High-Intensity Focused Ultrasound Induces Adipogenesis via Control of Cilia in Adipose-Derived Stem Cells in Subcutaneous Adipose Tissue
Abstract
1. Introduction
2. Results
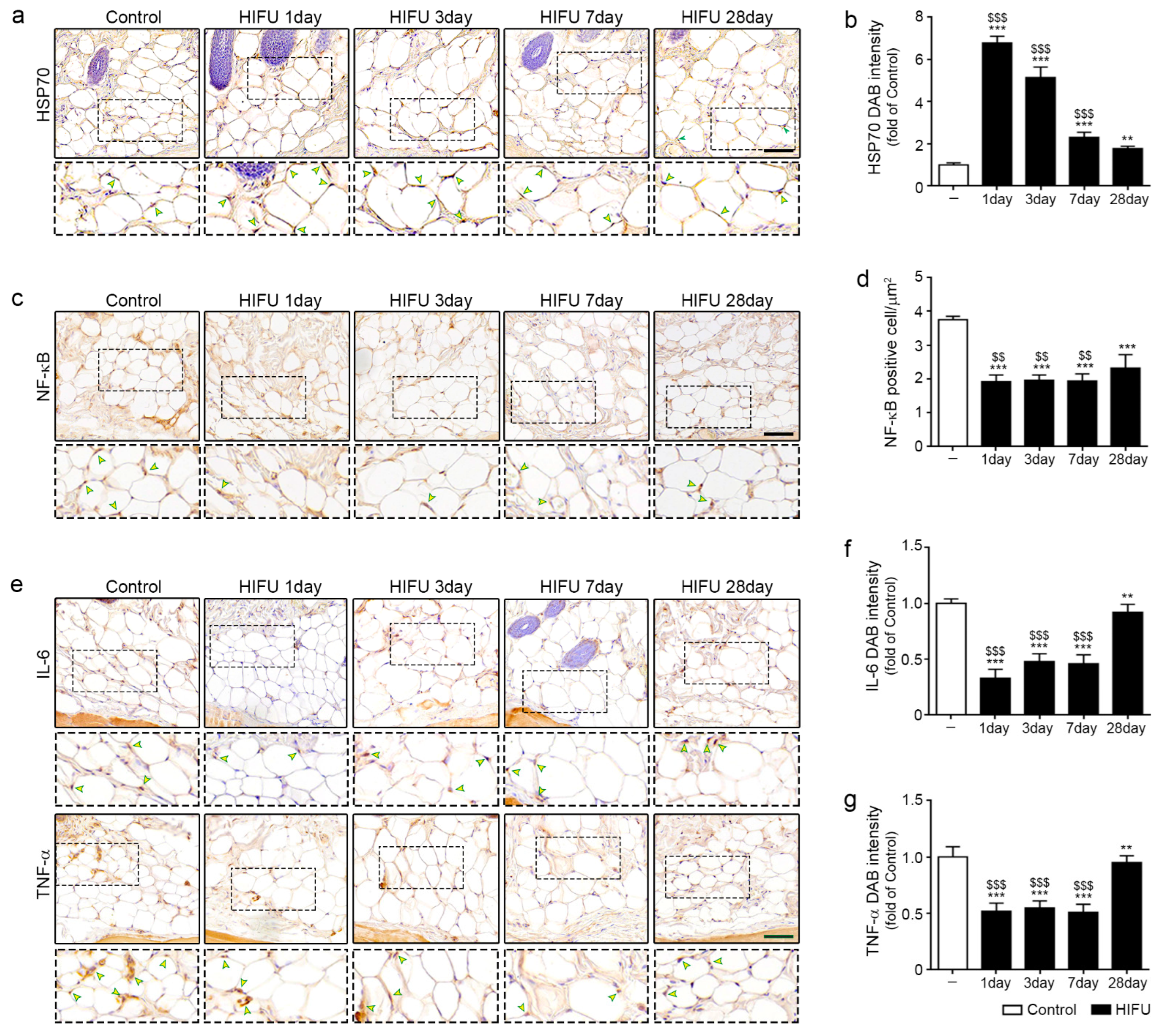
2.1. HIFU Increased Levels of HSP70 and Decreased Those of NF-κB, IL-6, and TNF-α
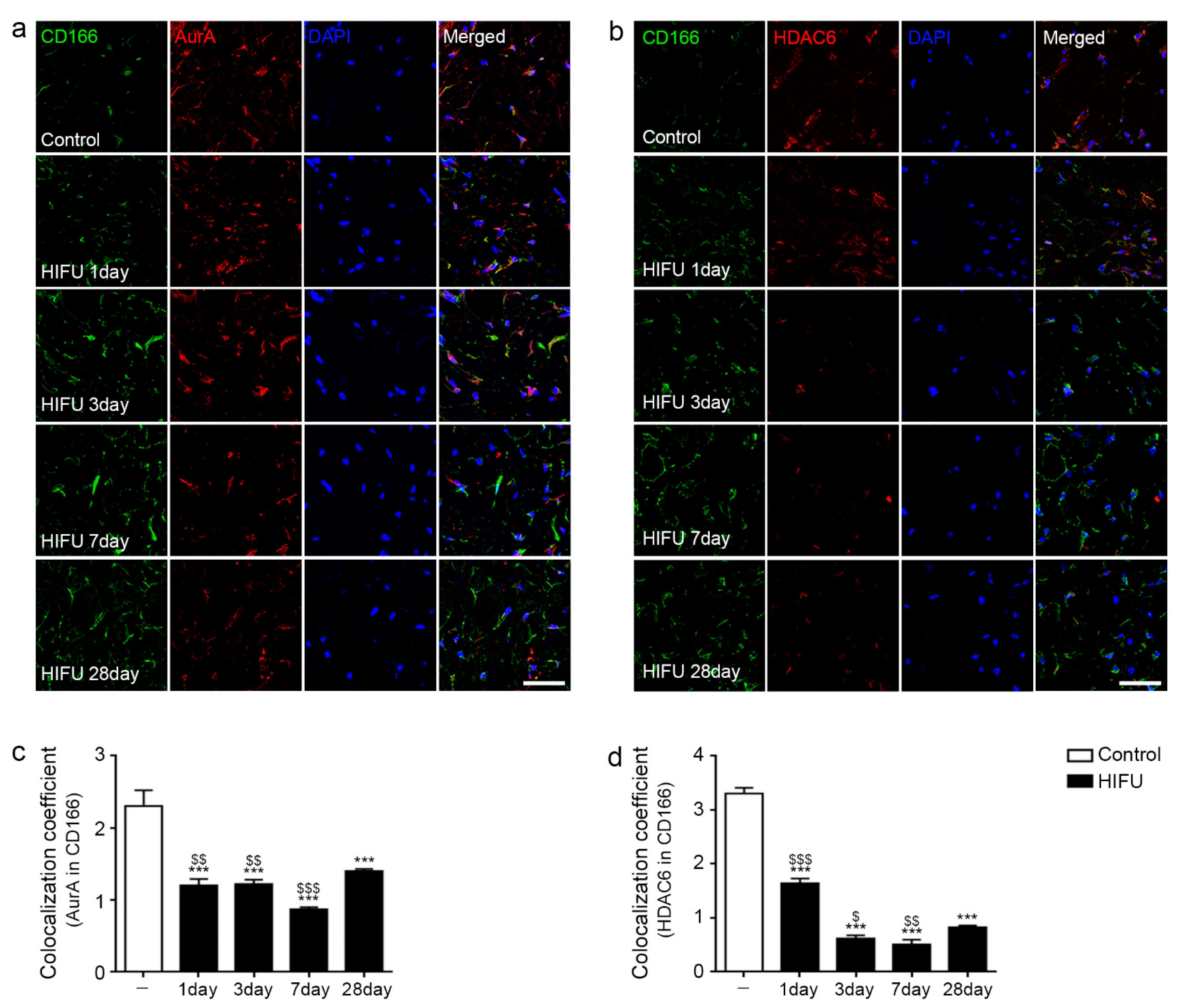
2.2. HIFU Decreased Expression of Cilia Disassembly Protein in the ASCs
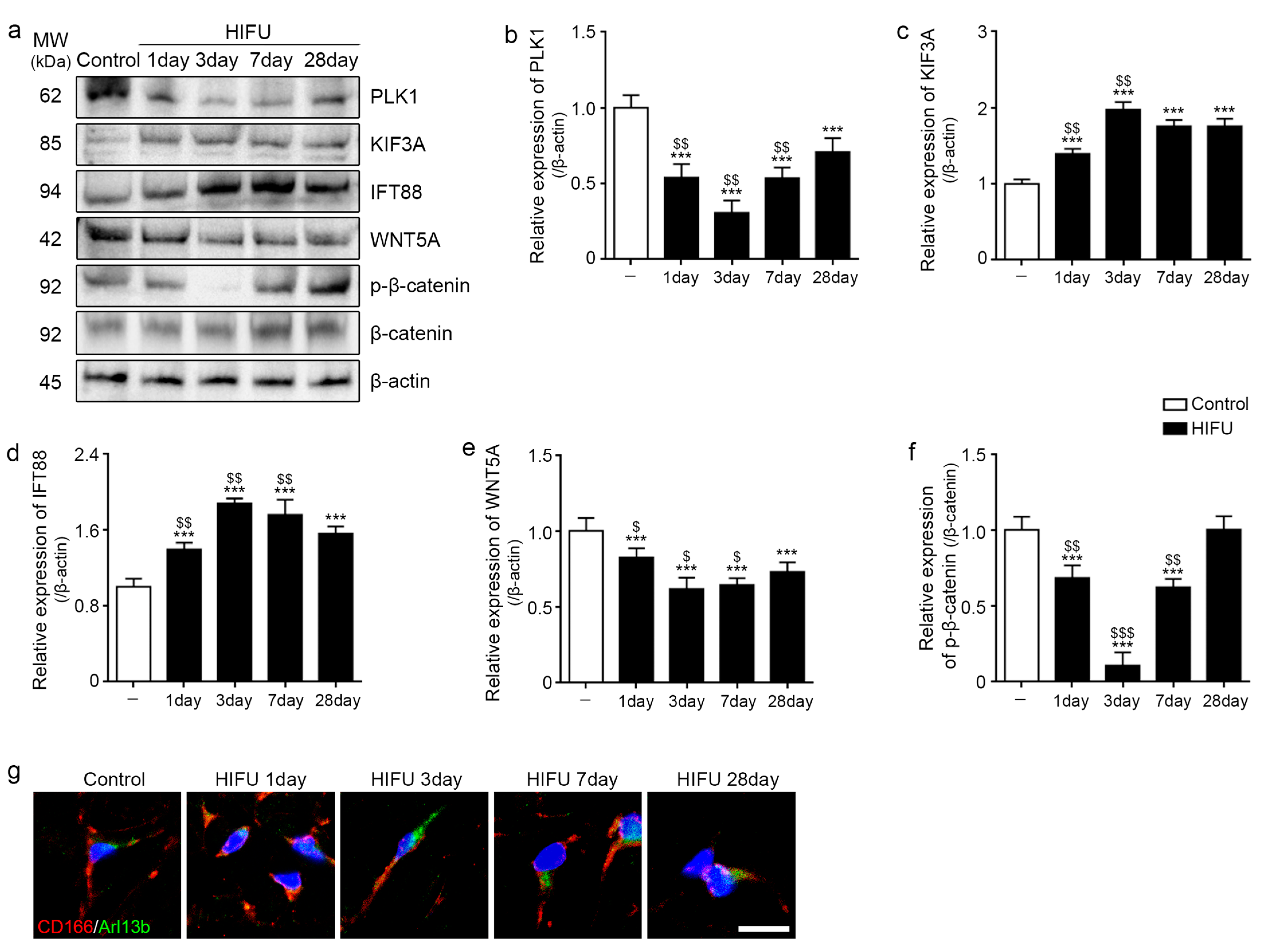
2.3. HIFU Increased Levels of Cilia Assembly Proteins and Decreased Those of Wnt5a/β-Catenin
2.4. HIFU Induced Adipogenesis in sWAT
3. Discussion
4. Materials and Methods
4.1. HIFU System
4.2. Method of Temperature Measurement
4.3. Animal Experiments and HIFU Application
4.4. Preparation of Paraffin-Embedded Skin and sWAT Tissue Sections
4.5. DAB Staining
4.6. Immunofluorescence
4.7. Western Blots
4.8. Hematoxylin and Eosin Staining
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wölfle, U.; Seelinger, G.; Bauer, G.; Meinke, M.C.; Lademann, J.; Schempp, C.M. Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Skin Pharmacol. Physiol. 2014, 27, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Dahmane, R.G.; Godić, A. Intrinsic skin aging: The role of oxidative stress. Acta Dermatovenerol. Alp. Pannonica Adriat. 2012, 21, 33–36. [Google Scholar] [PubMed]
- Nishigori, C.; Hattori, Y.; Arima, Y.; Miyachi, Y. Photoaging and oxidative stress. Exp. Dermatol. 2003, 12, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Binic, I.; Lazarevic, V.; Ljubenovic, M.; Mojsa, J.; Sokolovic, D. Skin ageing: Natural weapons and strategies. Evid. Based Complement Alternat. Med. 2013, 2013, 827248. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Rodríguez, S.A.; Grochová, D.; McKenna, T.; Borate, B.; Trivedi, N.S.; Erdos, M.R.; Eriksson, M. Global genome splicing analysis reveals an increased number of alternatively spliced genes with aging. Aging Cell 2016, 15, 267–278. [Google Scholar] [CrossRef]
- Salzer, M.C.; Lafzi, A.; Berenguer-Llergo, A.; Youssif, C.; Castellanos, A.; Solanas, G.; Peixoto, F.O.; Attolini, C.S.-O.; Prats, N.; Aguilera, M.; et al. Identity noise and adipogenic traits characterize dermal fibroblast aging. Cell 2018, 175, 1575–1590.22. [Google Scholar] [CrossRef]
