Functionalized 3D-Printed ST2/Gelatin Methacryloyl/Polcaprolactone Scaffolds for Enhancing Bone Regeneration with Vascularization
Abstract
:1. Introduction
2. Results
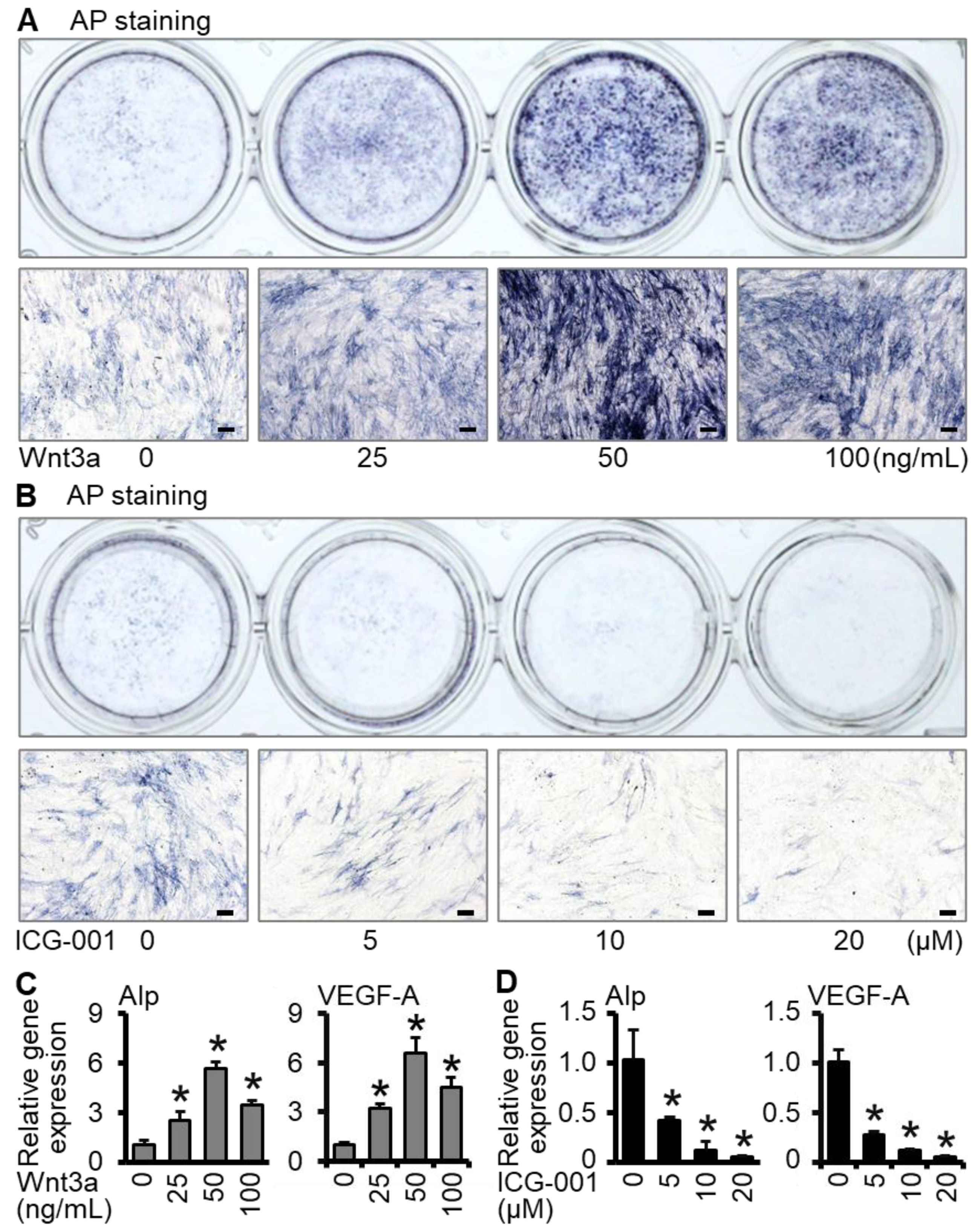
2.1. Wnt3a Promotes Osteogenic Differentiation and VEGF-A Expression of ST2 Cells in Two-Dimensional Culture
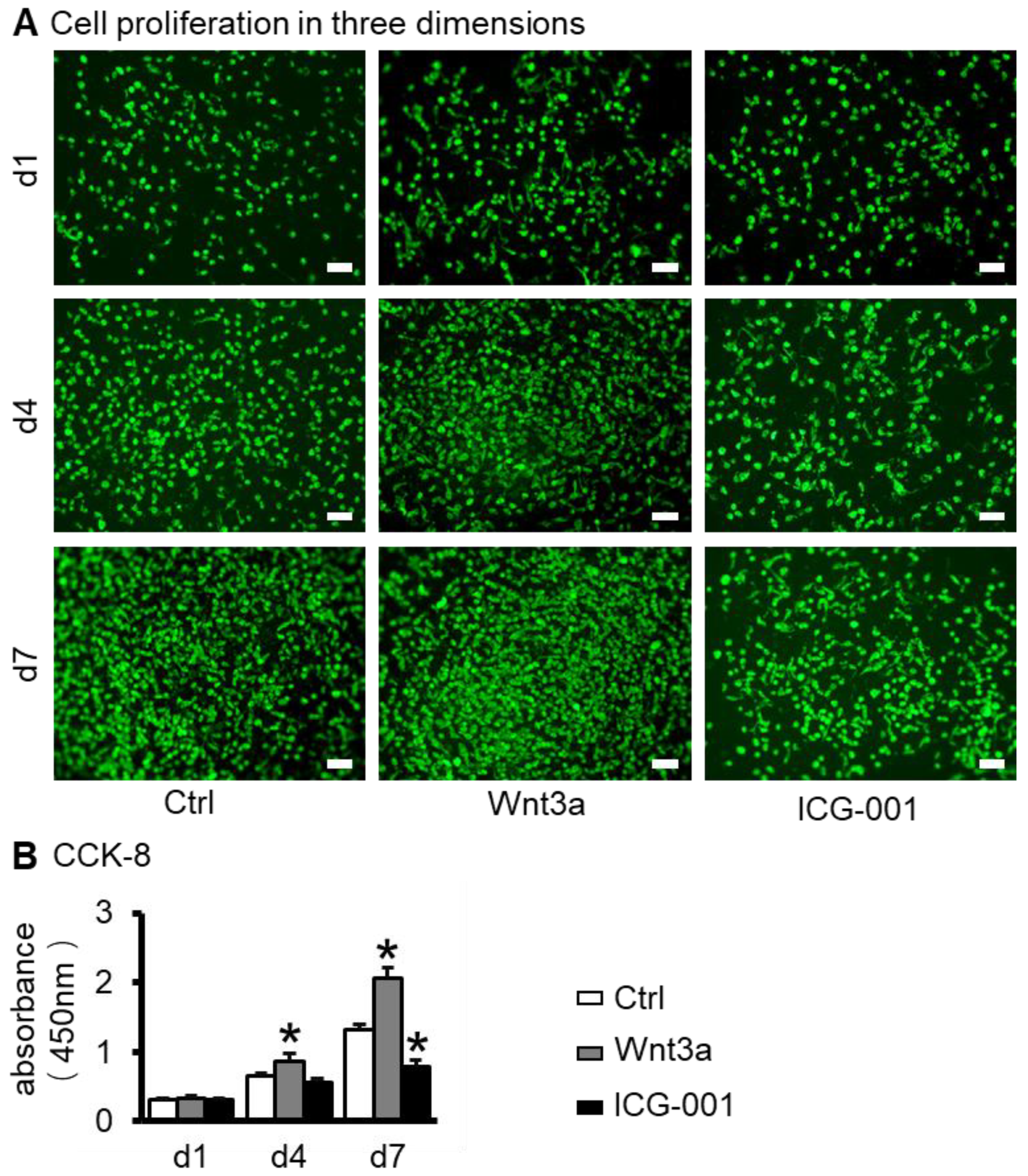
2.2. Preparation of ST2/GelMA/PCL Scaffolds by 3D Bioprinting and Their Biocompatibility Testing
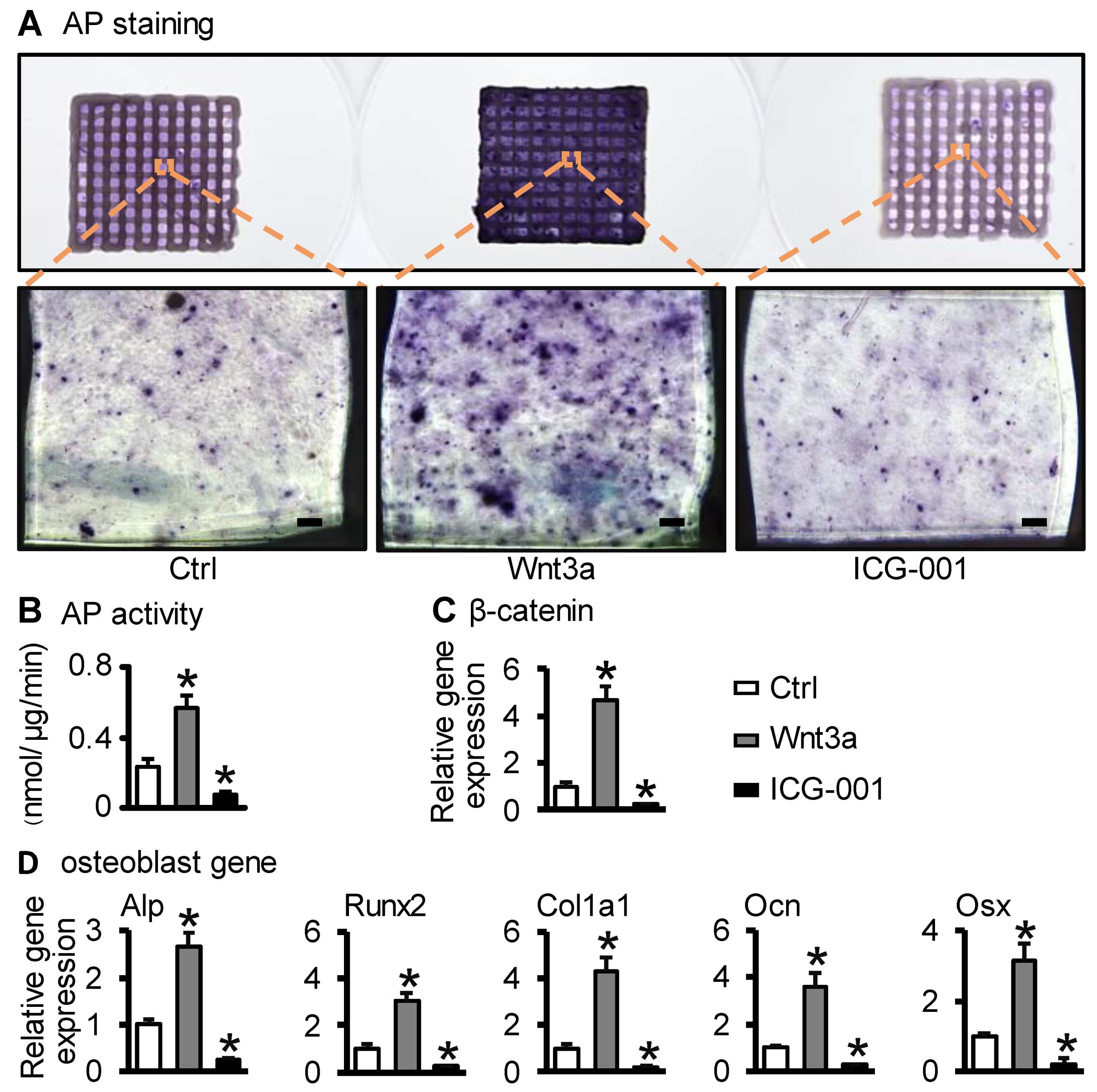
2.3. Wnt3a Pretreating ST2/GelMA/PCL Bone Repair Scaffold Enhanced Osteogenic Differentiation
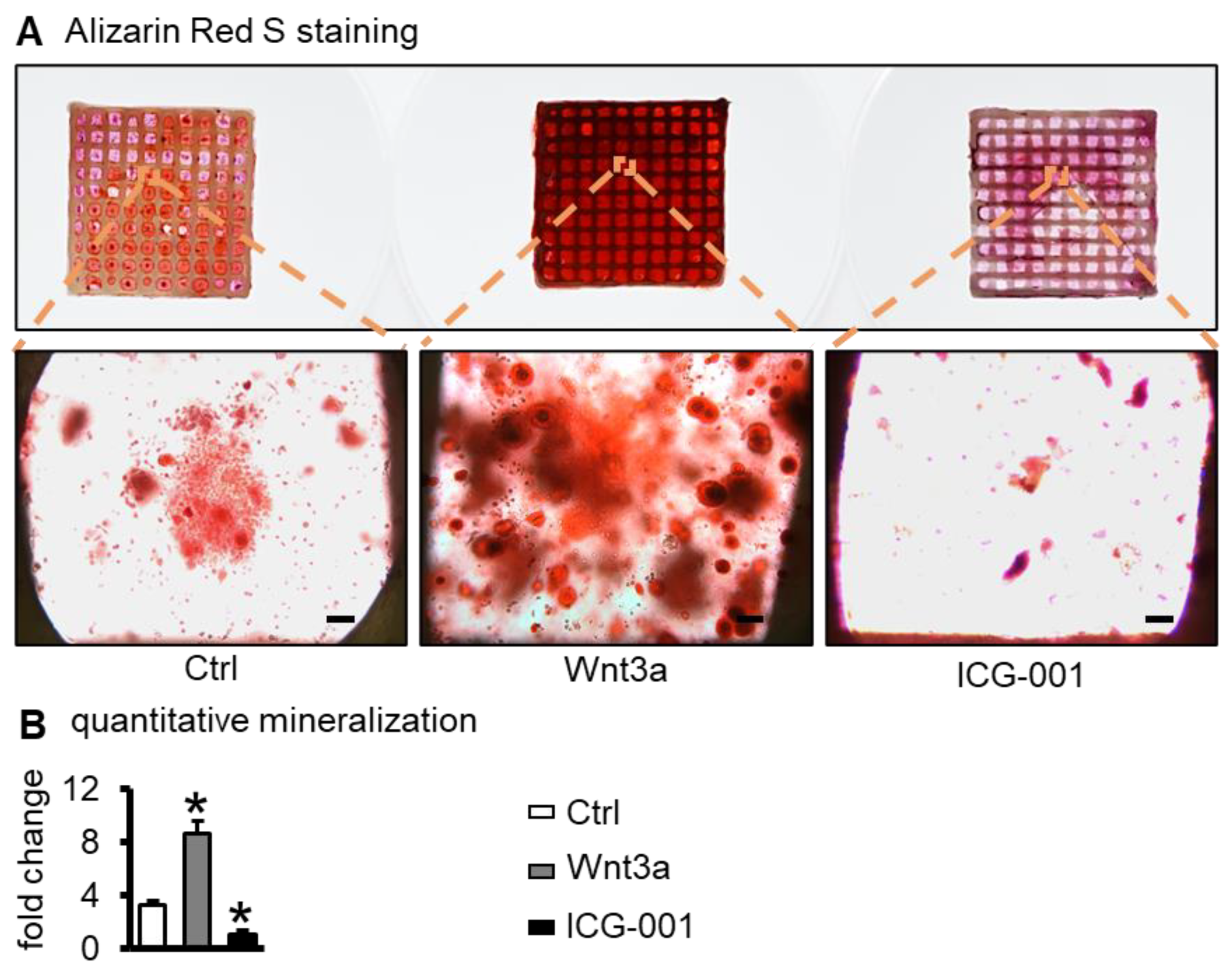
2.4. Wnt3a Pretreating Enhances the Mineralization of ST2 Cells in Bone Repair Scaffolds
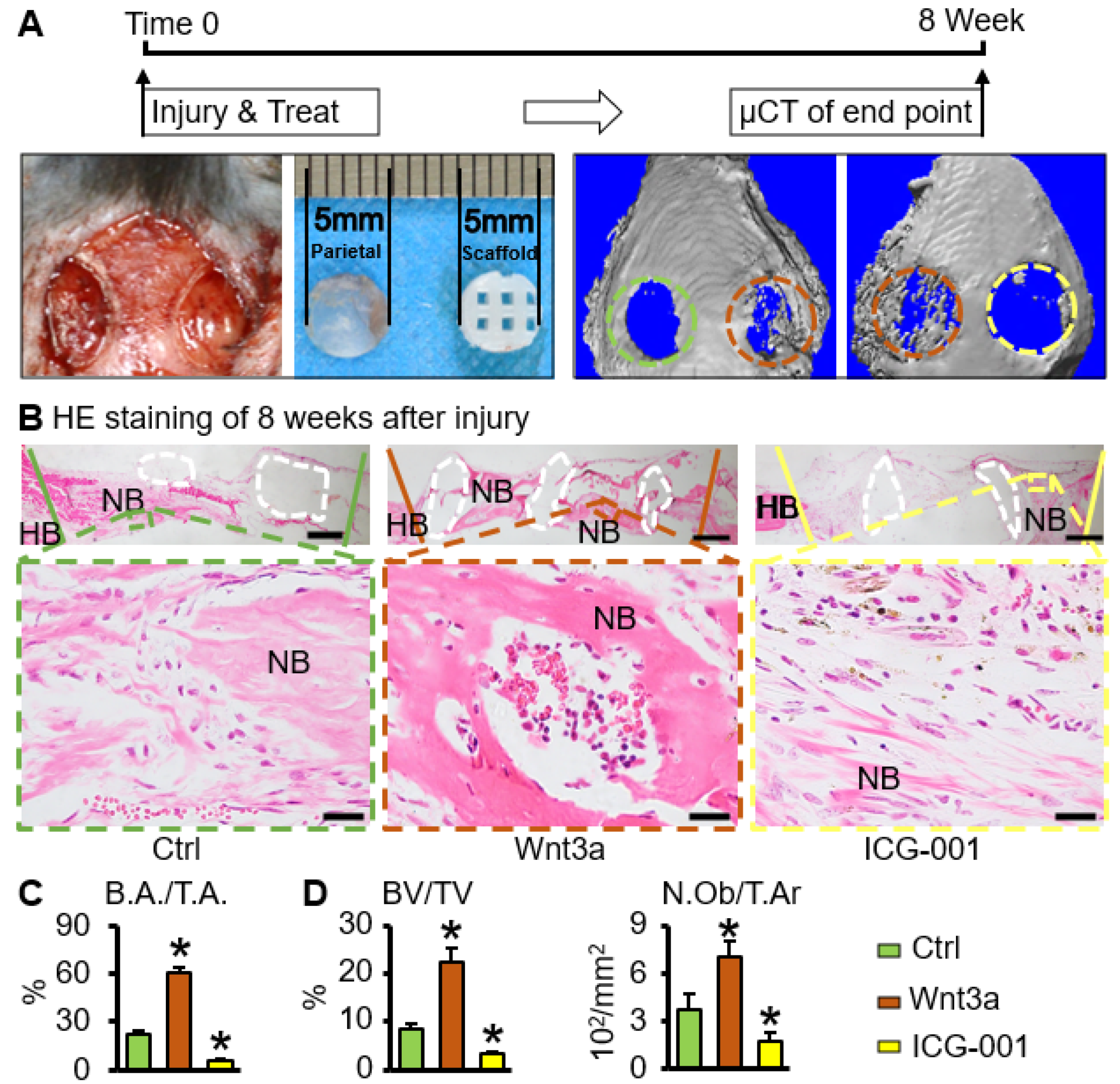
2.5. Wnt3a Pretreated Bone Repair Scaffold Accelerates Critical-Size Bone Defect Repair
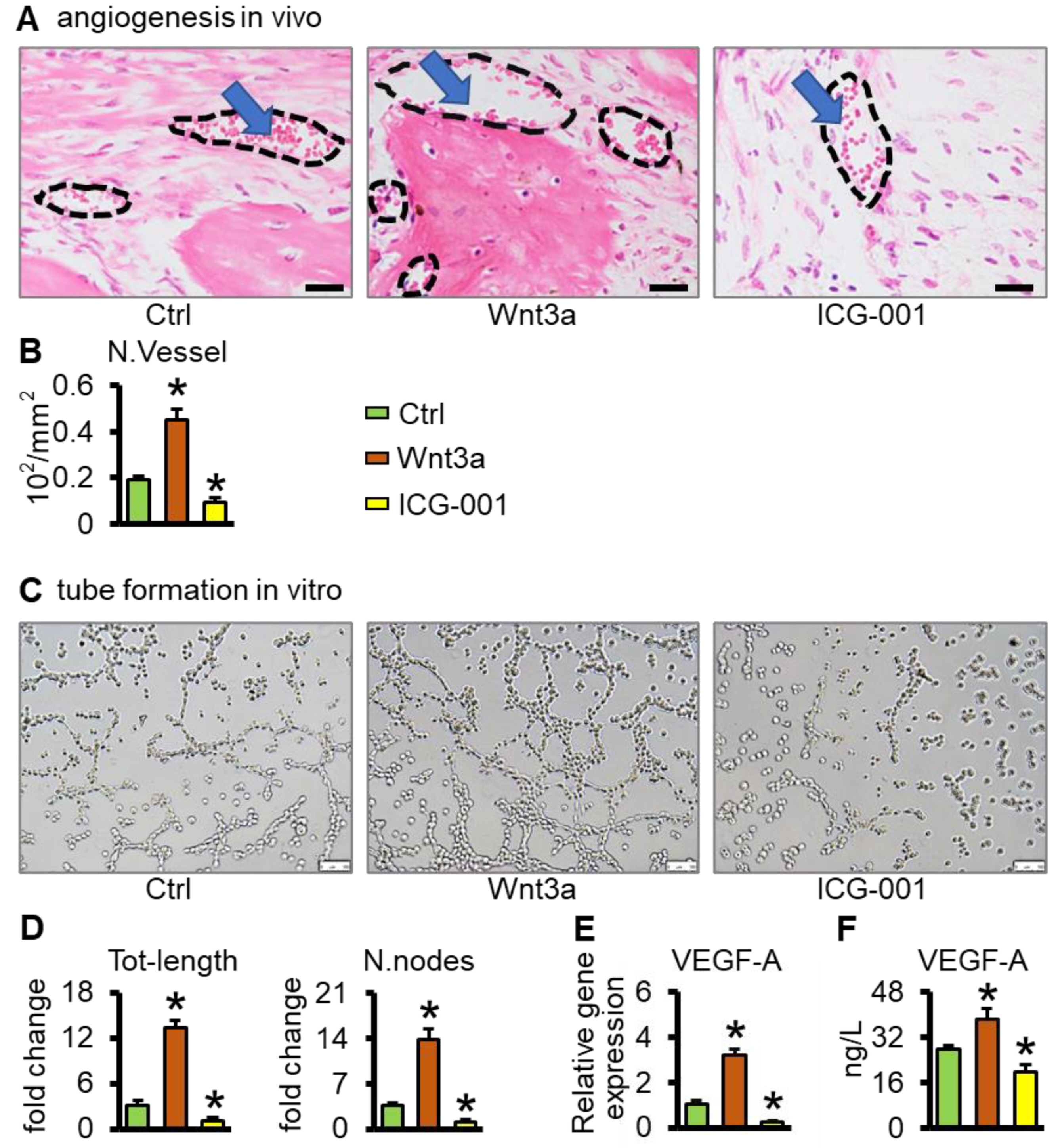
2.6. Wnt3a Pretreated Bone Repair Scaffolds Promote Vascularization
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. 3D-Bioprinting and Culture of ST2/GelMA/PCL Scaffolds
4.3. Cell Viability
4.4. Cell Proliferation
4.5. Cell Osteogenic Differentiation Assays
4.6. Mineralization Assay
4.7. Animal Experiments
4.8. Analysis of Bone Regeneration In Vivo
4.9. Tubular Formation Experiment
4.10. ELISA
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue Engineering and Cell-Based Therapies for Fractures and Bone Defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjelić, D.; Finšgar, M. The Role of Growth Factors in Bioactive Coatings. Pharmaceutics 2021, 13, 1083. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bai, J.; Wang, Q.; Ge, G.; Lin, J.; Xu, N.; Xu, C.; Xu, Y.; Wang, Y.; Geng, D. Inhibition of protein phosphatase 2A attenuates titanium-particle induced suppression of bone formation. Int. J. Biol. Macromol. 2020, 142, 142–151. [Google Scholar] [CrossRef]
