Dual Function of Secreted APE1/Ref-1 in TNBC Tumorigenesis: An Apoptotic Initiator and a Regulator of Chronic Inflammatory Signaling
Abstract
:1. Introduction
2. Results
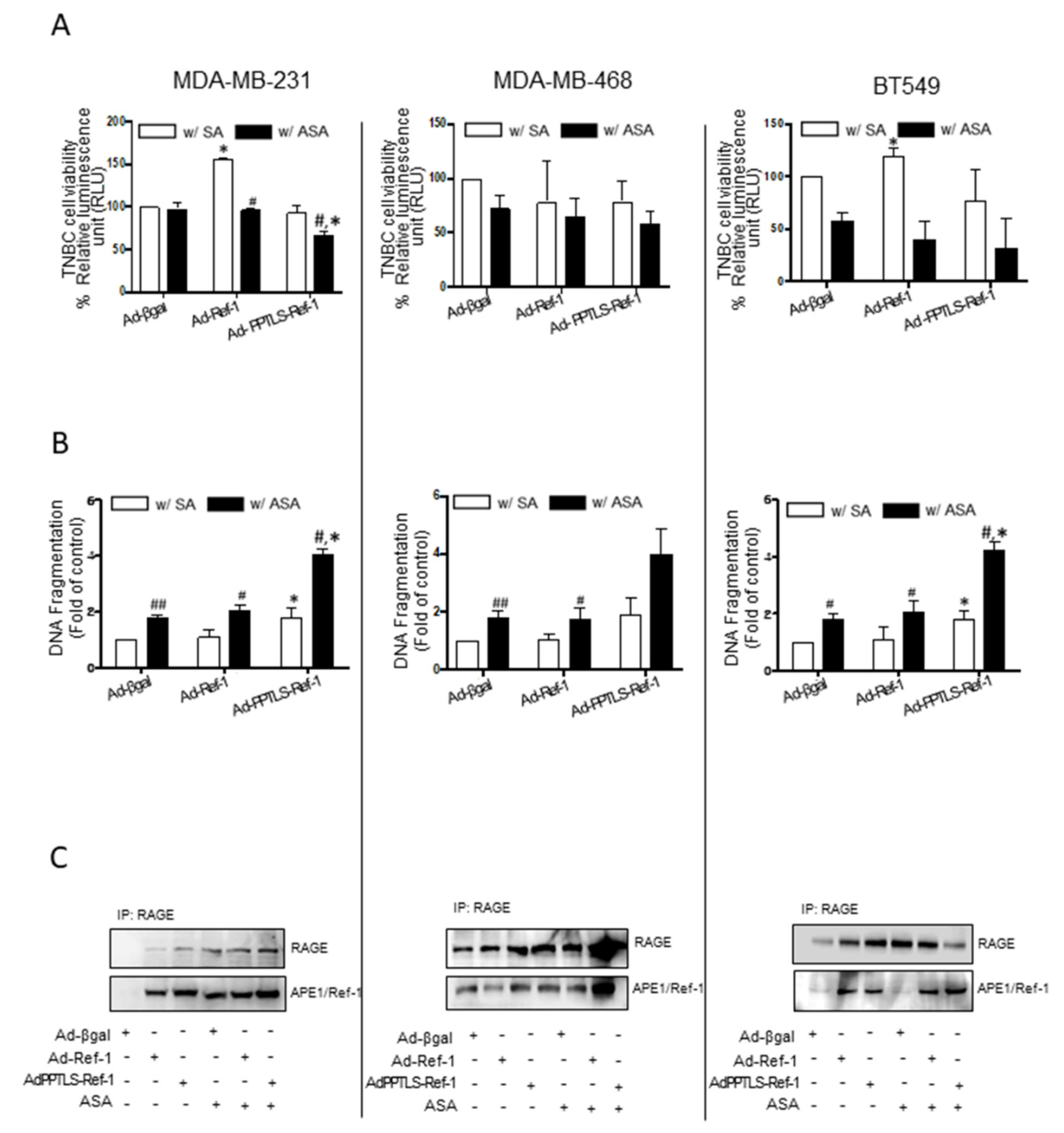
2.1. Adenovirus-Mediated PPTLS-APE1/Ref-1 Bound to RAGE in Response to Acetylation-Caused Apoptotic Cell Death
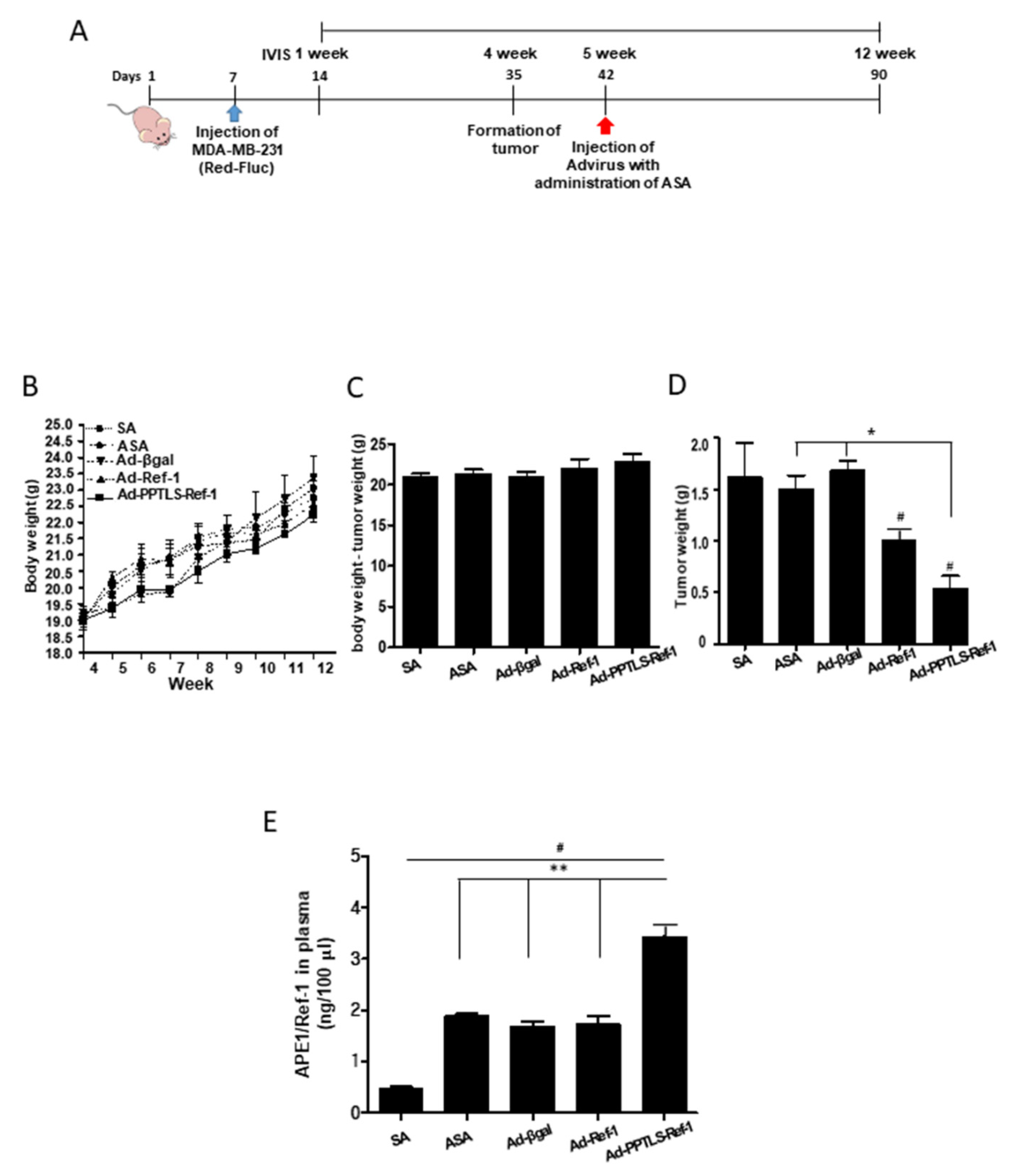
2.2. Correlation between PPTLS-APE1/Ref-1 Levels in Blood and the Inhibition of Tumor Growth
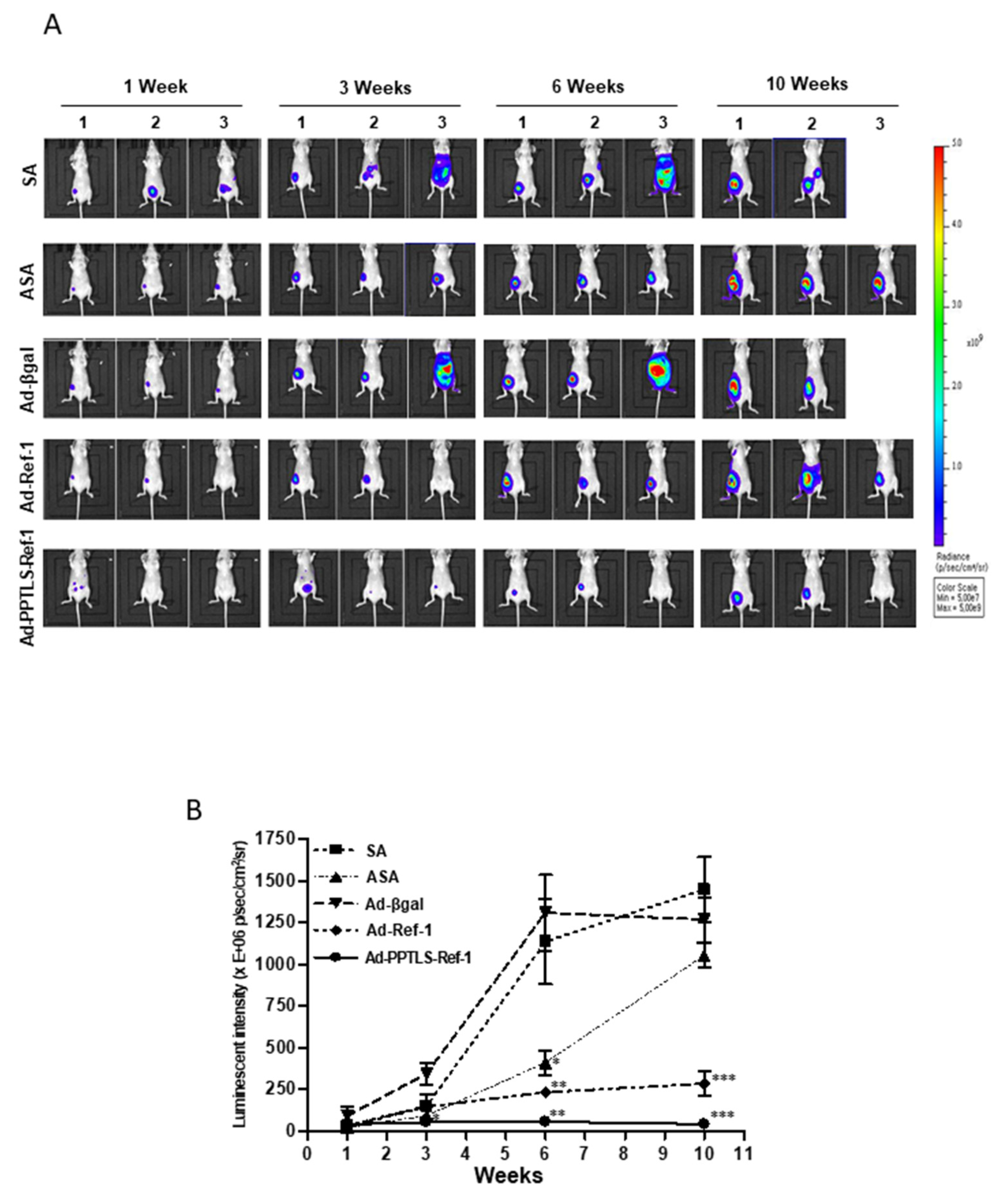
2.3. Retarded Tumor Cell Growth in Xenografts Secreting PPTLS-APE1/Ref-1 into the Blood
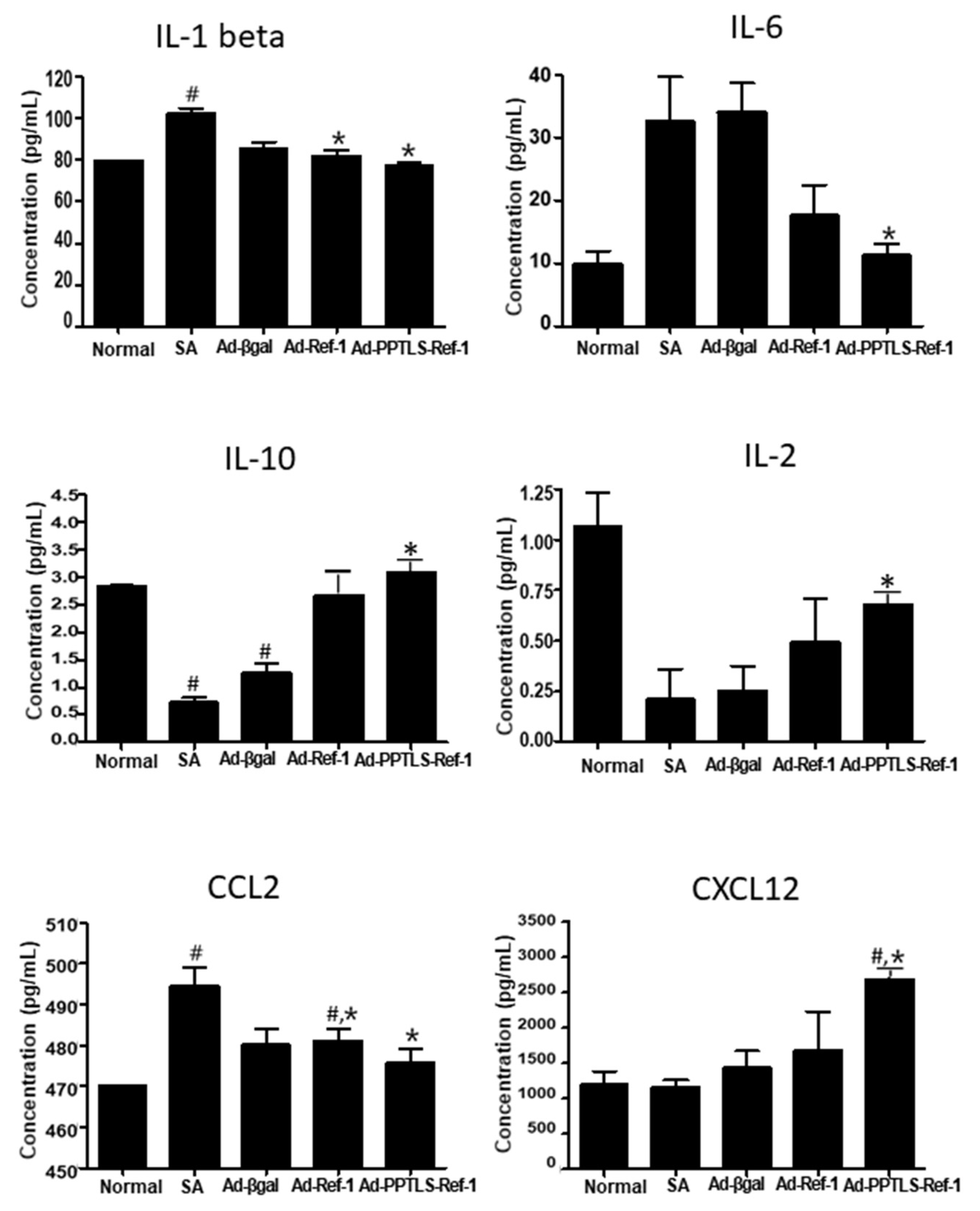
2.4. Plasma PPTLS-APE1/Ref-1 in Cytokine Regulation
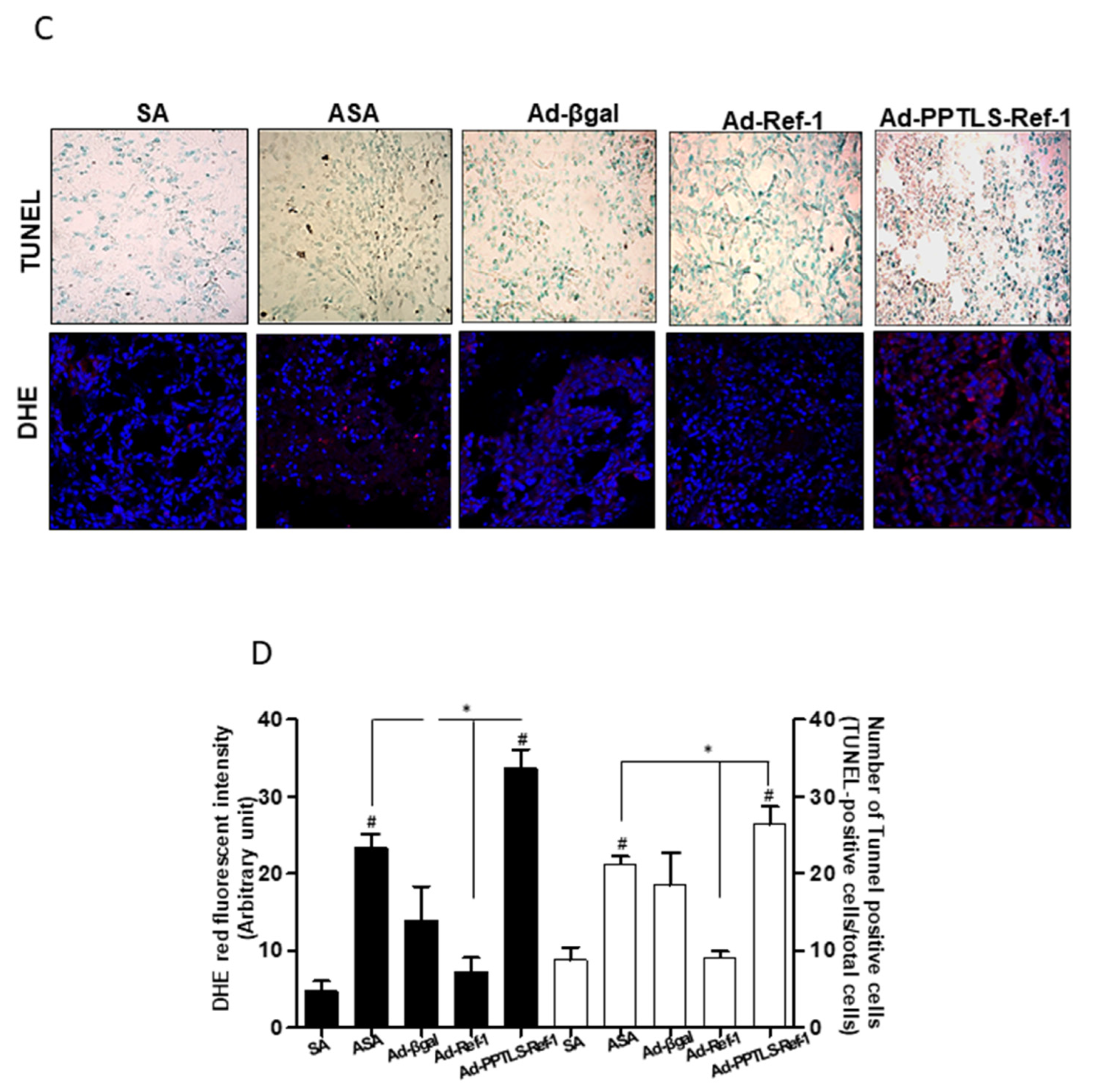
2.5. Anti-Inflammatory Signaling in Tumors Expressing PPTLS-APE1/Ref-1 Derived from MDA-MB-231 Orthotopic Xenografts
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability and Apoptotic DNA Fragmentation Assay
4.3. Animal Experiments
4.4. Measurement of APE1/Ref-1 and Cytokine Levels in Xenograft Plasma
4.5. Histological Analysis
4.6. Immunoblotting
