Regenerative Strategies for Retinal Neurons: Novel Insights in Non-Mammalian Model Organisms
Abstract
:1. Introduction
2. Alternative Organism Models for Retina Neuroregeneration
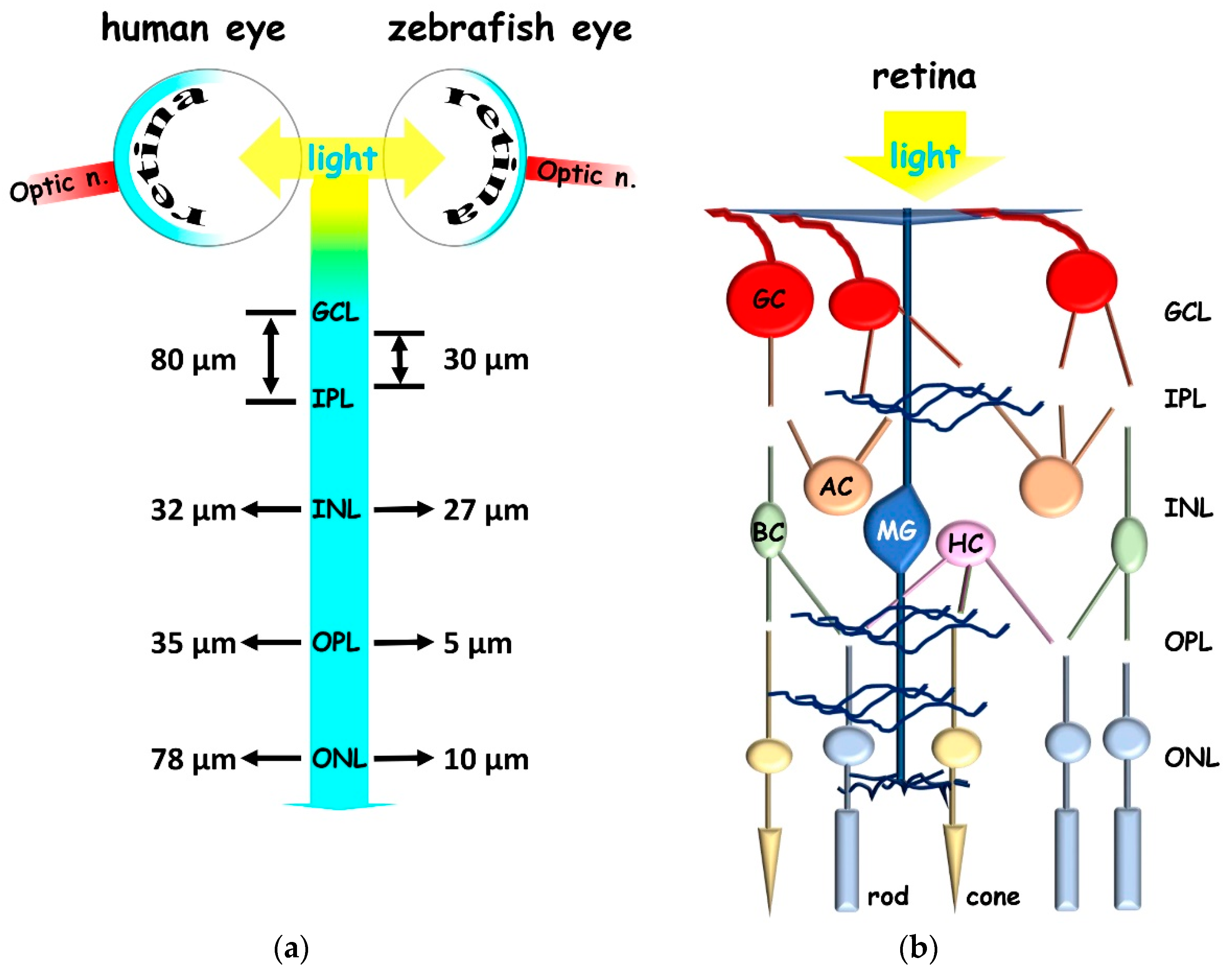
2.1. Zebrafish to Gain Insight in Vertebrate Retina
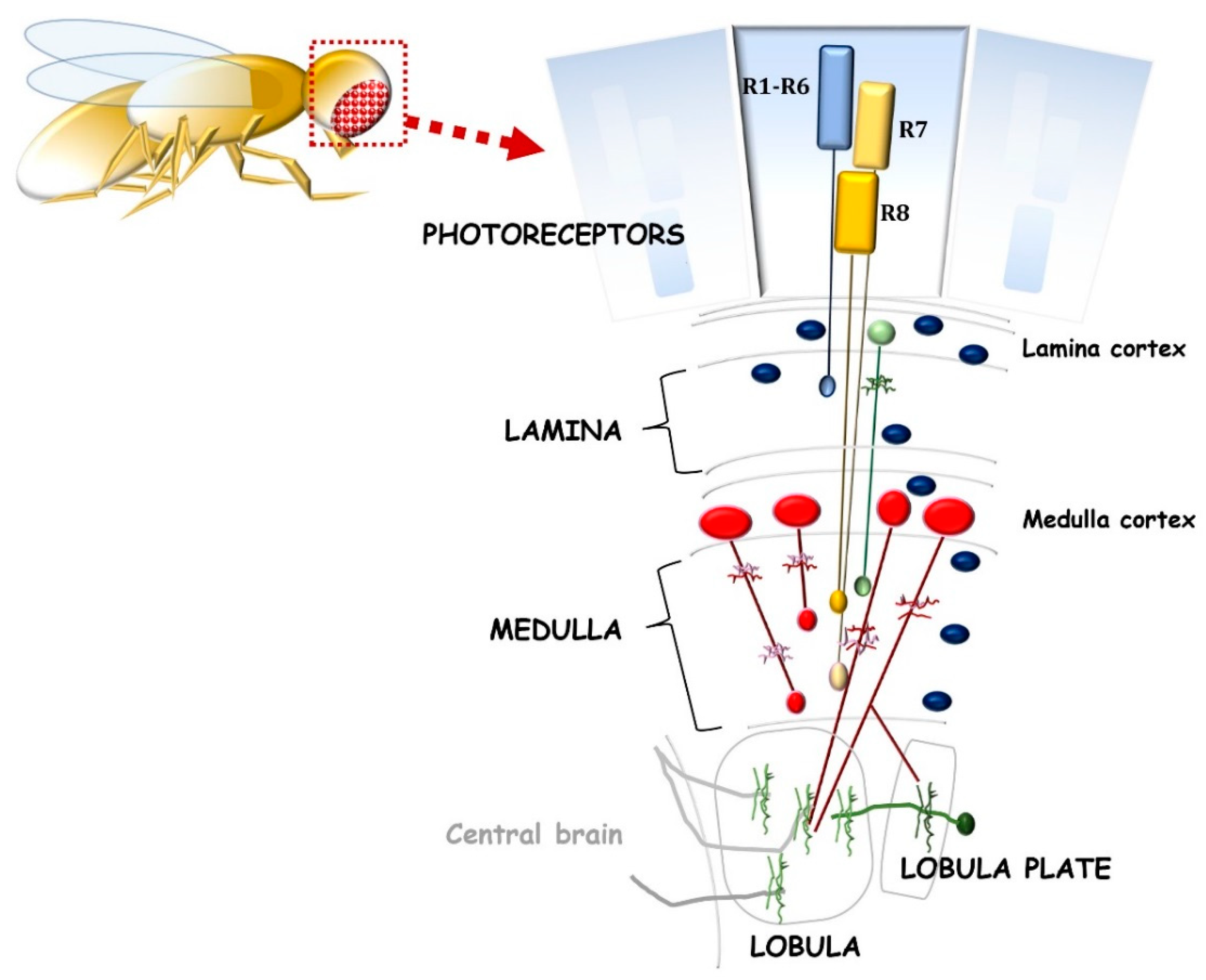
2.2. The Opportunity of D. melanogaster for Neuroregenerative Strategies
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maneu, V.; Lax, P.; Cuenca, N. Current and future therapeutic strategies for the treatment of retinal neurodegenerative diseases. Neural Regen. Res. 2022, 17, 103–104. [Google Scholar] [PubMed]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving neurodegeneration: Common mechanisms and strategies for new treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; You, Y. Editorial: Retinal Changes in Neurological Diseases. Front. Neurosci. 2021, 15, 813044. [Google Scholar] [CrossRef]
- Chan, J.W.; Chan, N.C.Y.; Sadun, A.A. Glaucoma as Neurodegeneration in the Brain. Eye Brain 2021, 13, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Alber, J.; Alt, C.; Bain, L.J.; Bouma, B.E.; Bouwman, F.H.; DeBuc, D.C.; Campbell, M.C.W.; Carrillo, M.C.; Chew, E.Y.; et al. Retinal imaging in Alzheimer’s and neurodegenerative diseases. Alzheimers Dement. 2021, 17, 103–111. [Google Scholar] [CrossRef]
- Andries, L.; Masin, L.; Salinas-Navarro, M.; Zaunz, S.; Claes, M.; Bergmans, S.; Brouwers, V.; Lefevere, E.; Verfaillie, C.; Movahedi, K.; et al. MMP2 Modulates Inflammatory Response during Axonal Regeneration in the Murine Visual System. Cells 2021, 10, 1672. [Google Scholar] [CrossRef] [PubMed]
- Schnichels, S.; Paquet-Durand, F.; Loscher, M.; Tsai, T.; Hurst, J.; Joachim, S.C.; Klettner, A. Retina in a dish: Cell cultures, retinal explants and animal models for common diseases of the retina. Prog. Retin. Eye Res. 2021, 81, 100880. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.; Fietz, A.; Tsai, T.; Joachim, S.C.; Schnichels, S. Organ Cultures for Retinal Diseases. Front. Neurosci. 2020, 14, 583392. [Google Scholar] [CrossRef] [PubMed]
- Schnichels, S.; Kiebler, T.; Hurst, J.; Maliha, A.M.; Loscher, M.; Dick, H.B.; Bartz-Schmidt, K.U.; Joachim, S.C. Retinal Organ Cultures as Alternative Research Models. Altern. Lab. Anim. 2019, 47, 19–29. [Google Scholar] [CrossRef]