- Nambiar, J.; Jana, S.; Nandi, S.K. Strategies for Enhancing Vascularization of Biomaterial-Based Scaffold in Bone Regeneration. Chem. Rec. 2022, 22, e202200008. [Google Scholar] [CrossRef]
- Kolan, K.C.R.; Li, J.; Roberts, S.; Semon, J.A.; Park, J.; Day, D.E.; Leu, M.C. Near-field electrospinning of a polymer/bioactive glass composite to fabricate 3D biomimetic structures. Int. J. Bioprint. 2019, 5, 163. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Hanim, Y.U.; Sipaut, C.S.; Jan, N.B.A.; Arshad, S.E.; How, S.E. Fabrication of Hydroxyapatite with Bioglass Nanocomposite for Human Wharton’s-Jelly-Derived Mesenchymal Stem Cell Growing Substrate. Int. J. Mol. Sci. 2021, 22, 9637. [Google Scholar] [CrossRef]
- Wu, Y.-H.A.; Chiu, Y.-C.; Lin, Y.-H.; Ho, C.-C.; Shie, M.-Y.; Chen, Y.-W. 3D-Printed Bioactive Calcium Silicate/Poly-ε-Caprolactone Bioscaffolds Modified with Biomimetic Extracellular Matrices for Bone Regeneration. Int. J. Mol. Sci. 2019, 20, 942. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-W.; Shen, Y.-F.; Ho, C.-C.; Yu, J.; Wu, Y.-H.A.; Wang, K.; Shih, C.-T.; Shie, M.-Y. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater. Sci. Eng. C 2018, 91, 679–687. [Google Scholar] [CrossRef]
- Fitzpatrick, V.; Martín-Moldes, Z.; Deck, A.; Torres-Sanchez, R.; Valat, A.; Cairns, D.; Li, C.; Kaplan, D.L. Functionalized 3D-printed silk-hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization. Biomaterials 2021, 276, 120995. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Seims, K.B.; Hunt, N.K.; Chow, L.W. Strategies to Control or Mimic Growth Factor Activity for Bone, Cartilage, and Osteochondral Tissue Engineering. Bioconjug. Chem. 2021, 32, 861–878. [Google Scholar] [CrossRef]
- Gillman, C.E.; Jayasuriya, A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C 2021, 130, 112466. [Google Scholar] [CrossRef]
