Abstract
The melanocortin receptors are G-protein-coupled receptors, which are essential components of the hypothalamic–pituitary–adrenal axis, and they mediate the actions of melanocortins (melanocyte-stimulating hormones: α-MSH, β-MSH, and γ-MSH) as well as the adrenocorticotropin hormone (ACTH) in skin pigmentation, adrenal steroidogenesis, and stress response. Three melanocortin receptor genes (MC1R, MC2R, and MC5R) contribute to the risk of major depressive disorder (MDD), and one melanocortin receptor gene (MC4R) contributes to the risk of type 2 diabetes (T2D). MDD increases T2D risk in drug-naïve patients; thus, MDD and T2D commonly coexist. The five melanocortin receptor genes might confer risk for both disorders. However, they have never been investigated jointly to evaluate their potential contributing roles in the MDD-T2D comorbidity, specifically within families. In 212 Italian families with T2D and MDD, we tested 11 single nucleotide polymorphisms (SNPs) in the MC1R gene, 9 SNPs in MC2R, 3 SNPs in MC3R, 4 SNPs in MC4R, and 2 SNPs in MC5R. The testing used 2-point parametric linkage and linkage disequilibrium (LD) (i.e., association) analysis with four models (dominant with complete penetrance (D1), dominant with incomplete penetrance (D2), recessive with complete penetrance (R1), and recessive with incomplete penetrance (R2)). We detected significant (p ≤ 0.05) linkage and/or LD (i.e., association) to/with MDD for one SNP in MC2R (rs111734014) and one SNP in MC5R (rs2236700), and to/with T2D for three SNPs in MC1R (rs1805007 and rs201192930, and rs2228479), one SNP in MC2R (rs104894660), two SNPs in MC3R (rs3746619 and rs3827103), and one SNP in MC4R genes (Chr18-60372302). The linkage/LD/association was significant across different linkage patterns and different modes of inheritance. All reported variants are novel in MDD and T2D. This is the first study to report risk variants in MC1R, MC2R, and MC3R genes in T2D. MC2R and MC5R genes are replicated in MDD, with one novel variant each. Within our dataset, only the MC2R gene appears to confer risk for both MDD and T2D, albeit with different risk variants. To further clarity the role of the melanocortin receptor genes in MDD-T2D, these findings should be sought among other ethnicities as well.
1. Introduction
The melanocortin receptors are G-protein-coupled receptors mediating the actions of melanocortins (melanocyte-stimulating hormones: α-MSH, β-MSH, and γ-MSH) and the adrenocorticotropin hormone (ACTH) in skin pigmentation, adrenal steroidogenesis, and stress response []. Melanocyte-stimulating hormones (α-MSH, β-MSH, and γ-MSH) and ACTH are synthesized in peptidergic neurons in the arcuate nuclei of the hypothalamus and the pituitary gland, respectively, from the same precursor: pro-opiomelanocortin (POMC) via post-translational modifications [,]. This central melanocortin system is part of the hypothalamic–pituitary–adrenal (HPA) axis, which is involved in stress responses [] and metabolic regulation [] and is expressed in both central (e.g., brain) and peripheral tissues (e.g., skin) []. There are five known melanocortin receptors in humans (MC1R-MC5R) []. MC1R is predominantly expressed in skin melanocytes, adrenal glands, kidneys, and immune cells [,]. MC2R is mainly expressed in the adrenal cortex []. MC3R, MC4R, and MC5R are expressed in the brain and other tissues (e.g., MC3R in macrophages []); MC5R is also present in adipose tissue, kidneys, and skeletal muscles [,].
The melanocortin receptors are encoded by five different genes (MC1R-MC5R) that exert different physiological functions in both humans and domestic animals []. MC1R is best known for regulating skin and coat pigmentation, and MC2R is the main receptor for ACTH []. Mutations in the MC2R gene can cause familial glucocorticoid deficiency []. MC3R and MC4R play important roles in energy and lipid metabolism []. MC4R dysfunction causes obesity in both humans [] and knockout mice []. The role of MC3R in energy homeostasis is less clear, and MC3R-knockout mice have normal weight and normal or low appetite []. While the MC5R function is the least understood, the evidence so far suggests its role in energy metabolism, inflammatory responses, and exocrine functions [].
T2D and MDD are two prevalent chronic complex diseases associated with significant worldwide morbidity and mortality []. They cumulatively affect 14% of adult populations [,], and their etiologies can be attributed to interactions between environmental and genetic risk factors [,,]. Genetic overlap exists between MDD and T2D and can be linked to at least a few genes [,].
Melanocortin receptors mediate the action of the hypothalamic–pituitary–adrenal (HPA) axis in response to superimposed stresses and cortisol feedback, which have been linked to depression (MDD) [] and type 2 diabetes (T2D) []. In humans, polymorphisms in the melanocortin receptor genes have been previously reported in patients with major depressive disorder (MDD) (MC1R [], MC2R [], and MC5R []), emotional eating and food craving (MC4R []), obesity (MC1R [], MC3R [], MC4R [], and MC5R [] via linkage studies in Quebec families []), and T2D (MC4R) [], but never in the MDD-T2D comorbidity. In this study, we evaluate the contribution of variants in the melanocortin receptor genes to the familial comorbidity of T2D and MDD.
2. Results and Discussion
Linkage, LD/Association Analysis, and LD among SNPs
We detected significant (p ≤ 0.05) linkage to and/or LD (i.e., association) with MDD for one SNP in MC2R and one SNP in MC5R and to/with T2D for three SNPs in MC1R, one SNP in MC2R, two SNPs in MC3R, and one SNP in MC4R. Table 1 shows information on the significant parametric models and chromosome and base pair location, Ref/Alt alleles and risk alleles, gene sites, and functional consequences of the specific risk variants. Moreover, Table 1 reports if the risk variant is independent or within a LD block, and whether it has been previously published in MDD or T2D.

