Comparison of Mitochondrial Genomes between a Cytoplasmic Male-Sterile Line and Its Restorer Line for Identifying Candidate CMS Genes in Gossypium hirsutum
Abstract
:1. Introduction
2. Results
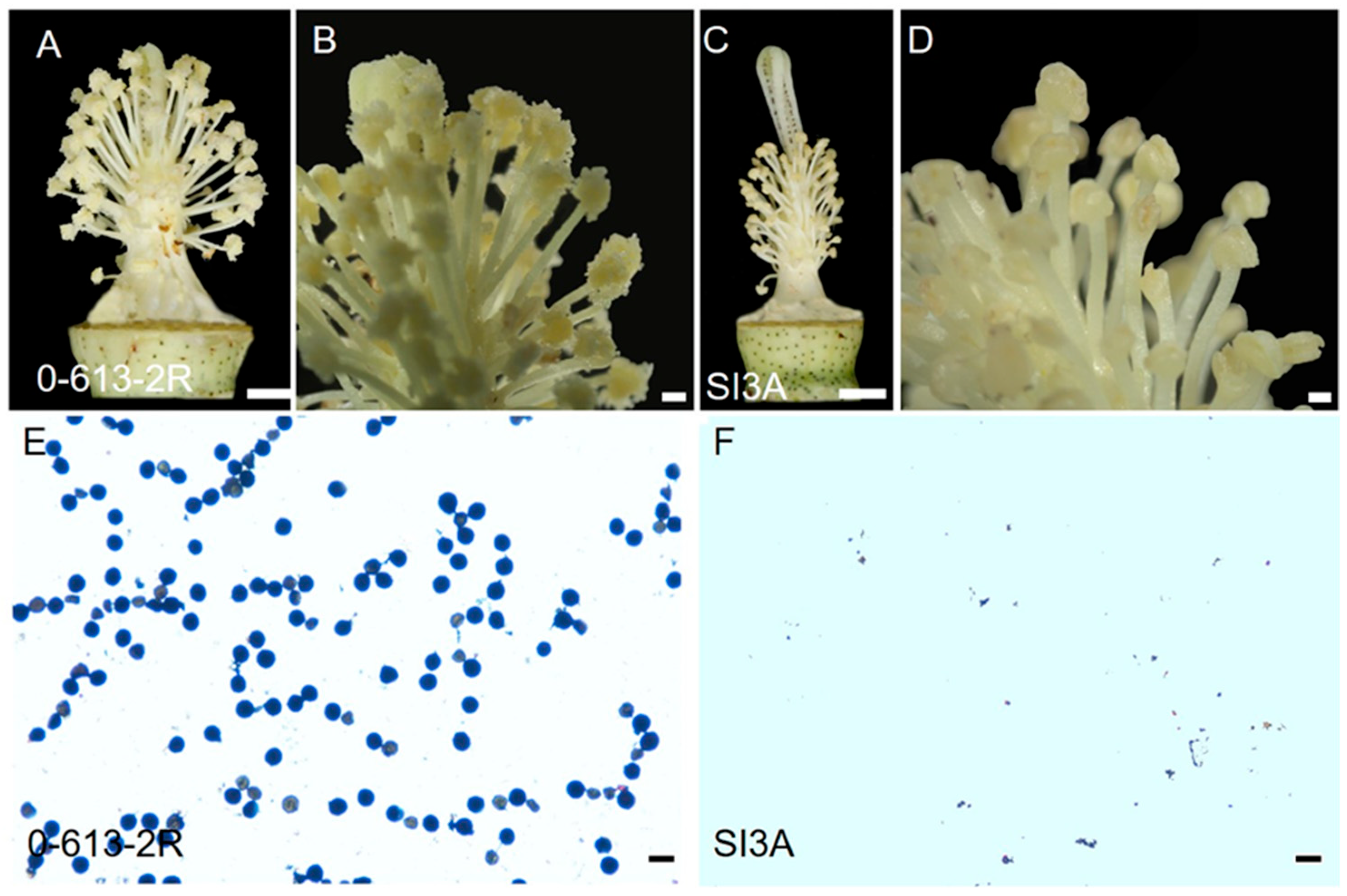
2.1. Flower Morphology of the CMS-D2 Line SI3A and Its Restorer Line 0-613-2R