- El Bialy, I.; Jiskoot, W.; Reza Nejadnik, M. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef] [Green Version]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Controlled release strategies for bone, cartilage, and osteochondral engineering--Part II: Challenges on the evolution from single to multiple bioactive factor delivery. Tissue Eng. Part B Rev. 2013, 19, 327–352. [Google Scholar] [CrossRef] [Green Version]
- Sreekumar, V.; Aspera-Werz, R.H.; Tendulkar, G.; Reumann, M.K.; Freude, T.; Breitkopf-Heinlein, K.; Dooley, S.; Pscherer, S.; Ochs, B.G.; Flesch, I.; et al. BMP9 a possible alternative drug for the recently withdrawn BMP7? New perspectives for (re-)implementation by personalized medicine. Arch. Toxicol. 2017, 91, 1353–1366. [Google Scholar] [CrossRef]
- Xiong, A.; He, Y.; Gao, L.; Li, G.; Weng, J.; Kang, B.; Wang, D.; Zeng, H. Smurf1-targeting miR-19b-3p-modified BMSCs combined PLLA composite scaffold to enhance osteogenic activity and treat critical-sized bone defects. Biomater. Sci. 2020, 8, 6069–6081. [Google Scholar] [CrossRef]
- Tu, X.; Delgado-Calle, J.; Condon, K.W.; Maycas, M.; Zhang, H.; Carlesso, N.; Taketo, M.M.; Burr, D.B.; Plotkin, L.I.; Bellido, T. Osteocytes mediate the anabolic actions of canonical Wnt/beta-catenin signaling in bone. Proc. Natl. Acad. Sci. USA 2015, 112, E478–E486. [Google Scholar] [CrossRef] [Green Version]
- Leucht, P.; Minear, S.; Berge, D.T.; Nusse, R.; Helms, J. Translating insights from development into regenerative medicine: The function of Wnts in bone biology. Semin. Cell Dev. Biol. 2008, 19, 434–443. [Google Scholar] [CrossRef]
- Zhou, Y.; Snead, M.L.; Tamerler, C. Bio-inspired hard-to-soft interface for implant integration to bone. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 431–434. [Google Scholar] [CrossRef]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef]
- Huang, X.; Li, C.; Zhu, B.; Wang, H.; Luo, X.; Wei, L. Co-cultured hBMSCs and HUVECs on human bio-derived bone scaffolds provide support for the long-term ex vivo culture of HSC/HPCs. J. Biomed. Mater. Res. Part A 2016, 104, 1221–1230. [Google Scholar] [CrossRef]