- Chhetri, J.; Jacobson, G.; Gueven, N. Zebrafish—On the move towards ophthalmological research. Eye 2014, 28, 367–380. [Google Scholar] [CrossRef] [Green Version]
- Stella, S.L., Jr.; Geathers, J.S.; Weber, S.R.; Grillo, M.A.; Barber, A.J.; Sundstrom, J.M.; Grillo, S.L. Neurodegeneration, Neuroprotection and Regeneration in the Zebrafish Retina. Cells 2021, 10, 633. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Tracey-White, D.; Webster, A.; Moosajee, M. The zebrafish eye-a paradigm for investigating human ocular genetics. Eye 2017, 31, 68–86. [Google Scholar] [CrossRef] [Green Version]
- Baden, T.; Euler, T.; Berens, P. Understanding the retinal basis of vision across species. Nat. Rev. Neurosci. 2020, 21, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Gallina, D.; Todd, L.; Fischer, A.J. A comparative analysis of Muller glia-mediated regeneration in the vertebrate retina. Exp. Eye Res. 2014, 123, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Singh, N.K. The Role of Inflammation in Retinal Neurodegeneration and Degenerative Diseases. Int. J. Mol. Sci. 2021, 23, 386. [Google Scholar] [CrossRef] [PubMed]
- Leach, L.L.; Hanovice, N.J.; George, S.M.; Gabriel, A.E.; Gross, J.M. The immune response is a critical regulator of zebrafish retinal pigment epithelium regeneration. Proc. Natl. Acad. Sci. USA 2021, 118, e2017198118. [Google Scholar] [CrossRef]
- Hammer, J.; Roppenack, P.; Yousuf, S.; Schnabel, C.; Weber, A.; Zoller, D.; Koch, E.; Hans, S.; Brand, M. Visual Function is Gradually Restored During Retina Regeneration in Adult Zebrafish. Front. Cell Dev. Biol. 2021, 9, 831322. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.C.; Gurdziel, K.; Thummel, R. A Comparative Analysis of Gene and Protein Expression Throughout a Full 28-Day Retinal Regeneration Time-Course in Adult Zebrafish. Front. Cell Dev. Biol. 2021, 9, 741514. [Google Scholar] [CrossRef] [PubMed]
- Endeman, D.; Klaassen, L.J.; Kamermans, M. Action spectra of zebrafish cone photoreceptors. PLoS ONE 2013, 8, e68540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Luoden, A.; Huang, X.; Chen, X.; Xu, H. Muller Glia-Mediated Retinal Regeneration. Mol. Neurobiol. 2021, 58, 2342–2361. [Google Scholar] [CrossRef]