Table 1.
Melanocortin Receptor Genes: Risk SNPs for MDD and T2D.
The test statistics and specified significant models are reported in Figure 1 for T2D and Figure 2 for MDD. The MC3R risk variants rs3746619 and rs3827103 are within LD-block Set 01 (Table 1) and, thus, function as replicates of one another.
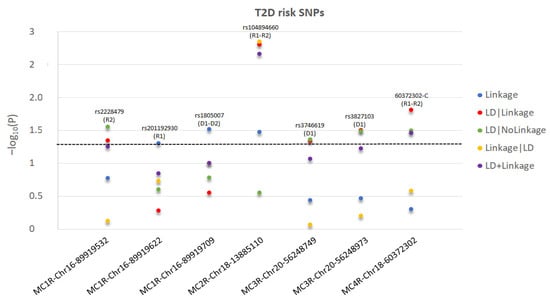
Figure 1.
For each significant T2D-risk SNP in MC2R-MC4R genes, we present the −log10(P) as a function of each test statistic (Linkage, LD|Linkage, LD|NoLinkage, Linkage|LD, and LD+Linkage) and label the significant inheritance model: D1: dominant, complete penetrance; D2: dominant, incomplete penetrance; R1: recessive, complete penetrance; R2: recessive, incomplete penetrance. For MC1R-rs1805007, the most significant test statistics between D1 and D2 are presented. For MC2R-rs104894660, the most significant test statistics between R1 and R2 are presented. For MC4R-60372302-C, R2 test statistics are presented as more significant than R1. The level of statistical significance is marked by the dotted line.
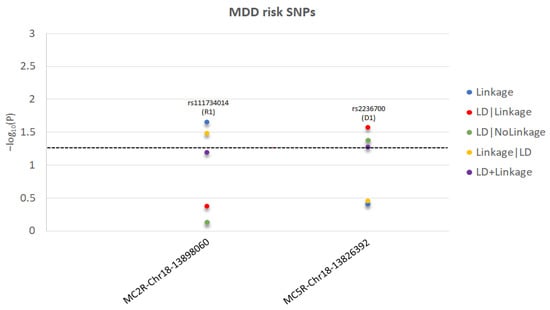
Figure 2.
For each significant MDD-risk SNP in MC2R and MC5R genes, we present the −log10(P) as a function of each test statistic (Linkage, LD|Linkage, LD|NoLinkage, Linkage|LD, and LD+Linkage) and label the significant inheritance model: D1: dominant, complete penetrance; R1: recessive, complete penetrance. The level of statistical significance is marked by the dotted line.
In this study, we reported nine variants in the melanocortin receptor genes that are significantly linked/in LD (or associated) to/with MDD and/or T2D in families with enriched T2D history. None of these variants have previously been reported in MDD or in T2D. This study pioneers the investigation of the five melanocortin receptor genes’ roles in familial MDD and T2D. Within our familial dataset, the MC2R and MC5R genes were significantly linked/in LD (or associated) to/with MDD, and four genes (MC1R, MC2R, MC3R, and MC4R) were significantly linked/in LD (or associated) to/with T2D. Of interest, the MC2R gene was significantly linked/in LD (or associated) to/with both MDD and T2D, thereby indicating the MC2R gene’s possible role in their comorbidity, despite being mediated by independent variants. To our knowledge, this study reveals a novel link of MC1R, MC2R, and MC3R genes to T2D.
The MC1R gene has been extensively studied in relation to skin and hair color []. Its role in MDD and obesity might be explained by ultraviolet light-induced mood changes []. However, MC1R has a role in mediating anti-inflammatory response. A study shows that both interferon and lipopolysaccharide (LPS) trigger MC1R expression in human neutrophils, and MC1R mediates the anti-inflammatory effects of alpha-MSH, likely contributing to neutrophil chemotaxis direct inhibition and anti-inflammatory activity []. Agonists of MC1R play a role in inflammatory response []. In mice, a melanocortin-like peptide blocks, as much as alpha-MSH and ACTH, cytokines’ release in response to LPS, rescuing the animals from lethal LPS doses, thereby showing that MC1R may play an anti-inflammatory role in protecting against LPS-generating gut microbes []. Thus, MC1R might play a systemic role in mediating inflammation derived from the gut–brain axis []. The role that MC1R plays in inflammation might contribute to T2D. In the present study, we found for the first time that the MC1R gene is related to T2D. In fact, we found MC1R rs1805007 significantly linked to T2D under the D1 and D2 models. It is known that MC1R rs1805007 regulates skin pigmentation [] and is associated with red hair [] and morbid obesity []; as in the present study, a prior study also failed to find that it confers MDD risk []. We also detected two additional MC1R variants. We found that the variant rs2228479 is linked/in LD (or associated) to/with T2D, specifically under the recessive incomplete penetrance model (R2); previously, it was associated with morbid obesity [] and with antidepressant response in MDD patients []. Furthermore, we found that the rs201192930 G allele is linked/in LD (or associated) to/with T2D, specifically under the recessive, complete penetrance model (R1). Previously, the A allele was reported as contributing to melanoma [].