- Rather, H.A.; Jhala, D.; Vasita, R. Dual functional approaches for osteogenesis coupled angiogenesis in bone tissue engineering. Mater. Sci. Eng. C 2019, 103, 109761. [Google Scholar] [CrossRef]
- Jing, W.; Smith, A.A.; Liu, B.; Li, J.; Hunter, D.J.; Dhamdhere, G.; Salmon, B.; Jiang, J.; Cheng, D.; Johnson, C.A.; et al. Reengineering autologous bone grafts with the stem cell activator WNT3A. Biomaterials 2015, 47, 29–40. [Google Scholar] [CrossRef]
- Fuster-Matanzo, A.; Manferrari, G.; Marchetti, B.; Pluchino, S. Wnt3a promotes pro-angiogenic features in macrophages in vitro: Implications for stroke pathology. Exp. Biol. Med. 2018, 243, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.Y.; Lee, J.H.; Gu, X.; Wei, Z.Z.; Harris, M.J.; Yu, S.P.; Wei, L. Intranasally Delivered Wnt3a Improves Functional Recovery after Traumatic Brain Injury by Modulating Autophagic, Apoptotic, and Regenerative Pathways in the Mouse Brain. J. Neurotrauma 2018, 35, 802–813. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Li, J.; Yang, S.; Xu, J.; Yokota, H.; Zhang, P. Wnt3a involved in the mechanical loading on improvement of bone remodeling and angiogenesis in a postmenopausal osteoporosis mouse model. FASEB J. 2019, 33, 8913–8924. [Google Scholar] [CrossRef]
- Tschaffon, M.E.A.; Reber, S.O.; Schoppa, A.; Nandi, S.; Cirstea, I.C.; Aszodi, A.; Ignatius, A.; Haffner-Luntzer, M. A novel in vitro assay to study chondrocyte-to-osteoblast transdifferentiation. Endocrine 2022, 75, 266–275. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, S.; Kang, Y.; Shan, X.; Li, Q.; Cai, Z. Biocompatibility evaluation of a 3D-bioprinted alginate-GelMA-bacteria nanocellulose (BNC) scaffold laden with oriented-growth RSC96 cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112393. [Google Scholar]