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- O’Brien, K.M.; Cole, S.R.; Tse, C.K.; Perou, C.M.; Carey, L.A.; Foulkes, W.D.; Dressler, L.G.; Geradts, J.; Millikan, R.C. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin. Cancer Res. 2010, 16, 6100–6110. [Google Scholar] [CrossRef]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2011, 2, 98. [Google Scholar] [CrossRef]
- Ben-Neriah, Y.; Karin, M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat. Immunol. 2011, 12, 715–723. [Google Scholar] [CrossRef]
- Luo, J.L.; Maeda, S.; Hsu, L.C.; Yagita, H.; Karin, M. Inhibition of NF-kappaB in cancer cells converts inflammation-induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell 2004, 6, 297–305. [Google Scholar] [CrossRef]
- Ham, B.; Fernandez, M.C.; D’Costa, Z.; Brodt, P. The diverse roles of the TNF axis in cancer progression and metastasis. Trends Cancer Res. 2016, 11, 1–27. [Google Scholar]
- Rabinovich, A.; Medina, L.; Piura, B.; Segal, S.; Huleihel, M. Regulation of ovarian carcinoma SKOV-3 cell proliferation and secretion of MMPs by autocrine IL-6. Anticancer Res. 2007, 27, 267–272. [Google Scholar]
- Hirano, T.; Ishihara, K.; Hibi, M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000, 19, 2548–2556. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X. Association between serum cytokines and progression of breast cancer in Chinese population. Medicine 2017, 96, e8840. [Google Scholar] [CrossRef]