MC2R binds to ACTH and mediates the release of cortisol from the adrenal glands []. Variants in the MC2R gene have been reported in Chinese patients with MDD []. In the Italian families under study, we found for the first time that MC2R variants confer risk for T2D. Namely, the study revealed that rs111734014 is significantly linked/in LD (or associated) to/with MDD under the recessive complete penetrance model (R1). Additionally, we detected that the MC2R rs104894660 G allele is significantly linked/in LD (or associated) to/with T2D, under the recessive complete (R1) and incomplete (R2) penetrance models. Of note, the MC2R rs104894660 A allele is reported in Clinvar and Uniprot as “pathogenic”, causing familial glucocorticoid deficiency via a recessive model of inheritance []. The MC2R rs104894660 G allele linkage/LD/association to/with T2D is, therefore, novel and might be explained by an increased MC2R affinity to ACTH, leading to higher cortisol secretion and subsequent insulin resistance, which together contribute to T2D. The potentially higher cortisol level can also explain the predisposition to stress-related MDD by its negative impact on mood, as it has been demonstrated in humans [].
The MC3R gene is involved in obesity [] and a marker near this gene has been reported having a role in insulin secretion []. Deficiency of MC3R in mice cause increased fat deposition and obesity, despite the decreased appetite []. Obesity may lead to T2D. In our study, we detected two MC3R closely linked variants, rs3746619 and rs3827103, contained within the same LD-block Set01, that are significantly linked/in LD (or associated) to/with T2D under the D1 model. As rs3746619 is located in the 5′UTR MC3R region and rs3827103 is a missense variant, their pathogenetic effect might be unrelated, as the first might affect gene transcription and the second may affect protein conformation. These two variants have been previously studied in obesity with inconsistent results. Both variants have been negatively associated with obesity in studies involving Caucasian [], Chilean [], and Thai [] populations; rs3746619 has been positively associated with obesity in a study in a Singaporean [] population; rs3827103 has been positively associated with obesity in a study in Caucasians [] and with body fat percentage in a study in Malaysian adolescents [] and African-Americans [], implying a potential role in body fat composition in various ethnic groups []). These inconsistent results might be due to underlying allelic population differences specifically reported for these two variants [], potential different LD blocks carrying the risk variant across populations, or differences in sample sizes and detecting power.
Variants in the MC4R gene—well-known as the human obesity gene []—have been reported in Chinese patients with T2D [], and MC4R knockout mice are hyperphagic and obese [] and have marked insulin resistance [], all of which may contribute to T2D. We detected a novel variant, chr18-60372302-C, in linkage/LD/association to/with T2D under the recessive complete (R1) and incomplete (R2) penetrance models. The alternative allele T, not conferring risks within our families for T2D, causes a W16X stop variant that was previously reported in a mother and child with early-onset obesity, not confirmed in the other overweight/obese family members, and absent in the control subjects [,]. This T allele confers impaired MC4R expression and signaling, both in vitro and in vivo [], while the stop signal is rescued in vitro by aminoglycoside-mediated read-throughs of stop codons [].
The MC5R was the last of the melanocortin receptors to be cloned, and it is potentially implicated in energy metabolism and inflammatory responses []. Variants in the MC5R gene have been previously associated with MDD [] and T2D in Finns []. We detected the MC5R rs2236700 SNP as significantly linked/in LD (or associated) to/with MDD under the dominant complete penetrance model. While a previous study reported no association of rs2236700 with bipolar disorder [], interestingly, the rs2236700 T2D-risk G allele we detected confers susceptibility to schizophrenia when present with two variants of two other genes (tryptophan 2,3-dioxygenase [TDO2] and melanin-concentrating hormone receptor 2 [MCHR2]) []. Of note, the TDO2 gene is activated by glucocorticoids [] and is a candidate gene in other neuropsychiatric disorders (i.e., autism [] and alcohol use disorder []), as it mediates immunosuppressive effects of kynurenine and its metabolites, loss of effective immune surveillance [], and inflammation []. The pathogenic mechanism of MC5R-related MDD might be mediated by its role in inflammatory responses []; of note, MC5R-deficient mice display behavioral changes such as reduced aggression and more defensive behaviors []. Mutated MC5R in humans might cause similar behavioral and/or mood changes, but this remains to be confirmed.
As we and others have described [,], MDD, schizophrenia, bipolar disorder, and T2D share genetic comorbidity, but mental–metabolic comorbidity studies have begun in recent years [,]. Of equal interest, the globally recognized T2D risk gene TCF7L2 [] has been found via a linkage study to contribute to schizophrenia [], further supporting the existence of comorbid genetic pathogenesis of metabolic–mental disorders.
Our study suggests that the melanocortin receptors risk variants, detected as contributing to familial risk for MDD and/or T2D, might be part of a more complex pathway implicated in the shared comorbidity of metabolic and mental disorders [,]. While it is hard to disentangle the genes’ direct roles in the phenotypes tested from the possible underlying biological effect(s), the genes reported appear implicated in the investigated phenotypes, but only MC2R shows pleiotropic effects within our familial dataset. This might be explained by the mediating effect of the HPA-axis on the MC2R of the adrenal, triggering cortisol secretion. Hypercortisolism is implicated in MDD [] and T2D [], and as we previously hypothesized, it is most likely implicated in the MDD-T2D comorbidity []. However, we want to note the significance and intrinsic limitation of the present study. While variants in linkage with a disorder cosegregate with the disease, they are not necessarily associated with it; on the other hand, variants in LD with a disease are both in linkage and associated with it; thus, they cosegregate as well as associate with the disease under study across various families. Despite this, only in vitro or in vivo studies can prove the functional effects of the variants on the gene expression, translation, or downstream function. Thus, we cannot prove that the detected risk variants are indeed causative variants; they might be in LD with an unknown, yet-to-be identified pathogenic variant.
