potential Risk Factor in Primary Open-Angle Glaucoma: A Review of Current Evidence. Br. J. Ophthalmol. 2021, 105, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M.; Early Manifest Glaucoma Trial Group. Reduction of Intraocular Pressure and Glaucoma Progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K.; Wilson, M.R.; Gordon, M.O. The Ocular Hypertension Treatment Study: A Randomized Trial Determines That Topical Ocular Hypotensive Medication Delays or Prevents the Onset of Primary Open-Angle Glaucoma. Arch. Ophthalmol. 2002, 120, 701–713. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [Green Version]
- Broadway, D.C.; Grierson, I.; O’Brien, C.; Hitchings, R.A. Adverse Effects of Topical Antiglaucoma Medication. I. The Conjunctival Cell Profile. Arch. Ophthalmol. 1994, 112, 1437–1445. [Google Scholar] [CrossRef]
- Arita, R.; Itoh, K.; Maeda, S.; Maeda, K.; Furuta, A.; Tomidokoro, A.; Aihara, M.; Amano, S. Comparison of the Long-Term Effects of Various Topical Antiglaucoma Medications on Meibomian Glands. Cornea 2012, 31, 1229–1234. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Agnifili, L.; Mastropasqua, R.; Fasanella, V. Conjunctival Modifications Induced by Medical and Surgical Therapies in Patients with Glaucoma. Curr. Opin. Pharmacol. 2013, 13, 56–64. [Google Scholar] [CrossRef]
- Chung, S.-H.; Lee, S.K.; Cristol, S.M.; Lee, E.S.; Lee, D.W.; Seo, K.Y.; Kim, E.K. Impact of Short-Term Exposure of Commercial Eyedrops Preserved with Benzalkonium Chloride on Precorneal Mucin. Mol. Vis. 2006, 12, 415–421. [Google Scholar] [PubMed]
- Lee, S.Y.; Lee, H.; Bae, H.W.; Kim, T.-I.; Kim, C.Y. Tear Lipid Layer Thickness Change and Topical Anti-Glaucoma Medication Use. Optom. Vis. Sci. 2016, 93, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Labbé, A.; Terry, O.; Brasnu, E.; Van Went, C.; Baudouin, C. Tear Film Osmolarity in Patients Treated for Glaucoma or Ocular Hypertension. Cornea 2012, 31, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.W.; Medeiros, F.A.; Weinreb, R.N. Prevalence of Ocular Surface Disease in Glaucoma Patients. J. Glaucoma 2008, 17, 350–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, W.C.; Stewart, J.A.; Nelson, L.A. Ocular Surface Disease in Patients with Ocular Hypertension and Glaucoma. Curr. Eye Res. 2011, 36, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Uusitalo, H.; Chen, E.; Pfeiffer, N.; Brignole-Baudouin, F.; Kaarniranta, K.; Leino, M.; Puska, P.; Palmgren, E.; Hamacher, T.; Hofmann, G.; et al. Switching from a Preserved to a Preservative-Free Prostaglandin Preparation in Topical Glaucoma Medication. Acta Ophthalmol. 2010, 88, 329–336. [Google Scholar] [CrossRef]