- Blobe, G.C.; Schiemann, W.P.; Lodish, H.F. Role of transforming growth factor beta in human disease. N. Engl. J. Med. 2000, 342, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Hartman, Z.C.; Poage, G.M.; den Hollander, P.; Tsimelzon, A.; Hill, J.; Panupinthu, N.; Zhang, Y.; Mazumdar, A.; Hilsenbeck, S.G.; Mills, G.B.; et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. 2013, 73, 3470–3480. [Google Scholar] [CrossRef] [PubMed]
- Pileczki, V.; Braicu, C.; Gherman, C.D.; Berindan-Neagoe, I. TNF-alpha gene knockout in triple negative breast cancer cell line induces apoptosis. Int. J. Mol. Sci. 2012, 14, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Pham, E.; Yin, M.; Peters, C.G.; Lee, C.R.; Brown, D.; Xu, P.; Man, S.; Jayaraman, L.; Rohde, E.; Chow, A.; et al. Preclinical Efficacy of Bevacizumab with CRLX101, an Investigational Nanoparticle-Drug Conjugate, in Treatment of Metastatic Triple-Negative Breast Cancer. Cancer Res. 2016, 76, 4493–4503. [Google Scholar] [CrossRef] [PubMed]
- Gamucci, T.; Mentuccia, L.; Natoli, C.; Sperduti, I.; Cassano, A.; Michelotti, A.; Di Lauro, L.; Sergi, D.; Fabi, A.; Sarobba, M.G.; et al. A Real-World Multicentre Retrospective Study of Paclitaxel-Bevacizumab and Maintenance Therapy as First-Line for HER2-Negative Metastatic Breast Cancer. J. Cell Physiol. 2017, 232, 1571–1578. [Google Scholar] [CrossRef]