3. Materials and Methods
Our aim was to investigate the potential role of the MC1R, MC2R, MC3R, MC4R, and MC5R genes in the pathogenesis of T2D, MDD, and their comorbidity.
We studied previously recruited Italian families with T2D, and the dataset was deidentified and coded. The study was approved by the Jefferson Ethical Committee. The 212 families studied descended from at least three generations of Italians originating from the Italian peninsula. Families with identical twins and siblings with uncertain paternity were excluded. The families had an enriched history of T2D [,] and were phenotyped for the presence or absence of MDD using DSM-IV diagnostic criteria [].
In the family subjects, we amplified 11 single nucleotide polymorphisms (SNPs) in MC1R, 9 SNPs in MC2R, 3 SNPs in MC3R, 4 SNPs in MC4R, and 2 SNPs in MC5R using microarrays. We performed genotyping and Mendelian error exclusion by PLINK []. Using Pseudomarker, we analyzed the total 29 SNPs for 2-point parametric linkage and linkage disequilibrium (LD), which involve association with T2D and MDD using the following models: dominant with complete penetrance (D1), dominant with incomplete penetrance (D2), recessive with complete penetrance (R1), and recessive with incomplete penetrance (R2). To test the presence or absence of LD blocks within the variants showing statistically significant results in T2D or MDD (p ≤ 0.05), we computed LD correlations via LD matrices among the SNPs available in the Toscani Italian population from the 1000 Genomes Project (https://www.internationalgenome.org/data-portal/population/TSI (accessed on 28 May 2022)) (LDmatrix function-RDocumentation,). The SNPs that significantly correlated (r [] ≥ 0.9) with other SNPs were considered within the same LD block and labeled based on that unique LD block (e.g., Set 01 and Set 02). All SNPs that were not correlated with any other SNPs were designated as “Independent”.
4. Conclusions
Our study expanded the phenotypic spectra of melanocortin receptor genes. This is the first study to report risk variants in MC1R, MC2R, and MC3R genes in T2D. MC2R and MC5R genes are replicated in MDD; however, these appear with one novel variant each. Within our dataset, only the MC2R gene appears to confer risks for both MDD and T2D, albeit with different risk variants. To further clarify the role of the melanocortin receptor genes in MDD-T2D, these findings should be replicated in other ethnicities to improve our understanding of the comorbidity of MDD and T2D.
Author Contributions
C.G. conceived and supervised the project, including statistical analysis and manuscript drafting. J.O. helped with the statistical analysis and manuscript drafting. M.A. helped with data interpretation, figures, tables, and manuscript drafting. R.W. and T.T.P. critically helped with data interpretation and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
C.G. was supported by NICHD 5R01HD086911 (PI Gragnoli). R.W. was supported by NICHD 5R01HD086911 (PI Gragnoli). T.T.P. was supported in part by NICHD 5R01HD086911 (PI Gragnoli) and the Rocky Mountain MIRECC for Suicide Prevention. NICHD had no role in the study design, data collection, analysis, data interpretation, or manuscript writing.
Institutional Review Board Statement
The study was approved by the Jefferson Ethical Committee, which qualified the study as “non-human research” and “exempt” from a full review, as the families were deidentified and coded.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon reasonable request. The data are not publicly available due to privacy restrictions and lack of specific patients’ consent.
Acknowledgments
We thank the families who participated in the study, and we thank Bios Biotech Multi-Diagnostic Health Center, Rome, Italy, for data access and for financial, medical, and laboratory staff support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tao, Y.X. Melanocortin receptors. Biochim. Biophys. Acta—Mol. Basis Dis. 2017, 1863, 2411–2413. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Mizusawa, K. Posttranslational Modifications of Proopiomelanocortin in Vertebrates and Their Biological Significance. Front. Endocrinol. 2013, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Harno, E.; Ramamoorthy, T.G.; Coll, A.P.; White, A. POMC: The Physiological Power of Hormone Processing. Physiol. Rev. 2018, 98, 2381. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Vicennati, V.; Cacciari, M.; Pagotto, U. The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann. N. Y. Acad. Sci. 2006, 1083, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Cho, B.K.; Cho, D.H.; Park, H.J. Expression of hypothalamic-pituitary-adrenal axis in common skin diseases: Evidence of its association with stress-related disease activity. Acta Derm. Venereol. 2013, 93, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Switonski, M.; Mankowska, M.; Salamon, S. Family of melanocortin receptor (MCR) genes in mammals-mutations, polymorphisms and phenotypic effects. J. Appl. Genet. 2013, 54, 461–472. [Google Scholar] [CrossRef]