- Uusitalo, H.; Egorov, E.; Kaarniranta, K.; Astakhov, Y.; Ropo, A. Benefits of Switching from Latanoprost to Preservative-Free Tafluprost Eye Drops: A Meta-Analysis of Two Phase IIIb Clinical Trials. Clin. Ophthalmol. 2016, 10, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Jaenen, N.; Baudouin, C.; Pouliquen, P.; Manni, G.; Figueiredo, A.; Zeyen, T. Ocular Symptoms and Signs with Preserved and Preservative-Free Glaucoma Medications. Eur. J. Ophthalmol. 2007, 17, 341–349. [Google Scholar] [CrossRef]
- Pisella, P.-J.; Debbasch, C.; Hamard, P.; Creuzot-Garcher, C.; Rat, P.; Brignole, F.; Baudouin, C. Conjunctival Proinflammatory and Proapoptotic Effects of Latanoprost and Preserved and Unpreserved Timolol: An Ex Vivo and In Vitro Study. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1360–1368. [Google Scholar] [CrossRef] [Green Version]
- Sedlak, L.; Świerczyńska, M.; Borymska, W.; Zych, M.; Wyględowska-Promieńska, D. Impact of Dorzolamide, Benzalkonium-Preserved Dorzolamide and Benzalkonium-Preserved Brinzolamide on Selected Biomarkers of Oxidative Stress in the Tear Film. BMC Ophthalmol. 2021, 21, 319. [Google Scholar] [CrossRef]
- Sedlak, L.; Wojnar, W.; Zych, M.; Wyględowska-Promieńska, D. Influence of Timolol, Benzalkonium-Preserved Timolol, and Benzalkonium-Preserved Brimonidine on Oxidative Stress Biomarkers in the Tear Film. Cutan. Ocul. Toxicol. 2020, 39, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Acera, A.; Suárez, T.; Rodríguez-Agirretxe, I.; Vecino, E.; Durán, J.A. Changes in Tear Protein Profile in Patients with Conjunctivochalasis. Cornea 2011, 30, 42–49. [Google Scholar] [CrossRef]
- Vidal-Villegas, B.; Burgos-Blasco, B.; Alvarez, J.L.S.; Espino-Paisán, L.; Fernández-Vigo, J.; Andrés-Guerrero, V.; García-Feijoo, J.; Martínez-de-la-Casa, J.M. Proinflammatory Cytokine Profile Differences between Primary Open-Angle and Pseudoexfoliative Glaucoma. Ophthalmic Res. 2022, 65, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R.; Gurbaxani, A.; Sallam, A.; Lightman, S. Topical Prostaglandin Analogues and Conjunctival Inflammation in Uveitic Glaucoma. Open Ophthalmol. J. 2012, 6, 75–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malvitte, L.; Montange, T.; Vejux, A.; Baudouin, C.; Bron, A.M.; Creuzot-Garcher, C.; Lizard, G. Measurement of Inflammatory Cytokines by Multicytokine Assay in Tears of Patients with Glaucoma Topically Treated with Chronic Drugs. Br. J. Ophthalmol. 2007, 91, 29–32. [Google Scholar] [CrossRef]
- Burgos-Blasco, B.; Vidal-Villegas, B.; Saenz-Frances, F.; Morales-Fernandez, L.; Perucho-Gonzalez, L.; Garcia-Feijoo, J.; Martinez-de-la-Casa, J.M. Tear and Aqueous Humour Cytokine Profile in Primary Open-Angle Glaucoma. Acta Ophthalmol. 2020, 98, e768–e772. [Google Scholar] [CrossRef]
- Lopilly Park, H.-Y.; Kim, J.H.; Lee, K.M.; Park, C.K. Effect of Prostaglandin Analogues on Tear Proteomics and Expression of Cytokines and Matrix Metalloproteinases in the Conjunctiva and Cornea. Exp. Eye Res. 2012, 94, 13–21. [Google Scholar] [CrossRef]