- Tell, G.; Quadrifoglio, F.; Tiribelli, C.; Kelley, M.R. The many functions of APE1/Ref-1: Not only a DNA repair enzyme. Antioxid. Redox Signal. 2009, 11, 601–620. [Google Scholar] [CrossRef]
- Kelley, M.R.; Georgiadis, M.M.; Fishel, M.L. APE1/Ref-1 role in redox signaling: Translational applications of targeting the redox function of the DNA repair/redox protein APE1/Ref-1. Curr. Mol. Pharmacol. 2012, 5, 36–53. [Google Scholar] [CrossRef]
- Oliveira, T.T.; Coutinho, L.G.; de Oliveira, L.O.A.; Timoteo, A.R.S.; Farias, G.C.; Agnez-Lima, L.F. APE1/Ref-1 Role in Inflammation and Immune Response. Front. Immunol. 2022, 13, 793096. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kim, K.M.; Jeon, B.H.; Choi, S. Extracellularly secreted APE1/Ref-1 triggers apoptosis in triple-negative breast cancer cells via RAGE binding, which is mediated through acetylation. Oncotarget 2015, 6, 23383–23398. [Google Scholar] [CrossRef]
- Lee, Y.R.; Park, M.S.; Joo, H.K.; Kim, K.M.; Kim, J.; Jeon, B.H.; Choi, S. Therapeutic positioning of secretory acetylated APE1/Ref-1 requirement for suppression of tumor growth in triple-negative breast cancer in vivo. Sci. Rep. 2018, 8, 8701. