The Leaf Trichome, Venation, and Mesophyll Structural Traits Play Important Roles in the Physiological Responses of Oak Seedlings to Water-Deficit Stress
Abstract
:1. Introduction
2. Results
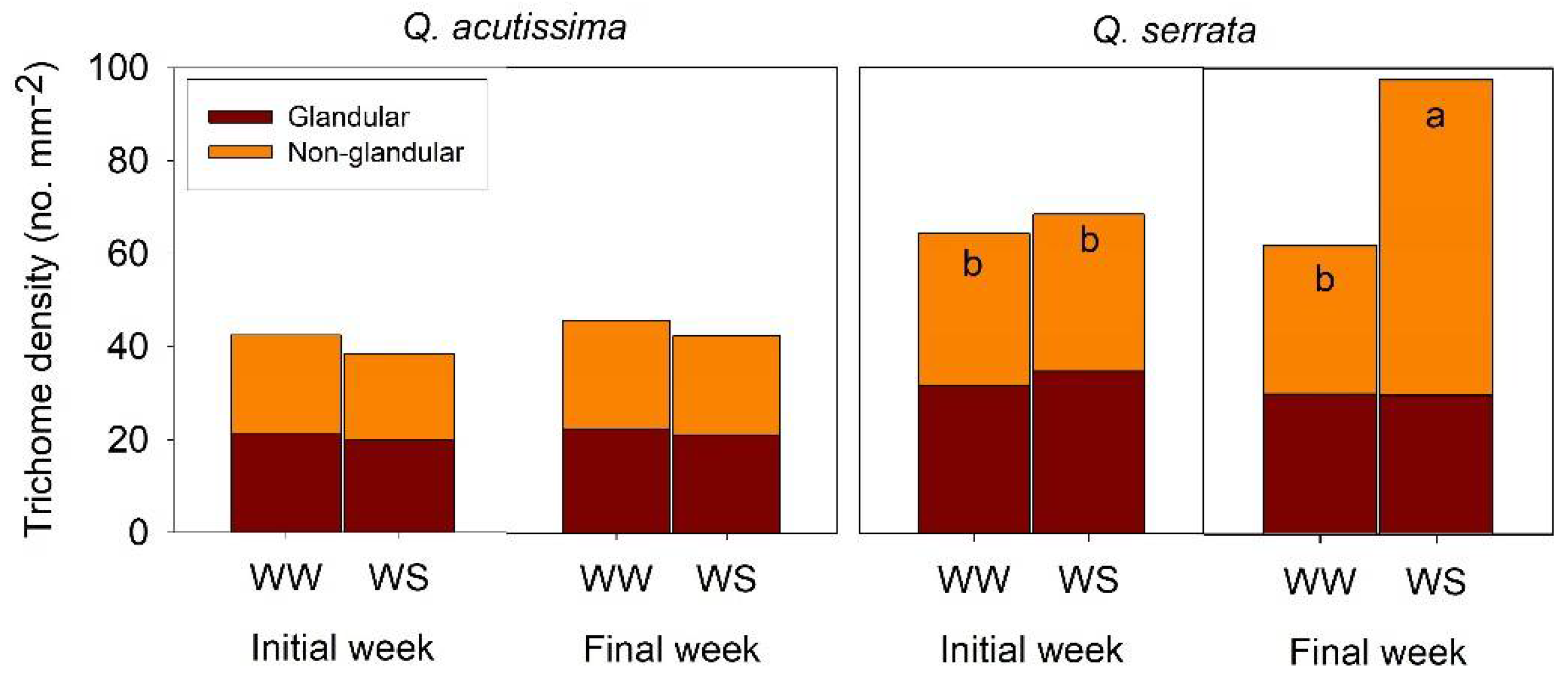
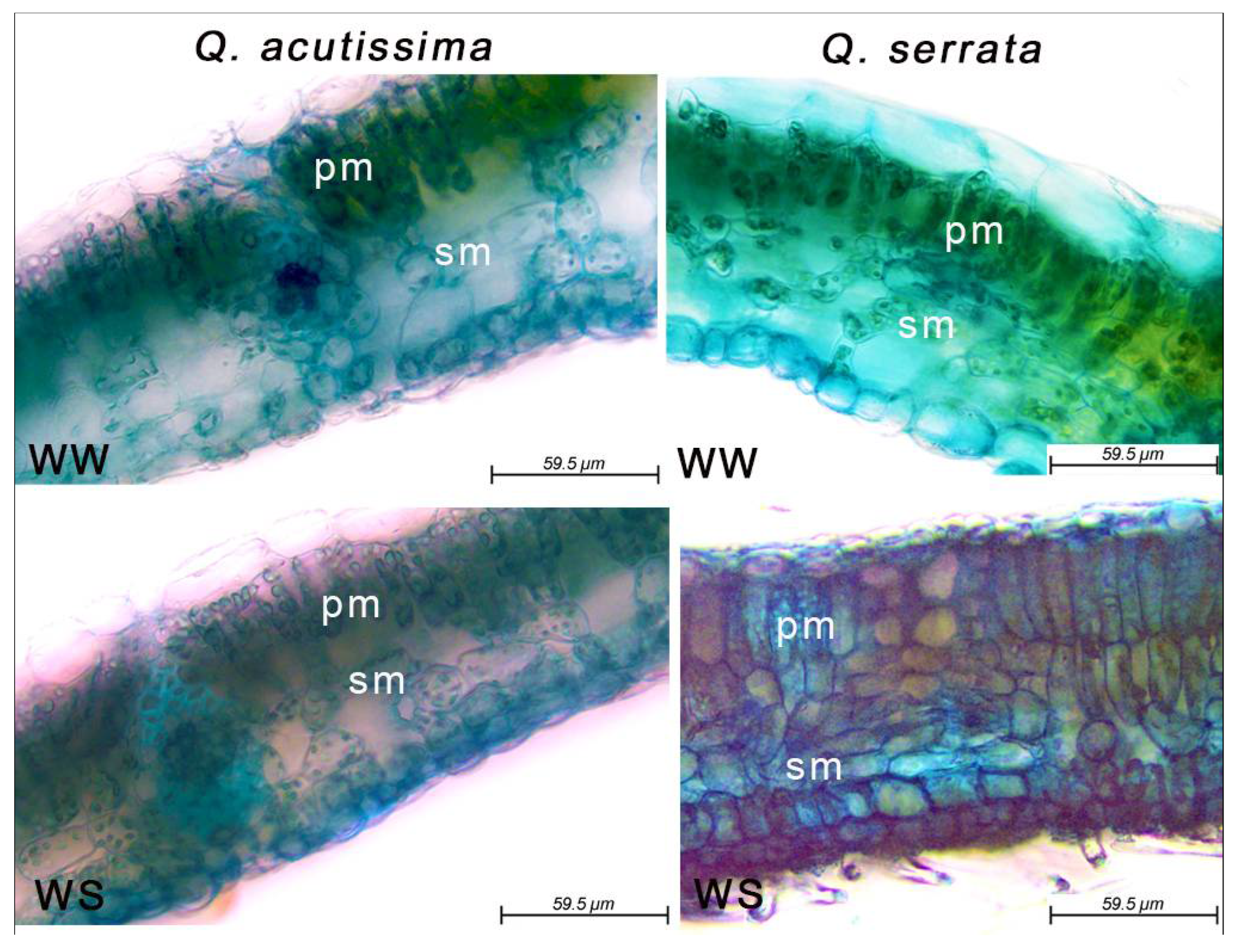
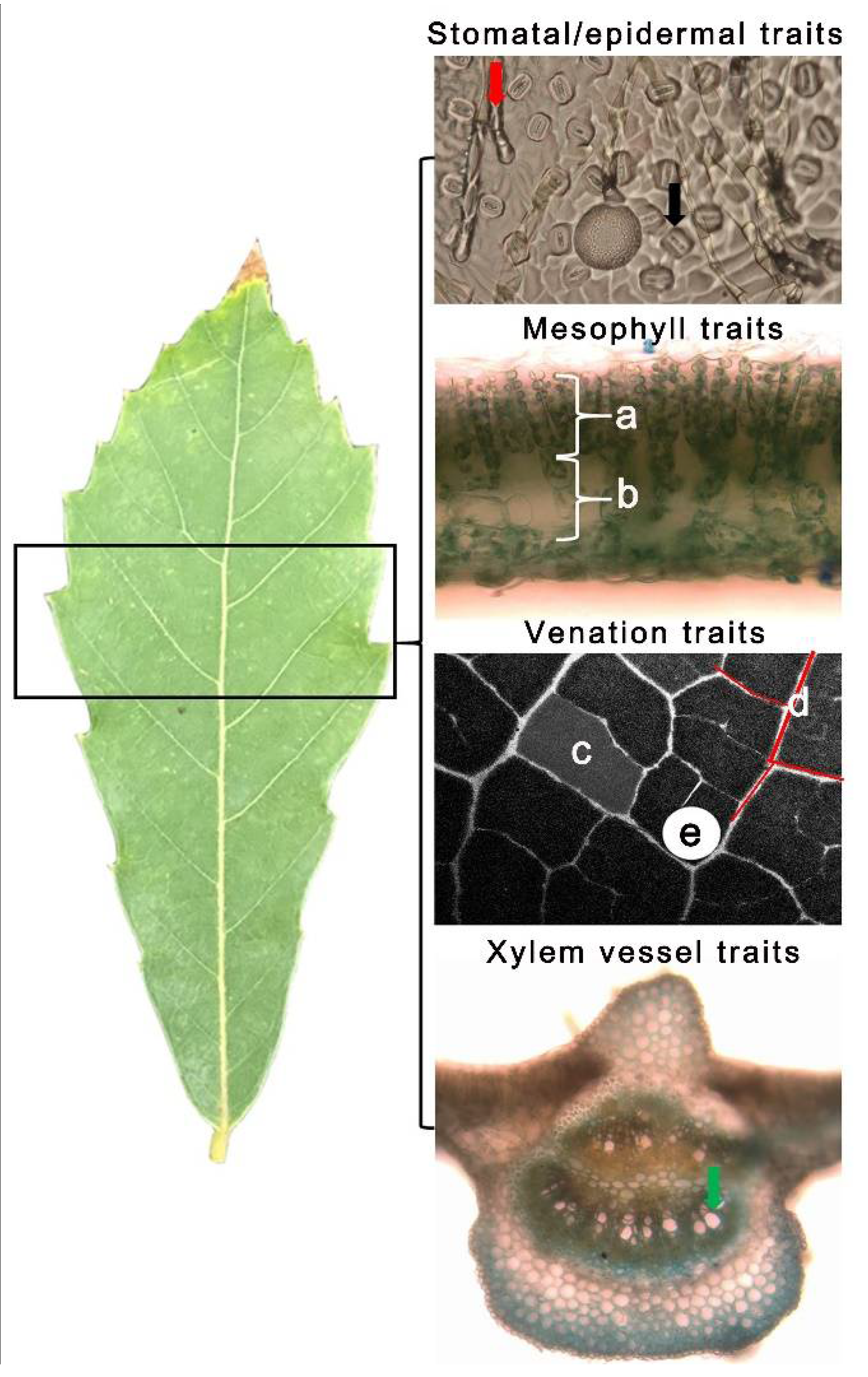
2.1. Leaf Anatomical Structures in Well-Watered and Water Deficit Stressed Seedlings
2.2. Leaf Physiological Traits in Well-Watered and Water-Stressed Seedlings
2.3. Principal Component Analysis Biplot
2.4. Starch Content in Well-Watered and Water-Stressed Seedlings
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Plant Materials and Experimental Design
4.3. Leaf Anatomical Traits Measurement
4.4. Leaf Gas Exchange Measurement
4.5. Starch Content Determination
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Convention to Combat Desertification [UNCCD]. Available online: https://www.unccd.int/news-stories/stories/latest-climate-report-underscores-urgent-need-act-drought (accessed on 2 May 2022).