- De la Fuente, M.; Rodríguez-Agirretxe, I.; Vecino, E.; Astigarraga, E.; Acera, A.; Barreda-Gómez, G. Elevation of Tear MMP-9 Concentration as a Biomarker of Inflammation in Ocular Pathology by Antibody Microarray Immunodetection Assays. Int. J. Mol. Sci. 2022, 23, 5639. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.T.; Zhou, L.; Li, J.; Tong, L.; Zhao, S.Z.; Li, X.R.; Yu, S.J.; Koh, S.K.; Beuerman, R.W. Proteomic Profiling of Inflammatory Signaling Molecules in the Tears of Patients on Chronic Glaucoma Medication. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7385–7391. [Google Scholar] [CrossRef] [PubMed]
- Martinez-de-la-Casa, J.M.; Perez-Bartolome, F.; Urcelay, E.; Santiago, J.L.; Moreno-Montañes, J.; Arriola-Villalobos, P.; Benitez-del-Castillo, J.M.; Garcia-Feijoo, J. Tear Cytokine Profile of Glaucoma Patients Treated with Preservative-Free or Preserved Latanoprost. Ocul. Surf. 2017, 15, 723–729. [Google Scholar] [CrossRef]
- Manni, G.; Centofanti, M.; Oddone, F.; Parravano, M.; Bucci, M.G. Interleukin-1β Tear Concentration in Glaucomatous and Ocular Hypertensive Patients Treated with Preservative-Free Nonselective Beta-Blockers. Am. J. Ophthalmol. 2005, 139, 72–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, S.; Sahay, P.; Padhy, D.; Sarangi, S.; Suar, M.; Modak, R.; Rao, A. Tear Biomarkers in Latanoprost and Bimatoprost Treated Eyes. PLoS ONE 2018, 13, e0201740. [Google Scholar] [CrossRef] [PubMed]
- Funke, S.; Beck, S.; Lorenz, K.; Kotterer, M.; Wolters, D.; Perumal, N.; Pfeiffer, N.; Grus, F.H. Analysis of the Effects of Preservative-Free Tafluprost on the Tear Proteome. Am. J. Transl. Res. 2016, 8, 4025–4039. [Google Scholar] [PubMed]
- Nättinen, J.; Jylhä, A.; Aapola, U.; Parkkari, M.; Mikhailova, A.; Beuerman, R.W.; Uusitalo, H. Patient Stratification in Clinical Glaucoma Trials Using the Individual Tear Proteome. Sci. Rep. 2018, 8, 12038. [Google Scholar] [CrossRef] [PubMed]
- Helin, M.; Rönkkö, S.; Puustjärvi, T.; Teräsvirta, M.; Ollikainen, M.; Uusitalo, H. Conjunctival Inflammatory Cells and Their Predictive Role for Deep Sclerectomy in Primary Open-Angle Glaucoma and Exfoliation Glaucoma. J. Glaucoma 2011, 20, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Helin-Toiviainen, M.; Rönkkö, S.; Puustjärvi, T.; Rekonen, P.; Ollikainen, M.; Uusitalo, H. Conjunctival Matrix Metalloproteinases and Their Inhibitors in Glaucoma Patients. Acta Ophthalmol. 2015, 93, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Sherwood, M.B.; Grierson, I.; Milgar, L.; Hitchings, R.A. Long-Term Morphologic Effects of Antiglaucoma Drugs on the Conjunctiva and Tenon’s Capsule in Glaucomatous Patients. Ophthalmology 1989, 96, 327–335. [Google Scholar] [CrossRef]
- Broadway, D.C.; Grierson, I.; O’Brien, C.; Hitchings, R.A. Adverse Effects of Topical Antiglaucoma Medication. II. The Outcome of Filtration Surgery. Arch. Ophthalmol. 1994, 112, 1446–1454. [Google Scholar] [CrossRef]