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, C.S.; Joo, H.K.; Lee, Y.R.; Kang, G.; Kim, S.J.; Choi, S.; Lee, S.D.; Park, J.B.; Jeon, B.H. Cytoplasmic localization and redox cysteine residue of APE1/Ref-1 are associated with its anti-inflammatory activity in cultured endothelial cells. Mol. Cells 2013, 36, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Choi, S.; Lee, Y.R.; Joo, H.K.; Kang, G.; Kim, C.S.; Kim, S.J.; Lee, S.D.; Jeon, B.H. Secreted APE1/Ref-1 inhibits TNF-alpha-stimulated endothelial inflammation via thiol-disulfide exchange in TNF receptor. Sci. Rep. 2016, 6, 23015. [Google Scholar] [CrossRef]
- Choi, S.; Kim, K.M.; Choi, E. APE1/Ref-1 with reducing activity induces mesenchymal-to-epithelial transition in TNF-α-stimulated breast cancer cells. Integr. Cancer Sci. Ther. 2022, 9, 1–8. [Google Scholar]
- Joo, H.K.; Lee, Y.R.; Lee, E.O.; Park, M.S.; Choi, S.; Kim, C.S.; Park, J.B.; Jeon, B.H. The extracellular role of Ref-1 as anti-inflammatory function in lipopolysaccharide-induced septic mice. Free Radic. Biol. Med. 2019, 139, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.R.; Cheng, L.; Foster, R.; Tritt, R.; Jiang, J.; Broshears, J.; Koch, M. Elevated and altered expression of the multifunctional DNA base excision repair and redox enzyme Ape1/ref-1 in prostate cancer. Clin. Cancer Res. 2001, 7, 824–830. [Google Scholar] [PubMed]