- Pellettieri, J.; Sánchez Alvarado, A. Cell turnover and adult tissue homeostasis: From humans to planarians. Annu. Rev. Genet. 2007, 41, 83–105. [Google Scholar] [CrossRef]
- Tchoukalova, Y.; Koutsari, C.; Jensen, M. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia 2007, 50, 151–157. [Google Scholar] [CrossRef]
- Liu, G.P.; Liao, C.H.; Xu, Y.P. Proliferation and adipogenic differentiation of human adipose-derived stem cells isolated from middle-aged patients with prominent orbital fat in the lower eyelids. Plast. Aesthet. Res. 2016, 3, 322–327. [Google Scholar] [CrossRef][Green Version]
- Malicki, J.J.; Johnson, C.A. The cilium: Cellular antenna and central processing unit. Trends Cell Biol. 2017, 27, 126–140. [Google Scholar] [CrossRef]
- He, M.; Agbu, S.; Anderson, K.V. Microtubule motors drive hedgehog signaling in primary cilia. Trends Cell Biol. 2017, 27, 110–125. [Google Scholar] [CrossRef]
- Praetorius, H.A. The primary cilium as sensor of fluid flow: New building blocks to the model. A review in the theme: Cell signaling: Proteins, pathways and mechanisms. Am. J. Physiol. Cell Physiol. 2015, 308, C198–C208. [Google Scholar] [CrossRef]
- Goto, H.; Inaba, H.; Inagaki, M. Mechanisms of ciliogenesis suppression in dividing cells. Cell Mol. Life Sci. 2017, 74, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Forcioli-Conti, N.; Lacas-Gervais, S.; Dani, C.; Peraldi, P. The primary cilium undergoes dynamic size modifications during adipocyte differentiation of human adipose stem cells. Biochem. Biophys. Res. Commun. 2015, 458, 117–122. [Google Scholar] [CrossRef]
- Bodle, J.C.; Loboa, E.G. Concise review: Primary cilia: Control centers for stem cell lineage specification and potential targets for cell-based therapies. Stem Cells 2016, 34, 1445–1454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dalbay, M.T.; Thorpe, S.D.; Connelly, J.T.; Chapple, J.P.; Knight, M.M. Adipogenic differentiation of hMSCs is mediated by recruitment of IGF-1r onto the primary cilium associated with cilia elongation. Stem Cells 2015, 33, 1952–1961. [Google Scholar] [CrossRef]