- Lavin, M.J.; Wormald, R.P.L.; Migdal, C.S.; Hitchings, R.A. The Influence of Prior Therapy on the Success of Trabeculectomy. Arch. Ophthalmol. 1990, 108, 1543–1548. [Google Scholar] [CrossRef]
- Agnifili, L.; Sacchi, M.; Figus, M.; Posarelli, C.; Lizzio, R.A.U.; Nucci, P.; Mastropasqua, L. Preparing the Ocular Surface for Glaucoma Filtration Surgery: An Unmet Clinical Need. Acta Ophthalmol. 2022; in press. [Google Scholar] [CrossRef]
- Cairns, J.E. Trabeculectomy. Preliminary Report of a New Method. Am. J. Ophthalmol. 1968, 66, 673–679. [Google Scholar] [CrossRef]
- Molteno, A.C. New Implant for Drainage in Glaucoma. Animal Trial. Br. J. Ophthalmol. 1969, 53, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendrinos, E.; Mermoud, A.; Shaarawy, T. Nonpenetrating Glaucoma Surgery. Surv. Ophthalmol. 2008, 53, 592–630. [Google Scholar] [CrossRef] [PubMed]
- Atreides, S.-P.A.; Skuta, G.L.; Reynolds, A.C. Wound Healing Modulation in Glaucoma Filtering Surgery. Int. Ophthalmol. Clin. 2004, 44, 61–106. [Google Scholar] [CrossRef]
- Landers, J.; Martin, K.; Sarkies, N.; Bourne, R.; Watson, P. A Twenty-Year Follow-up Study of Trabeculectomy: Risk Factors and Outcomes. Ophthalmology 2012, 119, 694–702. [Google Scholar] [CrossRef] [PubMed]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 11. Risk Factors for Failure of Trabeculectomy and Argon Laser Trabeculoplasty. Am. J. Ophthalmol. 2002, 134, 481–498. [Google Scholar] [CrossRef]
- Wong, J.K.W.; Leung, T.K.; Lai, J.S.-M.; Chan, J.C.-H. Evaluation of Adverse Effects of Topical Glaucoma Medications on Trabeculectomy Outcomes Using the Glaucoma Medications Intensity Index. Ophthalmol. Ther. 2022, 11, 387–401. [Google Scholar] [CrossRef]
- Kojima, S.; Inoue, T.; Kawaji, T.; Tanihara, H. Tear Fluid Signs Associated with Filtration Blebs, as Demonstrated by Three-Dimensional Anterior Segment Optical Coherence Tomography. Clin. Ophthalmol. 2014, 8, 767–772. [Google Scholar] [CrossRef] [Green Version]
- Neves Mendes, C.R.; Hida, R.Y.; Kasahara, N. Ocular Surface Changes in Eyes with Glaucoma Filtering Blebs. Curr. Eye Res. 2012, 37, 309–311. [Google Scholar] [CrossRef]
- Mathalone, N.; Marmor, S.; Rahat, M.A.; Lahat, N.; Oron, Y.; Geyer, O. MMP Expression in Leaking Filtering Blebs and Tears after Glaucoma Filtering Surgery. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1047–1055. [Google Scholar] [CrossRef]
- Chong, R.S.; Jiang, Y.Z.; Boey, P.Y.; Yu, S.J.; Htoon, H.M.; Aung, T.; Khaw, P.T.; Wong, T.T. Tear Cytokine Profile in Medicated Glaucoma Patients: Effect of Monocyte Chemoattractant Protein 1 on Early Posttrabeculectomy Outcome. Ophthalmology 2010, 117, 2353–2358. [Google Scholar] [CrossRef]