- Powell, C.; Grant, A.R.; Cornblath, E.; Goldman, D. Analysis of DNA methylation reveals a partial reprogramming of the Muller glia genome during retina regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 19814–19819. [Google Scholar] [CrossRef] [Green Version]
- Van Dyck, A.; Bollaerts, I.; Beckers, A.; Vanhunsel, S.; Glorian, N.; van Houcke, J.; van Ham, T.J.; De Groef, L.; Andries, L.; Moons, L. Muller glia-myeloid cell crosstalk accelerates optic nerve regeneration in the adult zebrafish. Glia 2021, 69, 1444–1463. [Google Scholar] [CrossRef]
- Martins, R.R.; Zamzam, M.; Tracey-White, D.; Moosajee, M.; Thummel, R.; Henriques, C.M.; MacDonald, R.B. Muller Glia maintain their regenerative potential despite degeneration in the aged zebrafish retina. Aging Cell 2022, 21, e13597. [Google Scholar] [CrossRef]
- Yang, S.G.; Wang, X.W.; Qian, C.; Zhou, F.Q. Reprogramming neurons for regeneration: The fountain of youth. Prog. Neurobiol. 2022, 214, 102284. [Google Scholar] [CrossRef]
- Wang, F.; Cheng, L.; Zhang, X. Reprogramming Glial Cells into Functional Neurons for Neuro-regeneration: Challenges and Promise. Neurosci. Bull. 2021, 37, 1625–1636. [Google Scholar] [CrossRef]
- Singh, M.S.; Park, S.S.; Albini, T.A.; Canto-Soler, M.V.; Klassen, H.; MacLaren, R.E.; Takahashi, M.; Nagiel, A.; Schwartz, S.D.; Bharti, K. Retinal stem cell transplantation: Balancing safety and potential. Prog. Retin. Eye Res. 2020, 75, 100779. [Google Scholar] [CrossRef]
- Nair, D.S.R.; Thomas, B.B. Stem Cell-based Treatment Strategies for Degenerative Diseases of the Retina. Curr. Stem. Cell Res. Ther. 2022, 17, 214–225. [Google Scholar] [CrossRef]
- Salman, A.; McClements, M.E.; MacLaren, R.E. Insights on the Regeneration Potential of Muller Glia in the Mammalian Retina. Cells 2021, 10, 1957. [Google Scholar] [CrossRef]
- Perkins, B.D. Zebrafish models of inherited retinal dystrophies. J. Transl. Genet. Genom. 2022, 6, 95–110. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, B. Critical Examination of Muller Glia-Derived in vivo Neurogenesis in the Mouse Retina. Front. Cell Dev. Biol. 2022, 10, 830382. [Google Scholar] [CrossRef]
- Vanhunsel, S.; Beckers, A.; Moons, L. Designing neuroreparative strategies using aged regenerating animal models. Ageing Res. Rev. 2020, 62, 101086. [Google Scholar] [CrossRef]