- Neumann Andersen, G.; Nagaeva, O.; Mandrika, I.; Petrovska, R.; Muceniece, R.; Mincheva-Nilsson, L.; Wikberg, J.E.S. MC(1) receptors are constitutively expressed on leucocyte subpopulations with antigen presenting and cytotoxic functions. Clin. Exp. Immunol. 2001, 126, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, D.Y.; Lin, Y.J.; Tao, Y.X. Melanocortin Regulation of Inflammation. Front. Endocrinol. 2019, 10, 683. [Google Scholar] [CrossRef]
- Gorrigan, R.J.; Guasti, L.; King, P.; Clark, A.J.; Chan, L.F. Localisation of the melanocortin-2-receptor and its accessory proteins in the developing and adult adrenal gland. J. Mol. Endocrinol. 2011, 46, 227. [Google Scholar] [CrossRef]
- Getting, S.J.; Gibbs, L.; Clark, A.J.; Flower, R.J.; Perretti, M. POMC gene-derived peptides activate melanocortin type 3 receptor on murine macrophages, suppress cytokine release, and inhibit neutrophil migration in acute experimental inflammation—PubMed. J. Immunol. 1999, 162, 7446–7453. [Google Scholar] [PubMed]
- Chagnon, Y.C.; Chen, W.J.; Pérusse, L.; Chagnon, M.; Nadeau, A.; Wilkison, W.O.; Bouchard, C. Linkage and association studies between the melanocortin receptors 4 and 5 genes and obesity-related phenotypes in the Québec Family Study. Mol. Med. 1997, 3, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Novoselova, T.V.; Chan, L.F.; Clark, A.J.L. Pathophysiology of melanocortin receptors and their accessory proteins. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 93–106. [Google Scholar] [CrossRef]
- Akin, M.A.; Akin, L.; Coban, D.; Ozturk, M.A.; Bircan, R.; Kurtoglu, S. A novel mutation in the MC2R gene causing familial glucocorticoid deficiency type 1. Neonatology 2011, 100, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Girardet, C.; Butler, A.A. Neural melanocortin receptors in obesity and related metabolic disorders. Biochim. Biophys. Acta 2014, 1842, 482. [Google Scholar] [CrossRef]
- Ayers, K.L.; Glicksberg, B.S.; Garfield, A.S.; Longerich, S.; White, J.A.; Yang, P.; Du, L.; Chittenden, T.W.; Gulcher, J.R.; Roy, S.; et al. Melanocortin 4 Receptor Pathway Dysfunction in Obesity: Patient Stratification Aimed at MC4R Agonist Treatment. J. Clin. Endocrinol. Metab. 2018, 103, 2601–2612. [Google Scholar] [CrossRef] [PubMed]
- Srisai, D.; Gillum, M.P.; Panaro, B.L.; Zhang, X.M.; Kotchabhakdi, N.; Shulman, G.I.; Ellacott, K.L.J.; Cone, R.D. Characterization of the Hyperphagic Response to Dietary Fat in the MC4R Knockout Mouse. Endocrinology 2011, 152, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.A.; Girardet, C.; Mavrikaki, M.; Trevaskis, J.L.; Macarthur, H.; Marks, D.L.; Farr, S.A. A Life without Hunger: The Ups (and Downs) to Modulating Melanocortin-3 Receptor Signaling. Front. Neurosci. 2017, 11, 128. [Google Scholar] [CrossRef]
- Jeong, J.H.; Um, Y.H.; Ko, S.H.; Park, J.H.; Park, J.Y.; Han, K.; Ko, K.S. Depression and Mortality in People with Type 2 Diabetes Mellitus, 2003 to 2013: A Nationwide Population-Based Cohort Study. Diabetes Metab. J. 2017, 41, 296–302. [Google Scholar] [CrossRef]
- NIMH. Major Depression; National Institute of Mental Health: Bethesda, MD, USA, 2022.
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Kaabi, J.A. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- Bener, A.; Zirie, M.; Al-Rikabi, A. Genetics, obesity, and environmental risk factors associated with type 2 diabetes. Croat. Med. J. 2005, 46, 302–307. [Google Scholar] [PubMed]
- Murea, M.; Ma, L.; Freedman, B.I. Genetic and environmental factors associated with type 2 diabetes and diabetic vascular complications. Rev. Diabet. Stud. RDS 2012, 9, 6. [Google Scholar] [CrossRef]
- Peterson, R.E.; Cai, N.; Dahl, A.W.; Bigdeli, T.B.; Edwards, A.C.; Webb, B.T.; Bacanu, S.A.; Zaitlen, N.; Flint, J.; Kendler, K.S. Molecular Genetic Analysis Subdivided by Adversity Exposure Suggests Etiologic Heterogeneity in Major Depression. Am. J. Psychiatry 2018, 175, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Gragnoli, C. Hypothesis of the neuroendocrine cortisol pathway gene role in the comorbidity of depression, type 2 diabetes, and metabolic syndrome. Appl. Clin. Genet. 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Kan, C.; Pedersen, N.L.; Christensen, K.; Bornstein, S.R.; Licinio, J.; MacCabe, J.H.; Ismail, K.; Rijsdijk, F. Genetic overlap between type 2 diabetes and depression in Swedish and Danish twin registries. Mol. Psychiatry 2016, 21, 903–909. [Google Scholar] [CrossRef]