- Csősz, É.; Tóth, N.; Deák, E.; Csutak, A.; Tőzsér, J. Wound-Healing Markers Revealed by Proximity Extension Assay in Tears of Patients Following Glaucoma Surgery. Int. J. Mol. Sci. 2018, 19, 4096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csősz, É.; Deák, E.; Tóth, N.; Traverso, C.E.; Csutak, A.; Tőzsér, J. Comparative Analysis of Cytokine Profiles of Glaucomatous Tears and Aqueous Humour Reveals Potential Biomarkers for Trabeculectomy Complications. FEBS Open Bio 2019, 9, 1020–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgos-Blasco, B.; Vidal-Villegas, B.; Saenz-Frances, F.; Fernandez-Vigo, J.I.; Andres-Guerrero, V.; Espino, L.; Garcia-Feijoo, J.; Martinez-de-la-Casa, J.M. Cytokine Profile in Tear and Aqueous Humor of Primary Open-Angle Patients as a Prognostic Factor for Trabeculectomy Outcome. Eur. J. Ophthalmol. 2021, 11206721211055964. [Google Scholar] [CrossRef] [PubMed]
- Vaajanen, A.; Nättinen, J.; Aapola, U.; Gielen, F.; Uusitalo, H. The Effect of Successful Trabeculectomy on the Ocular Surface and Tear Proteomics—A Prospective Cohort Study with 1-Year Follow-Up. Acta Ophthalmol. 2021, 99, 160–170. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Lu, D.; Liang, J.; Xing, X.; Liu, A.; Zhao, S.; Li, X.; Ji, J. The Tear Fluid Mucin 5AC Change of Primary Angle-Closure Glaucoma Patients after Short-Term Medications and Phacotrabeculectomy. Mol. Vis. 2010, 16, 2342–2346. [Google Scholar]
- Pinazo-Durán, M.D.; García-Medina, J.J.; Bolarín, J.M.; Sanz-González, S.M.; Valero-Vello, M.; Abellán-Abenza, J.; Zanón-Moreno, V.; Moreno-Montañés, J. Computational Analysis of Clinical and Molecular Markers and New Theranostic Possibilities in Primary Open-Angle Glaucoma. J. Clin. Med. 2020, 9, 3032. [Google Scholar] [CrossRef]
| UniProt ID | Name | Symbol | Treatment-Naïve | Treated |
|---|---|---|---|---|
| P60709 | Actin beta | ACTB | ↑POAG [37] | |
| P63261 | Actin gamma 1 | ACTG1 | ↑POAG [37] | |
| P02768 | Albumin | ALB | ↑POAG [37] | |
| P04083 | Annexin A1 | ANXA1 | ↓POAG [43] | |
| P25311 | Alpha-2-glycoprotein, zinc-binding | AZGP1 | ↑POAG [37] | ↓POAG [43]; ↓PXG [43] |
| P61769 | Beta-2-microglobulin | B2M | ↑POAG [37] | |
| P23560 | Brain-derived neurotrophic factor | BDNF | ↓NTG [44] | |
| P37279 | Cellular communication network factor 2 | CCN2 | ↑PXG [45]; ↓PXF [45] | |
| P26441 | Ciliary neurotrophic factor | CNTF | ↓POAG [46] | |
| P20849 | Collagen type IX alpha 1 chain | COL9A1 | ↑PXF [39] | |
| P01037 | Cystatin SN | CST1 | ↓POAG [43]; ↓PXG [43] | |
| P09228 | Cystatin SA | CST2 | ↓POAG [43]; ↓PXG [43] | |
| P01036 | Cystatin S | CST4 | ↑POAG [37] | ↓POAG [43]; ↓PXG [43] |
| P02751 | Fibronectin 1 | FN1 | ↓PXG [40] | |
| P04792 | Heat shock protein family B (small) member 1 | HSPB1 | ↑POAG [37] | |
| P01876 | Immunoglobulin heavy constant alpha 1 | IGHA1 | ↑POAG [37] | ↓POAG [43]; ↓PXG [43] |
| P01877 | Immunoglobulin heavy constant alpha 2 | IGHA2 | ↑POAG [37] | ↓POAG [43] |
| P01857 | Immunoglobulin heavy constant gamma 1 | IGHG1 | ↑POAG [43] | |
| P01859 | Immunoglobulin heavy constant gamma 2 | IGHG2 | ↑POAG [43] | |
| P01861 | Immunoglobulin heavy constant gamma 4 | IGHG4 | ↑POAG [43] | |