- Bae, Y.K.; Kim, G.H.; Kwon, J.H.; Kim, M.; Choi, S.J.; Oh, W.; Um, S.; Jin, H.J. Primary cilia mediate Wnt5a/β-catenin signaling to regulate adipogenic differentiation of human umbilical cord blood-derived mesenchymal stem cells following calcium induction. Tissue Eng. Regen. Med. 2020, 17, 193–202. [Google Scholar] [CrossRef]
- Bae, Y.K.; Kwon, J.H.; Kim, M.; Kim, G.H.; Choi, S.J.; Oh, W.; Yang, Y.S.; Jin, H.J.; Jeon, H.B. Intracellular calcium determines the adipogenic differentiation potential of human umbilical cord blood-derived mesenchymal stem cells via the Wnt5a/β-Catenin signaling pathway. Stem Cells Int. 2018, 2018, 6545071. [Google Scholar] [CrossRef]
- Corbit, K.C.; Shyer, A.E.; Dowdle, W.E.; Gaulden, J.; Singla, V.; Reiter, J.F. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat. Cell Biol. 2008, 10, 70–76. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Zhu, T.; Shen, Y.; Tang, X.; Fang, L.; Xu, Y. Cross-talking between PPAR and WNT signaling and its regulation in mesenchymal stem cell differentiation. Curr. Stem Cell Res. Ther. 2016, 11, 247–254. [Google Scholar] [CrossRef]
- Ritter, A.; Roth, S.; Kreis, N.N.; Friemel, A.; Hoock, S.C.; Steglich Souto, A.; Eichbaum, C.; Neuhoff, A.; Chen, Q.; Solbach, C. Primary cilia in trophoblastic cells: Potential involvement in preeclampsia. Hypertension 2020, 76, 1491–1505. [Google Scholar] [CrossRef]
- Ritter, A.; Friemel, A.; Kreis, N.N.; Hoock, S.C.; Roth, S.; Kielland-Kaisen, U.; Brüggmann, D.; Solbach, C.; Louwen, F.; Yuan, J. Primary cilia are dysfunctional in obese adipose-derived mesenchymal stem cells. Stem Cell Rep. 2018, 10, 583–599. [Google Scholar] [CrossRef]
- Ritter, A.; Kreis, N.-N.; Roth, S.; Friemel, A.; Jennewein, L.; Eichbaum, C.; Solbach, C.; Louwen, F.; Yuan, J. Restoration of primary cilia in obese adipose-derived mesenchymal stem cells by inhibiting Aurora A or extracellular signal-regulated kinase. Stem Cell Res. Ther. 2019, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.I. Cells in stress: Transcriptional activation of heat shock genes. Science 1993, 259, 1409–1410. [Google Scholar] [CrossRef]
- Morimoto, R.I. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998, 12, 3788–3796. [Google Scholar] [CrossRef]