- Mitchell, D.M.; Lovel, A.G.; Stenkamp, D.L. Dynamic changes in microglial and macrophage characteristics during degeneration and regeneration of the zebrafish retina. J. Neuroinflamm. 2018, 15, 163. [Google Scholar] [CrossRef] [Green Version]
- Neriec, N.; Desplan, C. From the Eye to the Brain: Development of the Drosophila Visual System. Curr. Top. Dev. Biol. 2016, 116, 247–271. [Google Scholar]
- Malin, J.; Desplan, C. Neural specification, targeting, and circuit formation during visual system assembly. Proc. Natl. Acad. Sci. USA 2021, 118, e2101823118. [Google Scholar] [CrossRef]
- Fox, D.T.; Cohen, E.; Smith-Bolton, R. Model systems for regeneration: Drosophila. Development 2020, 147, dev173781. [Google Scholar] [CrossRef]
- Catalani, E.; Silvestri, F.; Cervia, D. A Drosophila perspective on retina functions and dysfunctions. Neural Regen. Res. 2022, 17, 341–343. [Google Scholar]
- Catalani, E.; Silvestri, F.; Bongiorni, S.; Taddei, A.R.; Fanelli, G.; Rinalducci, S.; De Palma, C.; Perrotta, C.; Prantera, G.; Cervia, D. Retinal damage in a new model of hyperglycemia induced by high-sucrose diets. Pharmacol. Res. 2021, 166, 105488. [Google Scholar] [CrossRef]
- Catalani, E.; Fanelli, G.; Silvestri, F.; Cherubini, A.; Del Quondam, S.; Bongiorni, S.; Taddei, A.R.; Ceci, M.; De Palma, C.; Perrotta, C.; et al. Nutraceutical Strategy to Counteract Eye Neurodegeneration and Oxidative Stress in Drosophila melanogaster Fed with High-Sugar Diet. Antioxidants 2021, 10, 1197. [Google Scholar] [CrossRef]
- Catalani, E.; Bongiorni, S.; Taddei, A.R.; Mezzetti, M.; Silvestri, F.; Coazzoli, M.; Zecchini, S.; Giovarelli, M.; Perrotta, C.; De Palma, C.; et al. Defects of full-length dystrophin trigger retinal neuron damage and synapse alterations by disrupting functional autophagy. Cell. Mol. Life Sci. 2021, 78, 1615–1636. [Google Scholar] [CrossRef]
- Kremer, M.C.; Jung, C.; Batelli, S.; Rubin, G.M.; Gaul, U. The glia of the adult Drosophila nervous system. Glia 2017, 65, 606–638. [Google Scholar] [CrossRef] [Green Version]
- Chotard, C.; Salecker, I. Glial cell development and function in the Drosophila visual system. Neuron Glia Biol. 2007, 3, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Charlton-Perkins, M.A.; Sendler, E.D.; Buschbeck, E.K.; Cook, T.A. Multifunctional glial support by Semper cells in the Drosophila retina. PLoS Genet. 2017, 13, e1006782. [Google Scholar] [CrossRef]