| P01591 | Joining chain of multimeric IgA and IgM | JCHAIN | ↑POAG [37] | ↓POAG [43] |
| P01834 | Immunoglobulin kappa constant | IGKC | ↑POAG [37] | ↓POAG [43] |
| P37459 | Interleukin12A | IL12A | ↓POAG [41] | |
| P05231 | Interleukin 6 | IL6 | ↑POAG [47,48,49] * | |
| P04264 | Keratin 1 | KRT1 | ↓POAG [43]; ↓PXG [43] | |
| Q9GZZ8 | Lacritin | LACRT | ↓PXG [43] | |
| P31025 | Lipocalin 1 | LCN1 | ↑POAG [37] | ↓POAG [43]; ↓PXG [43] |
| P02788 | Lactotransferrin | LTF | ↑POAG [37] | |
| P61626 | Lysozyme | LYZ | ↑POAG [37] | ↓POAG [43]; ↓PXG [43] |
| P03956 | Matrix metallopeptidase 1 | MMP1 | ↑PXG [40]; ↑PXF [40] | |
| P08253 | Matrix metallopeptidase 2 | MMP2 | ↑POAG [42] | |
| P14780 | Matrix metallopeptidase 9 | MMP9 | ↑POAG [42]; ↑PACG [42]; ↑PXF [40,42] | ↑POAG [50] |
| P01840 | Polymeric immunoglobulin receptor | PIGR | ↑POAG [37] | ↓POAG [43] |
| P12273 | Prolactin induced protein | PIP | ↑POAG [37] | ↓POAG [43]; ↓PXG [43] |
| A5A3E0 | POTE ankyrin domain family member F | POTEE/POTEF | ↑POAG [37] | |
| Q6S8J3 | POTE ankyrin domain family member E | POTEE/POTEF | ↑POAG [37] | |
| P0CG38 | POTE ankyrin domain family member I | POTEI | ↑POAG [37] | |
| P0CG39 | POTE ankyrin domain family member J | POTEJ | ↑POAG [37] | |
| Q06830 | Peroxiredoxin 1 | PRDX1 | ↑POAG [37] | |
| Q99935 | Opiorphin prepropeptide | OPRPN | ↑POAG [37] | ↓PXG [43] |
| Q16378 | Proline rich 4 | PRR4 | ↑POAG [37] | ↓POAG [43]; ↓PXG [43] |
| P26447 | S100 calcium-binding protein A4 | S100A4 | ↑PXG [43] | |
| O75556 | Secretoglobin family 2A member 1 | SCGB2A1 | ↓POAG [43]; ↓PXG [43] | |
| P02787 | Transferrin | TF | ↑POAG [37] | ↑PXG [43] |
| P01137 | Transforming growth factor beta 1 | TGFB1 | ↑PXG [40]; ↑PXF [40] | |
| Q96DA0 | Zymogen granule protein 16B | ZG16B | ↑POAG [37] | ↓PXG [43] |
| UniProt ID | Name | Symbol | Tear Protein Changes for Glaucoma Patients Using… | ||
|---|---|---|---|---|---|
| Unspecified Topical Glaucoma Medication | Topical Glaucoma Medication with Prostaglandin Analogs | Topical Glaucoma Medication with Preservatives | |||
| P04083 | Annexin A1 | ANXA1 | ↓[43] | ||
| P25311 | Alpha-2-glycoprotein, zinc-binding | AZGP1 | ↓[43] | ||
| P13500 | C-C motif chemokine ligand 2 | CCL2 | ↑[76] | ||
| P10147 | C-C motif chemokine ligand 3 | CCL3 | ↓[77] | ||
| P01037 | Cystatin SN | CST1 | ↓[43] | ||
| P09228 | Cystatin SA | CST2 | ↓[43] | ||
| P01036 | Cystatin S | CST4 | ↓[43] | ||
| P09038 | Fibroblast growth factor 2 | FGF2 | ↑[77] | ↑[81] | |
| P09919 | Colony stimulating factor 3 | CSF3 | ↑[76] | ||
| P01876 | Immunoglobulin heavy constant alpha 1 | IGHA1 | ↓[43] | ||
| P01877 | Immunoglobulin heavy constant alpha 2 | IGHA2 | ↓[43] | ||
| P01857 | Immunoglobulin heavy constant gamma 1 | IGHG1 | ↑[43] | ||
| P01859 | Immunoglobulin heavy constant gamma 2 | IGHG2 | ↑[43] | ||
| P01861 | Immunoglobulin heavy constant gamma 4 | IGHG4 | ↑[43] | ||