- Wu, C. Heat shock transcription factors: Structure and regulation. Annu. Rev. Cell Dev. Biol. 1995, 11, 441–469. [Google Scholar] [CrossRef]
- Oberringer, M.; Baum, H.; Jung, V.; Welter, C.; Frank, J.; Kuhlmann, M.; Mutschler, W.; Hanselmann, R. Differential expression of heat shock protein 70 in well healing and chronic human wound tissue. Biochem. Biophys. Res. Commun. 1995, 214, 1009–1014. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Y.; Zhang, Y.; Jin, L.; Luo, L.; Xue, B.; Lu, C.; Zhang, X.; Yin, Z. Hsp70 inhibits lipopolysaccharide-induced NF-kappaB activation by interacting with TRAF6 and inhibiting its ubiquitination. FEBS Lett. 2006, 580, 3145–3152. [Google Scholar] [CrossRef] [PubMed]
- Ferat-Osorio, E.; Sánchez-Anaya, A.; Gutiérrez-Mendoza, M.; Boscó-Gárate, I.; Wong-Baeza, I.; Pastelin-Palacios, R.; Pedraza-Alva, G.; Bonifaz, L.C.; Cortés-Reynosa, P.; Pérez-Salazar, E.; et al. Heat shock protein 70 down-regulates the production of toll-like receptor-induced pro-inflammatory cytokines by a heat shock factor-1/constitutive heat shock element-binding factor-dependent mechanism. J. Inflamm. 2014, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Lee, S.; Lee, C.T.; Kim, Y.W.; Han, S.K.; Shim, Y.S. Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. J. Immunol. 2000, 164, 5416–5523. [Google Scholar] [CrossRef]
- Fatemi, A. High-intensity focused ultrasound effectively reduces adipose tissue. Semin. Cutan. Med. Surg. 2009, 28, 257–262. [Google Scholar] [CrossRef]
- Haar, G.T.; Coussios, C. High intensity focused ultrasound: Physical principles and devices. Int. J. Hyperth. 2007, 23, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Jewell, M.L.; Desilets, C.; Smoller, B.R. Evaluation of a novel high-intensity focused ultrasound device: Preclinical studies in a porcine model. Aesthet. Surg. J. 2011, 31, 429–434. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.B.; Cao, Y.D.; Zhou, Q.; Zhang, Y.; Xu, Z.L.; Zhu, X.Q. Expression of tumor antigens and heat-shock protein 70 in breast cancer cells after high-intensity focused ultrasound ablation. Ann. Surg. Oncol. 2007, 14, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, X.Y.; Liu, Y.; Morse, M.A.; Lyerly, H.K.; Clay, T.M.; Zhong, P. Release of endogenous danger signals from HIFU-treated tumor cells and their stimulatory effects on APCs. Biochem. Biophys. Res. Commun. 2005, 335, 124–131. [Google Scholar] [CrossRef]