- Méndez-Toribio, M.; Ibarra-Manríquez, G.; Paz, H.; Lebrija-Trejos, E. Atmospheric and soil drought risks combined shape community assembly of trees in a tropical dry forest. J. Ecol. 2020, 108, 1347–1357. [Google Scholar] [CrossRef]
- Resende, A.F.; Piedade, M.T.F.; Feitosa, Y.O.; Andrade, V.H.F.; Trumbore, S.E.; Durgante, F.M.; Macedo, M.O.; Schöngart, J. Flood-pulse disturbances as a threat for long-living Amazonian trees. New Phytol. 2020, 227, 1790–1803. [Google Scholar]
- Han, J.; Singh, V.P. Forecasting of droughts and tree mortality under global warming: A review of causative mechanisms and modeling methods. J. Water Clim. Chang. 2020, 11, 600–632. [Google Scholar]
- Dai, A. Drought under global warming: A Review. WIREs Clim. Chang. 2020, 2, 45–65. [Google Scholar] [CrossRef] [Green Version]
- Matesanz, S.; Ramos-Muñoz, M.; Moncalvillo, B.; Rubio Teso, M.L.; García de Dionisio, S.L.; Romero, J.; Iriondo, J.M. Plasticity to drought and ecotypic differentiation in populations of a crop wild relative. AoB Plants 2020, 12, plaa006. [Google Scholar] [CrossRef]
- Chen, J.-J.; Sun, Y.; Kopp, K.; Oki, L.; Jones, S.B.; Hipps, L. Effects of water availability on leaf trichome density and plant growth and development of Shepherdia × utahensis. Front. Plant Sci. 2022, 13, 855858. [Google Scholar] [CrossRef]
- Fahn, A. Structure and Function of Secretory Cells. In Advances in Botanical Research, Incorporating Advances in Plant Pathology; Hallahan, D.L., Gray, J.C., Callow, J.A., Eds.; Academic Press: London, UK, 2000; Volume 31, pp. 37–66. [Google Scholar]
- Huchelmann, A.; Boutry, M.; Hachez, C. Plant glandular trichomes: Natural cell factories of high biotechnological interest. Plant Physiol. 2017, 175, 6–22. [Google Scholar] [CrossRef] [Green Version]
- Perez-Estrada, L.B.; Cano-Santana, Z.; Oyama, K. Variation in leaf trichomes of Wigandia urens: Environmental factors and physiological consequences. Tree Physiol. 2000, 20, 629–632. [Google Scholar] [CrossRef] [Green Version]
- Konrad, W.; Burkhardt, J.; Ebner, M.; Roth-Nebelsick, A. Leaf pubescence as a possibility to increase water use efficiency by promoting condensation. Ecohydrology 2014, 8, 480–492. [Google Scholar] [CrossRef]
- Galdon-Armero, J.; Fullana-Pericas, M.; Mulet, P.A.; Conesa, M.A.; Martin, C.; Galmes, J. The ratio of trichomes to stomata is associated with water use efficiency in Solanum lycopersicum (tomato). Plant J. 2018, 96, 607–619. [Google Scholar] [CrossRef] [Green Version]
- Gasparini, K.; da Silva, M.F.; Costa, L.C.; Martins, S.C.V.; Ribeiro, D.M.; Peres, L.E.P.; Zsögön, A. The lanata trichome mutation increases stomatal conductance and reduces leaf temperature in tomato. J. Plant Physiol. 2021, 260, 153413. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, S.; Chi, Y.; Li, Q.; Huang, K.; Yu, Q. Responses of photosynthetic parameters to drought in subtropical forest ecosystem of China. Sci. Rep. 2015, 5, 18254. [Google Scholar] [CrossRef]
- Hernandez, J.O.; Quimado, M.O.; Fernando, E.S.; Pulan, D.E.; Malabrigo, P.L.; Maldia, L.S.J. Functional traits of stem and leaf of Wrightia candollei S. Vidal. Philipp. J. Sci. 2019, 148, 307–314. [Google Scholar]