- Kakolyris, S.; Kaklamanis, L.; Engels, K.; Fox, S.B.; Taylor, M.; Hickson, I.D.; Gatter, K.C.; Harris, A.L. Human AP endonuclease 1 (HAP1) protein expression in breast cancer correlates with lymph node status and angiogenesis. Br. J. Cancer 1998, 77, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Dammann, K.; Khare, V.; Gasche, C. Tracing PAKs from GI inflammation to cancer. Gut 2014, 63, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42–61. [Google Scholar] [CrossRef]
- Asad, A.S.; Moreno Ayala, M.A.; Gottardo, M.F.; Zuccato, C.; Nicola Candia, A.J.; Zanetti, F.A.; Seilicovich, A.; Candolfi, M. Viral gene therapy for breast cancer: Progress and challenges. Expert Opin. Biol. Ther. 2017, 17, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.T.; Breidenbach, M.; Curiel, D.T. Current developments in adenovirus-based cancer gene therapy. Future Oncol. 2006, 2, 137–143. [Google Scholar] [CrossRef]
- Choi, S.; Lee, Y.R.; Park, M.S.; Joo, H.K.; Cho, E.J.; Kim, H.S.; Kim, C.S.; Park, J.B.; Irani, K.; Jeon, B.H. Histone deacetylases inhibitor trichostatin A modulates the extracellular release of APE1/Ref-1. Biochem. Biophys. Res. Commun. 