- Fang, H.; Luo, C.; Liu, S.; Zhou, M.; Zeng, Y.; Hou, J.; Chen, L.; Mou, S.; Sun, J.; Wang, Z. A biocompatible vascularized graphene oxide (GO)-collagen chamber with osteoinductive and anti-fibrosis effects promotes bone regeneration in vivo. Theranostics 2020, 10, 2759–2772. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef] [PubMed]
- Amler, A.-K.; Thomas, A.; Tüzüner, S.; Lam, T.; Geiger, M.-A.; Kreuder, A.-E.; Palmer, C.; Nahles, S.; Lauster, R.; Kloke, L. 3D bioprinting of tissue-specific osteoblasts and endothelial cells to model the human jawbone. Sci. Rep. 2021, 11, 4876. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Lakalayeh, G.; Rahvar, M.; Haririan, E.; Karimi, R.; Ghanbari, H. Comparative study of different polymeric coatings for the next-generation magnesium-based biodegradable stents. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1380–1389. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.; Liu, J.; Huang, Y.; Peng, Y.; Xu, Y.; Ren, L. Photo-crosslinked GelMA/collagen membrane loaded with lysozyme as an antibacterial corneal implant. Int. J. Biol. Macromol. 2021, 191, 1006–1016. [Google Scholar] [CrossRef]
- Aquino-Martínez, R.; Rodríguez-Carballo, E.; Gámez, B.; Artigas, N.; Carvalho-Lobato, P.; Manzanares-Céspedes, M.C.; Rosa, J.L.; Ventura, F. Mesenchymal Stem Cells Within Gelatin/CaSO4 Scaffolds Treated Ex Vivo with Low Doses of BMP-2 and Wnt3a Increase Bone Regeneration. Tissue Eng. Part A 2016, 22, 41–52. [Google Scholar] [CrossRef]
- Leucht, P.; Lee, S.; Yim, N. Wnt signaling and bone regeneration: Can’t have one without the other. Biomaterials 2019, 196, 46–50. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Z.; Huang, B.; Yan, H.; Yang, C.; Li, Q.; Jin, D. Orcinol glucoside facilitates the shift of MSC fate to osteoblast and prevents adipogenesis via Wnt/β-catenin signaling pathway. Drug Des. Dev. Ther. 2019, 13, 2703–2713. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.; Guo, M.; Han, Z.; Wang, Y.; Yang, P.; Xu, C.; Wang, Q.; Du, L.; Li, Q.; Zhao, H.; et al. Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell Death Dis. 2016, 7, e2387. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Liang, H.; Han, Y. Cyclooxygenase-2 and β-Catenin as Potential Diagnostic and Prognostic Markers in Endometrial Cancer. Front. Oncol. 2020, 10, 56. [Google Scholar] [CrossRef]
- Vijaykumar, A.; Root, S.; Mina, M. Wnt/β-Catenin Signaling Promotes the Formation of Preodontoblasts In Vitro. J. Dent. Res. 2020, 100, 387–396. [Google Scholar] [CrossRef]
- Morsczeck, C.; Reck, A.; Reichert, T. WNT3A and the induction of the osteogenic differentiation in adipose tissue derived mesenchymal stem cells. Tissue Cell 2017, 49, 489–494. [Google Scholar] [CrossRef]
- Jiang, B.; Xu, J.; Zhou, Y.; Mao, J.; Guan, G.; Xu, X.; Mei, L. Estrogen Enhances Osteogenic Differentiation of Human Periodontal Ligament Stem Cells by Activating the Wnt/β-Catenin Signaling Pathway. J. Craniofacial Surg. 2020, 31, 583–587. [Google Scholar] [CrossRef]
- Moschouris, P.; Retzepi, M.; Petrie, A.; Donos, N. Effect of Wnt3a delivery on early healing events during guided bone regeneration. Clin. Oral Implant. Res. 2017, 28, 283–290. [Google Scholar] [CrossRef]
- Liu, W.C.; Chen, S.; Zheng, L.; Qin, L. Angiogenesis Assays for the Evaluation of Angiogenic Properties of Orthopaedic Biomaterials—A General Review. Adv. Healthc. Mater. 2017, 6, 1600434. [Google Scholar] [CrossRef]
- McBride, J.D.; Rodriguez-Menocal, L.; Guzman, W.; Candanedo, A.; Garcia-Contreras, M.; Badiavas, E.V. Bone Marrow Mesenchymal Stem Cell-Derived CD63(+) Exosomes Transport Wnt3a Exteriorly and Enhance Dermal Fibroblast Proliferation, Migration, and Angiogenesis In Vitro. Stem. Cells Dev. 2017, 26, 1384–1398. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W.; et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem. Cells Transl. Med. 2015, 4, 513–522. [Google Scholar] [CrossRef]