- Kulkarni, M.; Deshpande, U. Anatomical breeding for altered leaf parameters in tomato genotypes imparting drought resistance using leaf strength index. Asian J. Plant Sci. 2006, 5, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Cai, D.; Wang, J.; Cao, J.; Wen, Y.; He, J.; Zhao, L.; Wang, D.; Zhang, S. Physiological and anatomical changes in two rapeseed (Brassica napus L.) genotypes under drought stress conditions. Oil Crop. Sci. 2021, 6, 97–104. [Google Scholar] [CrossRef]
- Vollenweider, P.; Menard, T.; Arend, M.; Kuster, T.M.; Günthardt-Goerg, M.S. Structural changes associated with drought stress symptoms in foliage of central European oaks. Trees 2015, 30, 883–900. [Google Scholar] [CrossRef]
- Falchi, R.; Petrussa, E.; Braidot, E.; Sivilotti, P.; Boscutti, F.; Vuerich, M.; Calligaro, C.; Filippi, A.; Herrera, J.C.; Sabbatini, P.; et al. Analysis of non-structural carbohydrates and xylem anatomy of leaf petioles offers new insights in the drought response of two grapevine cultivars. Int. J. Mol. Sci. 2020, 21, 1457. [Google Scholar] [CrossRef] [Green Version]
- Secchi, F.; Zwieniecki, M.A. Sensing embolism in xylem vessels: The role of sucrose as a trigger for refilling. Plant Cell Environ. 2011, 34, 514–524. [Google Scholar]
- Baek, S.G.; Park, J.H.; Na, C.S.; Lee, B.; Cheng, H.C.; Woo, S.Y. The morphological characteristics of Pterocarpus indicus induced by elevated ozone under well-watered and drought conditions. For. Sci. Technol. 2018, 14, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Harayama, H.; Kitao, M.; Agathokleous, E.; Ishida, A. Effects of major vein blockage and aquaporin inhibition on leaf hydraulics and stomatal conductance. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190799. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Avramova, V.; Baggerman, G.; Van Raemdonck, G.; Valkenborg, D.; Van Ostade, X.; Guisez, Y.; Prinsen, E.; Asard, H.; Van den Ende, W.; et al. Starch biosynthesis contributes to the maintenance of photosynthesis and leaf growth under drought stress in maize. Plant Cell Environ. 2020, 43, 2254–2271. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Olson, E.J.; Dodd, J.P.; Rivera, M.A. Prosopis sp. tree-ring oxygen and carbon isotope record of regional-scale hydroclimate variability during the last 9500 years in the Atacama Desert. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 538, 109408. [Google Scholar] [CrossRef]
- Burlett, R.; Parise, C.; Capdeville, G.; Cochard, H.; Lamarque, L.J.; King, A.; Delzon, S. Measuring xylem hydraulic vulnerability for long-vessel species: An improved methodology with the flow centrifugation technique. Ann. For. Sci. 2022, 79, 5. [Google Scholar] [CrossRef]
- Mediavilla, S.; Martín, I.; Babiano, J.; Escudero, A. Foliar plasticity related to gradients of heat and drought stress across crown orientations in three Mediterranean Quercus species. PLoS ONE 2019, 14, e0224462. [Google Scholar] [CrossRef]
- Gonzales, W.L.; Negritto, M.A.; Suarez, L.H.; Gianoli, E. Induction of glandular and non-glandular trichomes by damage in leaves of Madia sativa under contrasting water regimes. Acta Oecol. 2008, 33, 128–132. [Google Scholar]