- Ahmed-de-Prado, S.; Baonza, A. Drosophila as a Model System to Study Cell Signaling in Organ Regeneration. Biomed Res. Int. 2018, 2018, 7359267. [Google Scholar] [CrossRef]
- Klemm, J.; Stinchfield, M.J.; Harris, R.E. Necrosis-induced apoptosis promotes regeneration in Drosophila wing imaginal discs. Genetics 2021, 219, iyab144. [Google Scholar] [CrossRef]
- Kashio, S.; Miura, M. Kynurenine Metabolism in the Fat Body Non-autonomously Regulates Imaginal Disc Repair in Drosophila. iScience 2020, 23, 101738. [Google Scholar] [CrossRef]
- Walden, E.L.; Li, S. Metabolic reprogramming of glial cells as a new target for central nervous system axon regeneration. Neural Regen. Res. 2022, 17, 997–998. [Google Scholar]
- Li, F.; Sami, A.; Noristani, H.N.; Slattery, K.; Qiu, J.; Groves, T.; Wang, S.; Veerasammy, K.; Chen, Y.X.; Morales, J.; et al. Glial Metabolic Rewiring Promotes Axon Regeneration and Functional Recovery in the Central Nervous System. Cell Metab. 2020, 32, 767–785.e767. [Google Scholar] [CrossRef]
- Harrison, N.J.; Connolly, E.; Gascon Gubieda, A.; Yang, Z.; Altenhein, B.; Losada Perez, M.; Moreira, M.; Sun, J.; Hidalgo, A. Regenerative neurogenic response from glia requires insulin-driven neuron-glia communication. eLife 2021, 10, e58756. [Google Scholar] [CrossRef]
- Losada-Perez, M.; Garcia-Guillen, N.; Casas-Tinto, S. A novel injury paradigm in the central nervous system of adult Drosophila: Molecular, cellular and functional aspects. Dis. Model Mech. 2021, 14, dmm044669. [Google Scholar] [CrossRef]
- Kitatani, Y.; Tezuka, A.; Hasegawa, E.; Yanagi, S.; Togashi, K.; Tsuji, M.; Kondo, S.; Parrish, J.Z.; Emoto, K. Drosophila miR-87 promotes dendrite regeneration by targeting the transcriptional repressor Tramtrack69. PLoS Genet. 2020, 16, e1008942. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, D.; Farrelly, O.; Miles, L.; Li, F.; Kim, S.E.; Lo, T.Y.; Wang, F.; Li, T.; Thompson-Peer, K.L.; et al. The Mechanosensitive Ion Channel Piezo Inhibits Axon Regeneration. Neuron 2019, 102, 373–389.e376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crocker, K.L.; Marischuk, K.; Rimkus, S.A.; Zhou, H.; Yin, J.C.P.; Boekhoff-Falk, G. Neurogenesis in the adult Drosophila brain. Genetics 2021, 219, iyab092. [Google Scholar] [CrossRef] [PubMed]