- Qin, D.D.; Rizak, J.; Feng, X.L.; Yang, S.C.; Lü, L.B.; Pan, L.; Yin, Y.; Hu, X.T. Prolonged secretion of cortisol as a possible mechanism underlying stress and depressive behaviour. Sci. Rep. 2016, 6, 30187. [Google Scholar] [CrossRef]
- Ortiz, R.; Kluwe, B.; Odei, J.B.; Echouffo Tcheugui, J.B.; Sims, M.; Kalyani, R.R.; Bertoni, A.G.; Golden, S.H.; Joseph, J.J. The association of morning serum cortisol with glucose metabolism and diabetes: The Jackson Heart Study. Psychoneuroendocrinology 2019, 103, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.S.; Luo, H.R.; Dong, C.; Mastronardi, C.; Licinio, J.; Wong, M.L. Sequence polymorphisms of MC1R gene and their association with depression and antidepressant response. Psychiatr. Genet. 2011, 21, 14–18. [Google Scholar] [CrossRef]
- Yuan, F.; Hou, B.; Ji, L.; Ren, D.; Liu, L.; Bi, Y.; Guo, Z.; Ma, G.; Yang, F.; Dong, Z.; et al. Sex-Specific association of MC2R polymorphisms and the risk of major depressive disorder in Chinese Southern Han. Psychiatr. Genet. 2021, 31, 36–37. [Google Scholar] [CrossRef]
- Heinzman, J.T.; Hoth, K.F.; Cho, M.H.; Sakornsakolpat, P.; Regan, E.A.; Make, B.J.; Kinney, G.L.; Wamboldt, F.S.; Holm, K.E.; Bormann, N.; et al. GWAS and systems biology analysis of depressive symptoms among smokers from the COPDGene cohort. J. Affect. Disord. 2019, 243, 16–22. [Google Scholar] [CrossRef]
- Yilmaz, Z.; Davis, C.; Loxton, N.J.; Kaplan, A.S.; Levitan, R.D.; Carter, J.C.; Kennedy, J.L. Association between MC4R rs17782313 polymorphism and overeating behaviors. Int. J. Obes. 2015, 39, 114–120. [Google Scholar] [CrossRef]
- Gerhard, G.S.; Chu, X.; Wood, G.C.; Gerhard, G.M.; Benotti, P.; Petrick, A.T.; Gabrielsen, J.; Strodel, W.E.; Still, C.D.; Argyropoulos, G. Next-Generation sequence analysis of genes associated with obesity and nonalcoholic fatty liver disease-related cirrhosis in extreme obesity. Hum. Hered. 2013, 75, 144–151. [Google Scholar] [CrossRef]
- Koya, C.; Yu, T.; Strong, C.; Tsai, M.C. Association between Two Common Missense Substitutions, Thr6Lys and Val81Ile, in MC3R Gene and Childhood Obesity: A Meta-Analysis. Child. Obes. 2018, 14, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tong, R.; Xu, J.; Tian, Y.; Pan, J.; Cui, J.; Chen, H.; Peng, Y.; Fei, S.; Yang, S.; et al. PDX1 and MC4R genetic polymorphisms are associated with type 2 diabetes mellitus risk in the Chinese Han population. BMC Med. Genom. 2021, 14, 249. [Google Scholar] [CrossRef]
- Rees, J.L. Genetics of hair and skin color. Annu. Rev. Genet. 2003, 37, 67–90. [Google Scholar] [CrossRef] [PubMed]
- Veleva, B.I.; van Bezooijen, R.L.; Chel, V.G.M.; Numans, M.E.; Caljouw, M.A.A. Effect of ultraviolet light on mood, depressive disorders and well-being. Photodermatol. Photoimmunol. Photomed. 2018, 34, 288–297. [Google Scholar] [CrossRef]
- Catania, A.; Rajora, N.; Capsoni, F.; Minonzio, F.; Star, R.A.; Lipton, J.M. The neuropeptide alpha-MSH has specific receptors on neutrophils and reduces chemotaxis in vitro. Peptides 1996, 17, 675–679. [Google Scholar] [CrossRef]
- Lindskog Jonsson, A.; Granqvist, A.; Elvin, J.; Johansson, M.E.; Haraldsson, B.; Nyström, J. Effects of melanocortin 1 receptor agonists in experimental nephropathies. PLoS ONE 2014, 9, e87816. [Google Scholar] [CrossRef]
- Qiang, X.; Liotta, A.S.; Shiloach, J.; Gutierrez, J.C.; Wang, H.; Ochani, M.; Ochani, K.; Yang, H.; Rabin, A.; LeRoith, D.; et al. New melanocortin-like peptide of E. coli can suppress inflammation via the mammalian melanocortin-1 receptor (MC1R): Possible endocrine-like function for microbes of the gut. NPJ Biofilms Microbiomes 2017, 3, 31. [Google Scholar] [CrossRef][Green Version]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef]
- Jones, P.; Lucock, M.; Scarlett, C.J.; Veysey, M.; Beckett, E. Environmental UVR Levels and Skin Pigmentation Gene Variants Associated with Folate and Homocysteine Levels in an Elderly Cohort. Int. J. Environ. Res. Public Health 2020, 17, 1545. [Google Scholar] [CrossRef]
- Zorina-Lichtenwalter, K.; Lichtenwalter, R.N.; Zaykin, D.V.; Parisien, M.; Gravel, S.; Bortsov, A.; Diatchenko, L. A study in scarlet: MC1R as the main predictor of red hair and exemplar of the flip-flop effect. Hum. Mol. Genet. 2019, 28, 2093–2106. [Google Scholar] [CrossRef]
- Demenais, F.; Mohamdi, H.; Chaudru, V.; Goldstein, A.M.; Newton Bishop, J.A.; Bishop, D.T.; Kanetsky, P.A.; Hayward, N.K.; Gillanders, E.; Elder, D.E.; et al. Association of MC1R variants and host phenotypes with melanoma risk in CDKN2A mutation carriers: A GenoMEL study. J. Natl. Cancer Inst. 2010, 102, 1568–1583. [Google Scholar] [CrossRef] [PubMed]
- Flück, C.E.; Martens, J.W.M.; Conte, F.A.; Miller, W.L. Clinical, genetic, and functional characterization of adrenocorticotropin receptor mutations using a novel receptor assay. J. Clin. Endocrinol. Metab. 2002, 87, 4318–4323. [Google Scholar] [CrossRef] [PubMed]