- Madersbacher, S.; Gröbl, M.; Kramer, G.; Dirnhofer, S.; Steiner, G.E.; Marberger, M. Regulation of heat shock protein 27 expression of prostatic cells in response to heat treatment. Prostate 1998, 37, 174–181. [Google Scholar] [CrossRef]
- Kramer, G.; Steiner, G.E.; Gröbl, M.; Hrachowitz, K.; Reithmayr, F.; Paucz, L.; Newman, M.; Madersbacher, S.; Gruber, D.; Susani, M.; et al. Response to sublethal heat treatment of prostatic tumor cells and of prostatic tumor infiltrating T-cells. Prostate 2004, 58, 109–120. [Google Scholar] [CrossRef]
- Xia, J.Z.; Xie, F.L.; Ran, L.F.; Xie, X.P.; Fan, Y.M.; Wu, F. High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes. Ultrasound Med. Biol. 2012, 38, 1363–1371. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.B.; Lu, P.; Xu, Z.L.; Chen, W.Z.; Zhu, H.; Jin, C.B. Activated anti-tumor immunity in cancer patients after high intensity focused ultrasound ablation. Ultrasound Med. Biol. 2004, 30, 1217–1222. [Google Scholar] [CrossRef]
- Pugacheva, E.N.; Jablonski, S.A.; Hartman, T.R.; Henske, E.P.; Golemis, E.A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 2007, 129, 1351–1363. [Google Scholar] [CrossRef]
- Peng, Q.; Alipour, H.; Porsborg, S.; Fink, T.; Zachar, V. Evolution of ASC immunophenotypical subsets during expansion in vitro. Int. J. Mol. Sci. 2020, 21, 1408. [Google Scholar] [CrossRef]
- Sadick, N.S.; Dorizas, A.S.; Krueger, N.; Nassar, A.H. The facial adipose system: Its role in facial aging and approaches to volume restoration. Dermatol. Surg. 2015, 41, S333–S339. [Google Scholar] [CrossRef]
- Marten, T.J.; Elyassnia, D. Fat grafting in facial rejuvenation. Clin. Plast. Surg. 2015, 42, 219–252. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Scherer, P.E. Skin aging: Are adipocytes the next target? Aging 2016, 8, 1457–1469. [Google Scholar] [CrossRef]