- Hernandez, J.O.; Quimado, M.O.; Fernando, E.S.; Province, B. Xerophytic characteristics of Tectona philippinensis Benth. & Hook.f. Philipp. J. Sci. 2016, 145, 259–269. [Google Scholar]
- Fu, Q.S.; Yang, R.C.; Wang, H.S.; Zhao, B.; Zhou, C.L.; Ren, S.X.; Guo, Y.D. Leaf morphological and ultrastructural performance of eggplant (Solanum melongena L.) in response to water stress. Photosynthetica 2013, 51, 109–114. [Google Scholar] [CrossRef]
- Mo, Y.; Yang, R.; Liu, L.; Gu, X.; Yang, X.; Wang, Y.; Zhang, X.; Li, H. Growth, photosynthesis and adaptive responses of wild and domesticated watermelon genotypes to drought stress and subsequent re-watering. Plant Growth Regul. 2015, 79, 229–241. [Google Scholar] [CrossRef]
- Lenssen, A.W.; Banfield, J.D.; Cash, S.D. The influence of trichome density on the drying rate of alfalfa forage. Grass Forage Sci. 2001, 56, 1–9. [Google Scholar] [CrossRef]
- Sack, L.; Holbrook, N.M. Leaf hydraulics. Annu. Rev. Plant Biol. 2006, 57, 361–381. [Google Scholar]
- Shahzad, M.; Khan, Z.; Nazeer, W.; Arshad, S.F.; Ahmad, F. Effect of drought on trichome density and length in cotton (Gossypium hirsutum). J. Bioresour. Manag. 2021, 8, 154–167. [Google Scholar] [CrossRef]
- Jones, H.G. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Nadal, M.; Flexas, J. Mesophyll Conductance to CO2 Diffusion: Effects of Drought and Opportunities for Improvement. In Water Scarcity and Sustainable Agriculture in Semiarid Environment; Garciía-Tejero, I.F., Duraán, Z.V.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 403–438. [Google Scholar] [CrossRef]
- Théroux-Rancourt, G.; Roddy, A.B.; Earles, J.M.; Gilbert, M.E.; Zwieniecki, M.A.; Boyce, C.K.; Tholen, D.; McElrone, A.J.; Simonin, K.A.; Brodersen, C.R. Maximum CO2 diffusion inside leaves is limited by the scaling of cell size and genome size. Proc. R. Soc. B Biol. Sci. 2021, 288, 20203145. [Google Scholar] [CrossRef]
- Tosens, T.; Niinemets, Ü.; Westoby, M.; Wright, I.J. Anatomical basis of variation in mesophyll resistance in eastern Australian sclerophylls: News of a long and winding path. J. Exp. Bot. 2012, 63, 5105–5119. [Google Scholar] [CrossRef] [Green Version]
- Ennajeh, M.; Vadel, A.M.; Cochard, H.; Khemira, H. Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. J. Hortic. Sci. Biotechnol. 2010, 85, 289–294. [Google Scholar] [CrossRef]
- Binks, O.; Meir, P.; Rowland, L.; da Costa, A.C.; Vasconcelos, S.S.; de Oliveira, A.A.; Ferreira, L.; Mencuccini, M. Limited acclimation in leaf anatomy to experimental drought in Tropical Rainforest Trees. Tree Physiol. 2016, 36, 1550–1561. [Google Scholar] [CrossRef]
- Haworth, M.; Centritto, M.; Giovannelli, A.; Marino, G.; Proietti, N.; Capitani, D.; De Carlo, A.; Loreto, F. Xylem morphology determines the drought response of two Arundo donax ecotypes from contrasting habitats. GCB Bioenergy 2016, 9, 119–131. [Google Scholar] [CrossRef]
- Dietrich, L.; Hoch, G.; Kahmen, A.; Körner, C. Losing half the conductive area hardly impacts the water status of mature trees. Sci. Rep. 2018, 8, 15006. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, A.L.; Brandon Pratt, R.; Venturas, M.D.; Hacke, U.G.; Lens, F. Large volume vessels are vulnerable to water-stress-induced embolism in stems of Poplar. IAWA J. 2019, 40, 4-S4. [Google Scholar] [CrossRef] [Green Version]