- Crocker, K.L.; Ahern-Djamali, S.; Boekhoff-Falk, G. Stimulating and Analyzing Adult Neurogenesis in the Drosophila Central Brain. J. Vis. Exp. 2021, 176, e63182. [Google Scholar] [CrossRef]
- Fernandez-Hernandez, I.; Rhiner, C.; Moreno, E. Adult neurogenesis in Drosophila. Cell Rep. 2013, 3, 1857–1865. [Google Scholar] [CrossRef] [Green Version]
- Ramon-Canellas, P.; Peterson, H.P.; Morante, J. From Early to Late Neurogenesis: Neural Progenitors and the Glial Niche from a Fly’s Point of View. Neuroscience 2019, 399, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Janovjak, H.; Kleinlogel, S. Optogenetic neuroregeneration. Neural Regen. Res. 2022, 17, 1468–1470. [Google Scholar] [CrossRef]
- Wang, Q.; Fan, H.; Li, F.; Skeeters, S.S.; Krishnamurthy, V.V.; Song, Y.; Zhang, K. Optical control of ERK and AKT signaling promotes axon regeneration and functional recovery of PNS and CNS in Drosophila. eLife 2020, 9, e57395. [Google Scholar] [CrossRef]
- Ingles-Prieto, A.; Furthmann, N.; Crossman, S.H.; Tichy, A.M.; Hoyer, N.; Petersen, M.; Zheden, V.; Biebl, J.; Reichhart, E.; Gyoergy, A.; et al. Optogenetic delivery of trophic signals in a genetic model of Parkinson’s disease. PLoS Genet. 2021, 17, e1009479. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalani, E.; Cherubini, A.; Del Quondam, S.; Cervia, D. Regenerative Strategies for Retinal Neurons: Novel Insights in Non-Mammalian Model Organisms. Int. J. Mol. Sci. 2022, 23, 8180. https://doi.org/10.3390/ijms23158180
Catalani E, Cherubini A, Del Quondam S, Cervia D. Regenerative Strategies for Retinal Neurons: Novel Insights in Non-Mammalian Model Organisms. International Journal of Molecular Sciences. 2022; 23(15):8180. https://doi.org/10.3390/ijms23158180
Chicago/Turabian StyleCatalani, Elisabetta, Agnese Cherubini, Simona Del Quondam, and Davide Cervia. 2022. "Regenerative Strategies for Retinal Neurons: Novel Insights in Non-Mammalian Model Organisms" International Journal of Molecular Sciences 23, no. 15: 8180. https://doi.org/10.3390/ijms23158180
APA StyleCatalani, E., Cherubini, A., Del Quondam, S., & Cervia, D. (2022). Regenerative Strategies for Retinal Neurons: Novel Insights in Non-Mammalian Model Organisms. International Journal of Molecular Sciences, 23(15), 8180. https://doi.org/10.3390/ijms23158180