- Fiksdal, A.; Hanlin, L.; Kuras, Y.; Gianferante, D.; Chen, X.; Thoma, M.V.; Rohleder, N. Associations between symptoms of depression and anxiety and cortisol responses to and recovery from acute stress. Psychoneuroendocrinology 2019, 102, 44–52. [Google Scholar] [PubMed]
- Demidowich, A.P.; Jun, J.Y.; Yanovski, J.A. Polymorphisms and mutations in the melanocortin-3 receptor and their relation to human obesity. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Love, D.R.; Kyle, C.; Daniels, A.; White, M.; Stewart, A.W.; Schnell, A.H.; Elston, R.C.; Holdaway, I.M.; Mountjoy, K.G. Melanocortin-3 receptor gene variants in a Maori kindred with obesity and early onset type 2 diabetes. Diabetes Res. Clin. Pract. 2002, 58, 61–71. [Google Scholar] [CrossRef]
- Chen, A.S.; Marsh, D.J.; Trumbauer, M.E.; Frazier, E.G.; Guan, X.M.; Yu, H.; Rosenblum, C.I.; Vongs, A.; Feng, Y.; Cao, L.; et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 2000, 26, 97–102. [Google Scholar] [CrossRef]
- Zegers, D.; Beckers, S.; Mertens, I.L.; Van Gaal, L.F.; Van Hul, W. Common melanocortin-3 receptor variants are not associated with obesity, although rs3746619 does influence weight in obese individuals. Endocrine 2010, 38, 289–293. [Google Scholar]
- Manriquez, V.; Aviles, J.; Salazar, L.; Saavedra, N.; Seron, P.; Lanas, F.; Fajardo, C.M.; Hirata, M.H.; Hirata, R.D.C.; Cerda, A. Polymorphisms in Genes Involved in the Leptin-Melanocortin Pathway are Associated with Obesity-Related Cardiometabolic Alterations in a Southern Chilean Population. Mol. Diagn. Ther. 2018, 22, 101–113. [Google Scholar] [CrossRef]
- Wannaiampikul, S.; Phonrat, B.; Tungtrongchitr, A.; Limwongse, C.; Chongviriyaphan, N.; Santiprabhob, J.; Tungtrongchitr, R. Genetic variant screening of MC3R and MC4R genes in early-onset obese children and their relatives among a Thai population: Family-based study. Genet. Mol. Res. GMR 2015, 14, 18090–18102. [Google Scholar] [CrossRef] [PubMed]
- Aris, I.M.; Tint, M.T.; Teh, A.L.; Holbrook, J.D.; Quah, P.L.; Chong, M.F.F.; Lin, X.; Soh, S.E.; Saw, S.M.; Kwek, K.; et al. MC3R gene polymorphisms are associated with early childhood adiposity gain and infant appetite in an Asian population. Pediatr. Obes. 2016, 11, 450–458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zaharan, N.L.; Muhamad, N.H.; Jalaludin, M.Y.; Su, T.T.; Mohamed, Z.; Mohamed, M.N.A.; Majid, H.A. Non-Synonymous Single-Nucleotide Polymorphisms and Physical Activity Interactions on Adiposity Parameters in Malaysian Adolescents. Front. Endocrinol. 2018, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Demidowich, A.P.; Parikh, V.J.; Dedhia, N.; Branham, R.E.; Madi, S.A.; Marwitz, S.E.; Roberson, R.B.; Uhlman, A.J.; Levi, N.J.; Mi, S.J.; et al. Associations of the melanocortin 3 receptor C17A + G241A haplotype with body composition and inflammation in African-American adults. Ann. Hum. Genet. 2019, 83, 355–360. [Google Scholar] [CrossRef]
- Yoshiuchi, I. Evidence for natural selection at the melanocortin-3 receptor gene in European and African populations. Acta Diabetol. 2016, 53, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Li, L.; Zhang, L.; Guo, L.; Wang, C. Association between MC4R rs17782313 genotype and obesity: A meta-analysis. Gene 2020, 733, 144372. [Google Scholar] [CrossRef]
- Weide, K.; Christ, N.; Moar, K.M.; Arens, J.; Hinney, A.; Mercer, J.G.; Eiden, S.; Schmidt, I. Hyperphagia, not hypometabolism, causes early onset obesity in melanocortin-4 receptor knockout mice. Physiol. Genom. 2003, 13, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Trevaskis, J.L.; Gawronska-Kozak, B.; Sutton, G.M.; McNeil, M.; Stephens, J.M.; Smith, S.R.; Butler, A.A. Role of adiponectin and inflammation in insulin resistance of Mc3r and Mc4r knockout mice. Obesity 2007, 15, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.; Corbalán, M.S.; Forga, L.; Martinez, J.A.; Hinney, A.; Hebebrand, J. A novel nonsense mutation in the melanocortin-4 receptor associated with obesity in a Spanish population. Int. J. Obes. Relat. Metab. Disorders. 2003, 27, 385–388. [Google Scholar] [CrossRef]
- Tao, Y.X. Mutations in melanocortin-4 receptor and human obesity. Prog. Mol. Biol. Transl. Sci. 2009, 88, 173–204. [Google Scholar] [PubMed]
- Bolze, F.; Rink, N.; Brumm, H.; Kühn, R.; Mocek, S.; Schwarz, A.E.; Kless, C.; Biebermann, H.; Wurst, W.; Rozman, J.; et al. Characterization of the melanocortin-4-receptor nonsense mutation W16X in vitro and in vivo. Pharm. J. 2013, 13, 80–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brumm, H.; Mühlhaus, J.; Bolze, F.; Scherag, S.; Hinney, A.; Hebebrand, J.; Wiegand, S.; Klingenspor, M.; Grüters, A.; Krude, H.; et al. Rescue of melanocortin 4 receptor (MC4R) nonsense mutations by aminoglycoside-mediated read-through. Obesity 2012, 20, 1074–1081. [Google Scholar] [CrossRef]