- Trivisonno, A.; Rossi, A.; Monti, M.; Di Nunno, D.; Desouches, C.; Cannistra, C.; Toietta, G. Facial skin rejuvenation by autologous dermal microfat transfer in photoaged patients: Clinical evaluation and skin surface digital profilometry analysis. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 1118–1128. [Google Scholar] [CrossRef]
- Donato, A.J.; Henson, G.D.; Hart, C.R.; Layec, G.; Trinity, J.D.; Bramwell, R.C.; Enz, R.A.; Morgan, R.G.; Reihl, K.; Hazra, S.; et al. The impact of ageing on adipose structure, function and vasculature in the B6D2F1 mouse: Evidence of significant multisystem dysfunction. J. Physiol. 2014, 592, 4083–4096. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Scherer, P.E. Dermal adipocytes: From irrelevance to metabolic targets? Trends Endocrinol. Metab. 2016, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sepe, A.; Tchkonia, T.; Thomou, T.; Zamboni, M.; Kirkland, J.L. Aging and regional differences in fat cell progenitors—A mini-review. Gerontology 2011, 57, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Wetzker, R.; Abdel-Naser, M.B.; Kruglikov, I.L. Role of adipose tissue in facial aging. Clin. Interv. Aging 2017, 12, 2069–2076. [Google Scholar] [CrossRef]
- Liang, Y.; Meng, D.; Zhu, B.; Pan, J. Mechanism of ciliary disassembly. Cell Mol. Life Sci. 2016, 73, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, I.; Dynlacht, B.D. Cilium assembly and disassembly. Nat. Cell Biol. 2016, 18, 711–717. [Google Scholar] [CrossRef]
- Saket, P.; Shobeihi, S.; Mehrdadi, S. Study of efficacy of esthetic High-Intensity Focused Ultrasound system on Iranian skin for reducing the laxity and wrinkles of aging. J. Cosmet. Dermatol. 2017, 16, 336–341. [Google Scholar] [CrossRef]
- Aoki, S.; Harada, K.; Yoko, S.K.; Nagata, S.; Imataki, R.; Arita, K. Expression of heat shock protein in response to mild short-term heat shock in human deciduous dental pulp fibroblast-like cells. J. Hard. Tissue Biol. 2016, 25, 257–262. [Google Scholar] [CrossRef]
- Sart, S.; Ma, T.; Li, Y. Preconditioning stem cells for in vivo delivery. Biores. Open Access 2014, 3, 137–149. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Harris, D.T. Effect of mild heat stress on the proliferative and differentiative ability of human mesenchymal stromal cells. Cytotherapy 2015, 17, 359–368. [Google Scholar] [CrossRef]