- Olsen, J.J.; Pohl, S.; Deshmukh, A.; Visweswaran, M.; Ward, N.C.; Arfuso, F.; Agostino, M.; Dharmarajan, A. The Role of Wnt Signalling in Angiogenesis. Clin. Biochem. Rev. 2017, 38, 131–142. [Google Scholar]
- Bouland, C.; Philippart, P.; Dequanter, D.; Corrillon, F.; Loeb, I.; Bron, D.; Lagneaux, L.; Meuleman, N. Cross-Talk Between Mesenchymal Stromal Cells (MSCs) and Endothelial Progenitor Cells (EPCs) in Bone Regeneration. Front. Cell Dev. Biol. 2021, 9, 674084. [Google Scholar] [CrossRef]
- Pan, Y.; Hu, N.; Wei, X.; Gong, L.; Zhang, B.; Wan, H.; Wang, P. 3D cell-based biosensor for cell viability and drug assessment by 3D electric cell/matrigel-substrate impedance sensing. Biosens. Bioelectron. 2019, 130, 344–351. [Google Scholar] [CrossRef]
- Wu, X.; Chen, K.; Chai, Q.; Liu, S.; Feng, C.; Xu, L.; Zhang, D. Freestanding vascular scaffolds engineered by direct 3D printing with Gt-Alg-MMT bioinks. Biomater. Adv. 2022, 133, 112658. [Google Scholar] [CrossRef]
- Shao, P.-L.; Wu, S.-C.; Lin, Z.-Y.; Ho, M.-L.; Chen, C.-H.; Wang, C.-Z. Alpha-5 Integrin Mediates Simvastatin-Induced Osteogenesis of Bone Marrow Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 506. [Google Scholar] [CrossRef] [Green Version]
- López-González, I.; Zamora-Ledezma, C.; Sanchez-Lorencio, M.I.; Tristante Barrenechea, E.; Gabaldón-Hernández, J.A.; Meseguer-Olmo, L. Modifications in Gene Expression in the Process of Osteoblastic Differentiation of Multipotent Bone Marrow-Derived Human Mesenchymal Stem Cells Induced by a Novel Osteoinductive Porous Medical-Grade 3D-Printed Poly(ε-caprolactone)/β-tricalcium Phosphate Composite. Int. J. Mol. Sci. 2021, 22, 11216. [Google Scholar]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef]