| P01834 | Immunoglobulin kappa constant | IGKC | ↓[43] | ||
| P01591 | Joining chain of multimeric IgA and IgM | JCHAIN | ↓[43] | ||
| P01579 | Interferon gamma | IFNG | ↑[76] | ||
| P01584 | Interleukin 1 beta | IL1B | ↑[78] | ↑[76,82] * | |
| P60568 | Interleukin 2 | IL2 | ↑[76,81] | ||
| P05112 | Interleukin 4 | IL4 | ↑[77] | ↑[76] | |
| P05113 | Interleukin 5 | IL5 | ↑[76,81] | ||
| P05231 | Interleukin 6 | IL6 | ↑[78] | ↑[76] | |
| P13232 | Interleukin 7 | IL7 | ↑[76] | ||
| P10145 | Interleukin 8 | IL8 | ↑[76] | ||
| P22301 | Interleukin 10 | IL10 | ↑[76,81] | ||
| P29459 | Interleukin 12A | IL12A | ↑[77] | ↑[76,81] | |
| P35225 | Interleukin 13 | IL13 | ↑[76,81] | ||
| P40933 | Interleukin 15 | IL15 | ↑[77] | ↑[81] | |
| Q16552 | Interleukin 17A | IL17A | ↑[76,81] | ||
| P04264 | Keratin 1 | KRT1 | ↓[43] | ||
| Q9GZZ8 | Lacritin | LACRT | ↓[43] | ||
| P31025 | Lipocalin 1 | LCN1 | ↓[43] | ||
| P02788 | Lactotransferrin | LTF | ↓[43] | ||
| P61626 | Lysozyme | LYZ | ↓[43] | ||
| P03956 | Matrix metallopeptidase 1 | MMP1 | ↑[78] | ||
| P08254 | Matrix metallopeptidase 3 | MMP3 | ↑[78] | ||
| P14780 | Matrix metallopeptidase 9 | MMP9 | ↑[78,79] | ↑[79] | |
| Q99935 | Opiorphin prepropeptide | OPRPN | ↓[43] | ||
| P01127 | Platelet-derived growth factor subunit B | PDGFB | ↑[81] | ||
| P01833 | Polymeric immunoglobulin receptor | PIGR | ↓[43] | ||
| P12273 | Prolactin induced protein | PIP | ↓[43] | ||
| Q16378 | Proline rich 4 | PRR4 | ↓[80] | ↓[43] | |
| P26447 | S100 calcium-binding protein A4 | S100A4 | ↑[43] | ||
| P05109 | S100 calcium-binding protein A8 | S100A8 | ↑[80] | ||
| P06702 | S100 calcium-binding protein A9 | S100A9 | ↑[80] | ||
| O75556 | Secretoglobin family 2A member 1 | SCGB2A1 | ↑[80] | ↓[43] | |
| P02787 | Transferrin | TF | ↑[43] | ||
| P01033 | TIMP metallopeptidase inhibitor 1 | TIMP1 | ↓[78] | ||
| P16035 | TIMP metallopeptidase inhibitor 2 | TIMP2 | ↓[78] | ||
| P01375 | Tumor necrosis factor | TNF | ↑[76,81] | ||
| P15692 | Vascular endothelial growth factor A | VEGFA | ↑[77] | ||
| P63104 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta | YWHAZ | ↑[80] | ||
| Q96DA0 | Zymogen granule protein 16B | ZG16B | ↓[43] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nättinen, J.; Aapola, U.; Nukareddy, P.; Uusitalo, H. Clinical Tear Fluid Proteomics—A Novel Tool in Glaucoma Research. Int. J. Mol. Sci. 2022, 23, 8136. https://doi.org/10.3390/ijms23158136
Nättinen J, Aapola U, Nukareddy P, Uusitalo H. Clinical Tear Fluid Proteomics—A Novel Tool in Glaucoma Research. International Journal of Molecular Sciences. 2022; 23(15):8136. https://doi.org/10.3390/ijms23158136
Chicago/Turabian StyleNättinen, Janika, Ulla Aapola, Praveena Nukareddy, and Hannu Uusitalo. 2022. "Clinical Tear Fluid Proteomics—A Novel Tool in Glaucoma Research" International Journal of Molecular Sciences 23, no. 15: 8136. https://doi.org/10.3390/ijms23158136