- Xu, Y.; Guan, X.; Zhou, R.; Gong, R. Melanocortin 5 receptor signaling pathway in health and disease. Cell. Mol. Life Sci. 2020, 77, 3831. [Google Scholar] [CrossRef] [PubMed]
- Valli-Jaakola, K.; Suviolahti, E.; Schalin-Jäntti, C.; Ripatti, S.; Silander, K.; Oksanen, L.; Salomaa, V.; Peltonen, L.; Kontula, K. Further evidence for the role of ENPP1 in obesity: Association with morbid obesity in Finns. Obesity 2008, 16, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Lohoff, F.W.; Berrettini, W.H. Lack of association between variations in the melanocortin 5 receptor gene and bipolar disorder. Psychiatr. Genet. 2005, 15, 255–258. [Google Scholar] [CrossRef][Green Version]
- Miller, C.L.; Murakami, P.; Ruczinski, I.; Ross, R.G.; Sinkus, M.; Sullivan, B.; Leonard, S. Two complex genotypes relevant to the kynurenine pathway and melanotropin function show association with schizophrenia and bipolar disorder. Schizophr. Res. 2009, 113, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Soichot, M.; Vaast, A.; Vignau, J.; Guillemin, G.J.; Lhermitte, M.; Broly, F.; Allorge, D. Characterization of Functional Polymorphisms and Glucocorticoid-Responsive Elements in the Promoter of TDO2, a Candidate Gene for Ethanol-Induced Behavioural Disorders. Alcohol Alcohol. 2013, 48, 415–425. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nabi, R.; Serajee, F.J.; Chugani, D.C.; Zhong, H.; Huq, A.H.M.M. Association of tryptophan 2,3 dioxygenase gene polymorphism with autism. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2004, 125B, 63–68. [Google Scholar] [CrossRef]
- Comings, D.E. Serotonin and the biochemical genetics of alcoholism: Lessons from studies of attention deficit hyperactivity disorder (ADHD) and Tourette syndrome. Alcohol Alcohol. Suppl. 1993, 2, 237–241. [Google Scholar]
- Cheong, J.E.; Sun, L. Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy—Challenges and Opportunities. Trends Pharmacol. Sci. 2018, 39, 307–325. [Google Scholar] [CrossRef]
- Chang, Y.; Han, P.; Wang, Y.; Jia, C.; Zhang, B.; Zhao, Y.; Li, S.; Li, S.; Wang, X.; Yang, X.; et al. Tryptophan 2,3-dioxygenase 2 plays a key role in regulating the activation of fibroblast-like synoviocytes in autoimmune arthritis. Br. J. Pharmacol. 2021, 179, 3024–3042. [Google Scholar] [CrossRef]
- Morgan, C.; Thomas, R.E.; Cone, R.D. Melanocortin-5 receptor deficiency promotes defensive behavior in male mice. Horm. Behav. 2004, 45, 58–63. [Google Scholar] [CrossRef]
- Postolache, T.T.; del Bosque-Plata, L.; Jabbour, S.; Vergare, M.; Wu, R.; Gragnoli, C. Co-Shared genetics and possible risk gene pathway partially explain the comorbidity of schizophrenia, major depressive disorder, type 2 diabetes, and metabolic syndrome. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2019, 180, 186–203. [Google Scholar] [CrossRef]
- Mizuki, Y.; Sakamoto, S.; Okahisa, Y.; Yada, Y.; Hashimoto, N.; Takaki, M.; Yamada, N. Mechanisms Underlying the Comorbidity of Schizophrenia and Type 2 Diabetes Mellitus. Int. J. Neuropsychopharmacol. 2021, 24, 367. [Google Scholar] [CrossRef]
- Bahrami, S.; Steen, N.E.; Shadrin, A.; O’Connell, K.; Frei, O.; Bettella, F.; Wirgenes, K.V.; Krull, F.; Fan, C.C.; Dale, A.M.; et al. Shared Genetic Loci Between Body Mass Index and Major Psychiatric Disorders: A Genome-wide Association Study. JAMA Psychiatry 2020, 77, 503–512. [Google Scholar] [CrossRef]
- Strawbridge, R.J.; Johnston, K.J.A.; Bailey, M.E.S.; Baldassarre, D.; Cullen, B.; Eriksson, P.; de Faire, U.; Ferguson, A.; Gigante, B.; Giral, P.; et al. The overlap of genetic susceptibility to schizophrenia and cardiometabolic disease can be used to identify metabolically different groups of individuals. Sci. Rep. 2021, 11, 632. [Google Scholar] [CrossRef]
- Chen, X.; Ayala, I.; Shannon, C.; Fourcaudot, M.; Acharya, N.K.; Jenkinson, C.P.; Heikkinen, S.; Norton, L. The Diabetes Gene and Wnt Pathway Effector TCF7L2 Regulates Adipocyte Development and Function. Diabetes 2018, 67, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Alkelai, A.; Greenbaum, L.; Lupoli, S.; Kohn, Y.; Sarner-Kanyas, K.; Ben-Asher, E.; Lancet, D.; Macciardi, F.; Lerer, B. Association of the Type 2 Diabetes Mellitus Susceptibility Gene, TCF7L2, with Schizophrenia in an Arab-Israeli Family Sample. PLoS ONE 2012, 7, e29228. [Google Scholar] [CrossRef] [PubMed]
- Gragnoli, C. PSMD9 gene in the NIDDM2 locus is linked to type 2 diabetes in Italians. J. Cell. Physiol. 2010, 222, 265–267. [Google Scholar] [CrossRef]
- Gragnoli, C. Proteasome modulator 9 and depression in type 2 diabetes. Curr. Med. Chem. 2012, 19, 5178–5180. [Google Scholar] [CrossRef]
- APA. Schizophrenia. In Diagnostic and Statistical Manual of Mental Disorders; DSM-IV-TR; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).