2013, 435, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Kichina, J.V.; Goc, A.; Al-Husein, B.; Somanath, P.R.; Kandel, E.S. PAK1 as a therapeutic target. Expert Opin. Ther. Targets 2010, 14, 703–725. [Google Scholar] [CrossRef]
- Shrestha, Y.; Schafer, E.J.; Boehm, J.S.; Thomas, S.R.; He, F.; Du, J.; Wang, S.; Barretina, J.; Weir, B.A.; Zhao, J.J.; et al. PAK1 is a breast cancer oncogene that coordinately activates MAPK and MET signaling. Oncogene 2012, 31, 3397–3408. [Google Scholar] [CrossRef] [PubMed]
- Rane, C.; Senapedis, W.; Baloglu, E.; Landesman, Y.; Crochiere, M.; Das-Gupta, S.; Minden, A. A novel orally bioavailable compound KPT-9274 inhibits PAK4, and blocks triple negative breast cancer tumor growth. Sci. Rep. 2017, 7, 42555. [Google Scholar] [CrossRef] [PubMed]
- Bollrath, J.; Greten, F.R. IKK/NF-kappaB and STAT3 pathways: Central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009, 10, 1314–1319. [Google Scholar] [CrossRef]
- Gan, J.; Ke, X.; Jiang, J.; Dong, H.; Yao, Z.; Lin, Y.; Lin, W.; Wu, X.; Yan, S.; Zhuang, Y.; et al. Growth hormone-releasing hormone receptor antagonists inhibit human gastric cancer through downregulation of PAK1-STAT3/NF-kappaB signaling. Proc. Natl. Acad. Sci. USA 2016, 113, 14745–14750. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Lee, Y.-R.; Kim, K.-M.; Choi, E.; Jeon, B.-H. Dual Function of Secreted APE1/Ref-1 in TNBC Tumorigenesis: An Apoptotic Initiator and a Regulator of Chronic Inflammatory Signaling. Int. J. Mol. Sci. 2022, 23, 9021. https://doi.org/10.3390/ijms23169021
Choi S, Lee Y-R, Kim K-M, Choi E, Jeon B-H. Dual Function of Secreted APE1/Ref-1 in TNBC Tumorigenesis: An Apoptotic Initiator and a Regulator of Chronic Inflammatory Signaling. International Journal of Molecular Sciences. 2022; 23(16):9021. https://doi.org/10.3390/ijms23169021
Chicago/Turabian StyleChoi, Sunga, Yu-Ran Lee, Ki-Mo Kim, Euna Choi, and Byeong-Hwa Jeon. 2022. "Dual Function of Secreted APE1/Ref-1 in TNBC Tumorigenesis: An Apoptotic Initiator and a Regulator of Chronic Inflammatory Signaling" International Journal of Molecular Sciences 23, no. 16: 9021. https://doi.org/10.3390/ijms23169021
APA StyleChoi, S., Lee, Y. -R., Kim, K. -M., Choi, E., & Jeon, B. -H. (2022). Dual Function of Secreted APE1/Ref-1 in TNBC Tumorigenesis: An Apoptotic Initiator and a Regulator of Chronic Inflammatory Signaling. International Journal of Molecular Sciences, 23(16), 9021. https://doi.org/10.3390/ijms23169021