- Reyes, R.; Rodríguez, J.A.; Orbe, J.; Arnau, M.R.; Évora, C.; Delgado, A. Combined sustained release of BMP2 and MMP10 accelerates bone formation and mineralization of calvaria critical size defect in mice. Drug Deliv. 2018, 25, 750–756. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, M.; Güç, E.; Park, A.J.; Julier, Z.; Briquez, P.S.; Kuhn, G.A.; Müller, R.; Swartz, M.A.; Hubbell, J.A.; Martino, M.M. Growth factors with enhanced syndecan binding generate tonic signalling and promote tissue healing. Nat. Biomed. Eng. 2020, 4, 463–475. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Tu, X.; Pacheco-Costa, R.; McAndrews, K.; Edwards, R.; Pellegrini, G.G.; Kuhlenschmidt, K.; Olivos, N.; Robling, A.; Peacock, M.; et al. Control of Bone Anabolism in Response to Mechanical Loading and PTH by Distinct Mechanisms Downstream of the PTH Receptor. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017, 32, 522–535. [Google Scholar] [CrossRef] [Green Version]
- Piard, C.; Jeyaram, A.; Liu, Y.; Caccamese, J.; Jay, S.M.; Chen, Y.; Fisher, J. 3D printed HUVECs/MSCs cocultures impact cellular interactions and angiogenesis depending on cell-cell distance. Biomaterials 2019, 222, 119423. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Chen, J.; Wang, X.; Liu, Y.; Ma, Y.; Tu, X. Functionalized 3D-Printed ST2/Gelatin Methacryloyl/Polcaprolactone Scaffolds for Enhancing Bone Regeneration with Vascularization. Int. J. Mol. Sci. 2022, 23, 8347. https://doi.org/10.3390/ijms23158347
Liu G, Chen J, Wang X, Liu Y, Ma Y, Tu X. Functionalized 3D-Printed ST2/Gelatin Methacryloyl/Polcaprolactone Scaffolds for Enhancing Bone Regeneration with Vascularization. International Journal of Molecular Sciences. 2022; 23(15):8347. https://doi.org/10.3390/ijms23158347
Chicago/Turabian StyleLiu, Guangliang, Jie Chen, Xiaofang Wang, Yujiao Liu, Yufei Ma, and Xiaolin Tu. 2022. "Functionalized 3D-Printed ST2/Gelatin Methacryloyl/Polcaprolactone Scaffolds for Enhancing Bone Regeneration with Vascularization" International Journal of Molecular Sciences 23, no. 15: 8347. https://doi.org/10.3390/ijms23158347