- Qiao, P.F.; Yao, L.; Zhang, X.C.; Li, G.D.; Wu, D.Q. Heat shock pretreatment improves stem cell repair following ischemia-reperfusion injury via autophagy. World J. Gastroenterol. 2015, 21, 12822–12834. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Wang, Q.; Xie, J.; Xie, C.; Fu, X. Heat shock pretreatment improves mesenchymal stem cell viability by heat shock proteins and autophagy to prevent cisplatin-induced granulosa cell apoptosis. Stem Cell Res. Ther. 2019, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yuan, X.; Zhang, J.; Lu, T.; Yao, J.; Zheng, J.; Cai, J.; Xiao, J.; Chen, H.; Xie, S.; et al. Heat shock preconditioning mesenchymal stem cells attenuate acute lung injury via reducing NLRP3 inflammasome activation in macrophages. Stem Cell Res. Ther. 2021, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.; Kim, H.M.; Batsukh, S.; Sun, H.J.; Kim, T.; Kang, D.; Son, K.H.; Byun, K. High-Intensity Focused Ultrasound Induces Adipogenesis via Control of Cilia in Adipose-Derived Stem Cells in Subcutaneous Adipose Tissue. Int. J. Mol. Sci. 2022, 23, 8866. https://doi.org/10.3390/ijms23168866
Oh S, Kim HM, Batsukh S, Sun HJ, Kim T, Kang D, Son KH, Byun K. High-Intensity Focused Ultrasound Induces Adipogenesis via Control of Cilia in Adipose-Derived Stem Cells in Subcutaneous Adipose Tissue. International Journal of Molecular Sciences. 2022; 23(16):8866. https://doi.org/10.3390/ijms23168866
Chicago/Turabian StyleOh, Seyeon, Hyoung Moon Kim, Sosorburam Batsukh, Hye Jin Sun, Taehui Kim, Donghwan Kang, Kuk Hui Son, and Kyunghee Byun. 2022. "High-Intensity Focused Ultrasound Induces Adipogenesis via Control of Cilia in Adipose-Derived Stem Cells in Subcutaneous Adipose Tissue" International Journal of Molecular Sciences 23, no. 16: 8866. https://doi.org/10.3390/ijms23168866
APA StyleOh, S., Kim, H. M., Batsukh, S., Sun, H. J., Kim, T., Kang, D., Son, K. H., & Byun, K. (2022). High-Intensity Focused Ultrasound Induces Adipogenesis via Control of Cilia in Adipose-Derived Stem Cells in Subcutaneous Adipose Tissue. International Journal of Molecular Sciences, 23(16), 8866. https://doi.org/10.3390/ijms23168866