- Hacke, U.G.; Spicer, R.; Schreiber, S.G.; Plavcová, L. An ecophysiological and developmental perspective on variation in vessel diameter. Plant Cell Environ. 2016, 40, 831–845. [Google Scholar] [CrossRef]
- Guo, X.; Peng, C.; Li, T.; Huang, J.; Song, H.; Zhu, Q.; Wang, M. The effects of drought and re-watering on non-structural carbohydrates of pinus tabulaeformis seedlings. Biology 2021, 10, 281. [Google Scholar] [CrossRef]
- MacNeill, G.J.; Mehrpouyan, S.; Minow, M.A.; Patterson, J.A.; Tetlow, I.J.; Emes, M.J. Starch as a source, starch as a sink: The bifunctional role of starch in carbon allocation. J. Exp. Bot. 2017, 68, 4433–4453. [Google Scholar] [CrossRef]
- Kannenberg, S.A.; Phillips, R.P. Non-structural carbohydrate pools not linked to hydraulic strategies or carbon supply in tree saplings during severe drought and subsequent recovery. Tree Physiol. 2019, 40, 259–271. [Google Scholar] [CrossRef]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Hernandez, J.O.; An, J.Y.; Combalicer, M.S.; Chun, J.-P.; Oh, S.-K.; Park, B.B. Morpho-Anatomical traits and soluble sugar concentration largely explain the responses of three deciduous tree species to progressive water stress. Front. Plant Sci. 2021, 12, 738301. [Google Scholar] [CrossRef]
- Jimenez, S.; Dridi, J.; Gutierrez, D.; Moret, D.; Irigoyen, J.J.; Moreno, M.A.; Gogorcena, Y. Physiological, biochemical and molecular responses in four prunus rootstocks submitted to drought stress. Tree Physiol. 2013, 33, 1061–1075. [Google Scholar] [CrossRef]
- Snow, M.D.; Tingey, D.T. Evaluation of a system for the imposition of plant water stress. Plant Physiol. 1985, 77, 602–607. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Blonder, B.; Violle, C.; Bentley, L.P.; Enquist, B.J. Venation networks and the origin of the leaf economics spectrum. Ecol. Lett. 2011, 14, 91–100. [Google Scholar]
- Morris, D.L. Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef]








| Species | Treatment | Vein Density (mm mm−2) | Vein Distance (mm) | Vein Loopiness (Count mm−2) |
| Q. acutissima | WW | 5.93 (0.29) | 8.43 (0.20) | 8.61(0.43) |
| WS | 6.18 (0.23) | 8.43 (0.15) | 9.32 (0.21) | |
| Q. serrata | WW | 5.47 (0.27) b | 8.06 (0.28) a | 9.82 (0.59) b |
| WS | 12.73 (0.53) a | 5.15 (0.17) b | 13.74 (0.54) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, J.O.; Park, B.B. The Leaf Trichome, Venation, and Mesophyll Structural Traits Play Important Roles in the Physiological Responses of Oak Seedlings to Water-Deficit Stress. Int. J. Mol. Sci. 2022, 23, 8640. https://doi.org/10.3390/ijms23158640
Hernandez JO, Park BB. The Leaf Trichome, Venation, and Mesophyll Structural Traits Play Important Roles in the Physiological Responses of Oak Seedlings to Water-Deficit Stress. International Journal of Molecular Sciences. 2022; 23(15):8640. https://doi.org/10.3390/ijms23158640
Chicago/Turabian StyleHernandez, Jonathan O., and Byung Bae Park. 2022. "The Leaf Trichome, Venation, and Mesophyll Structural Traits Play Important Roles in the Physiological Responses of Oak Seedlings to Water-Deficit Stress" International Journal of Molecular Sciences 23, no. 15: 8640. https://doi.org/10.3390/ijms23158640