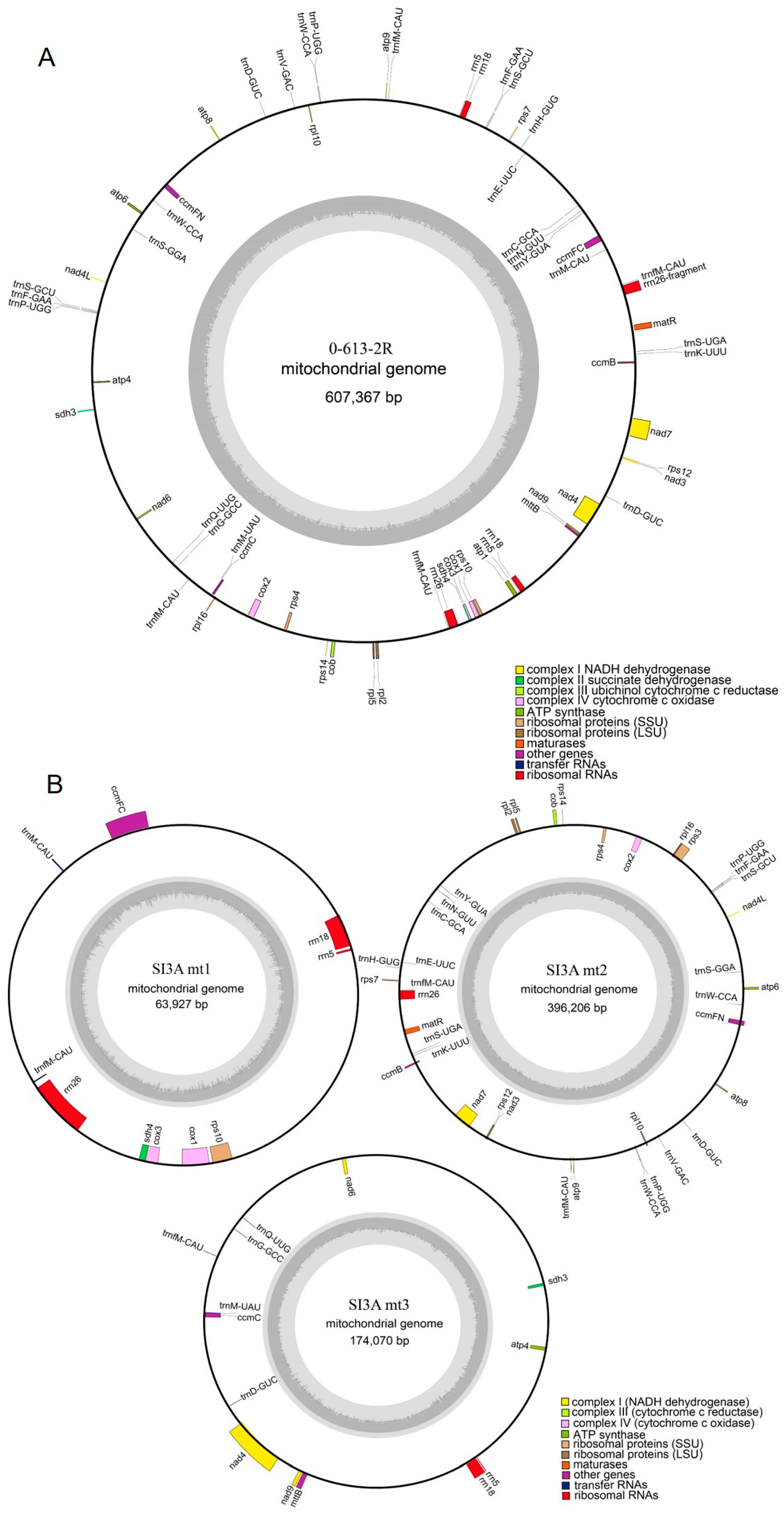
2.2. Mitochondrial Genome Assembly and Annotation
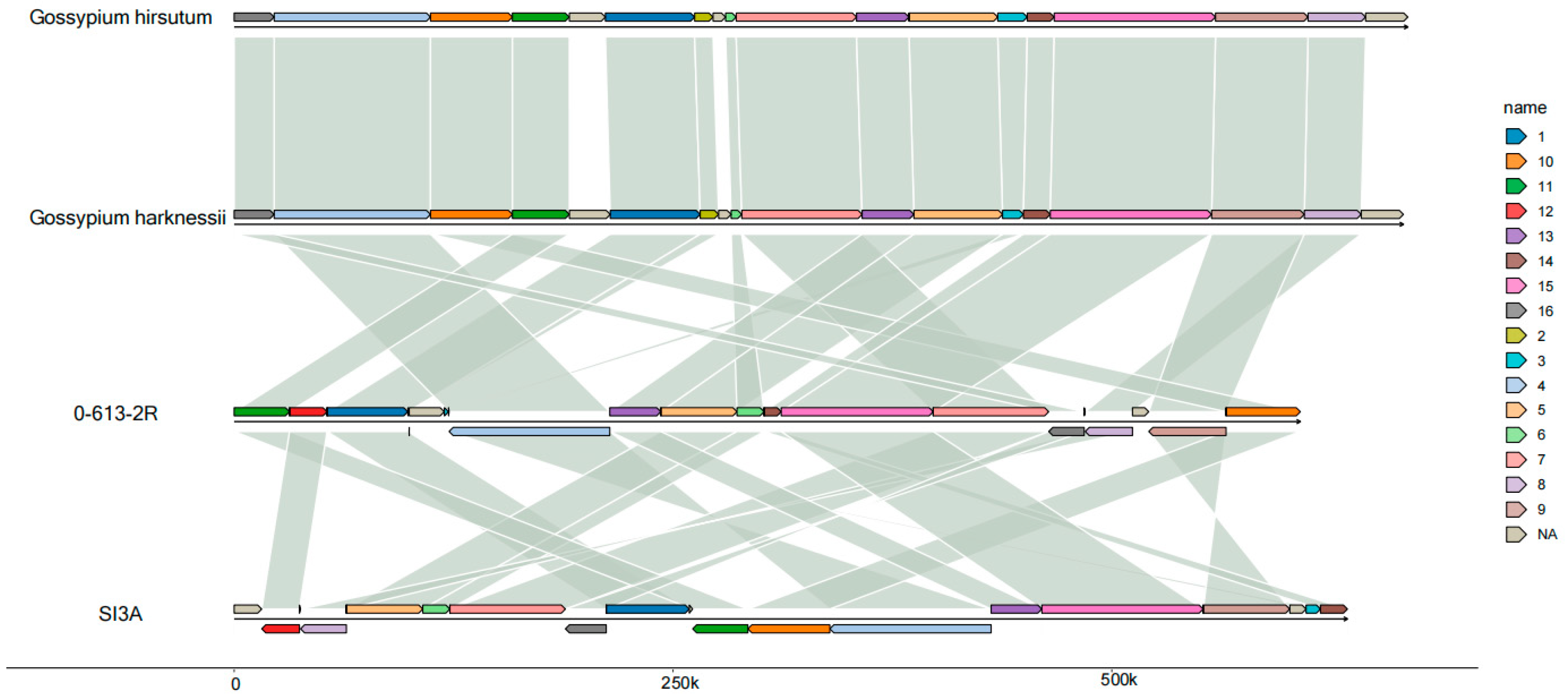
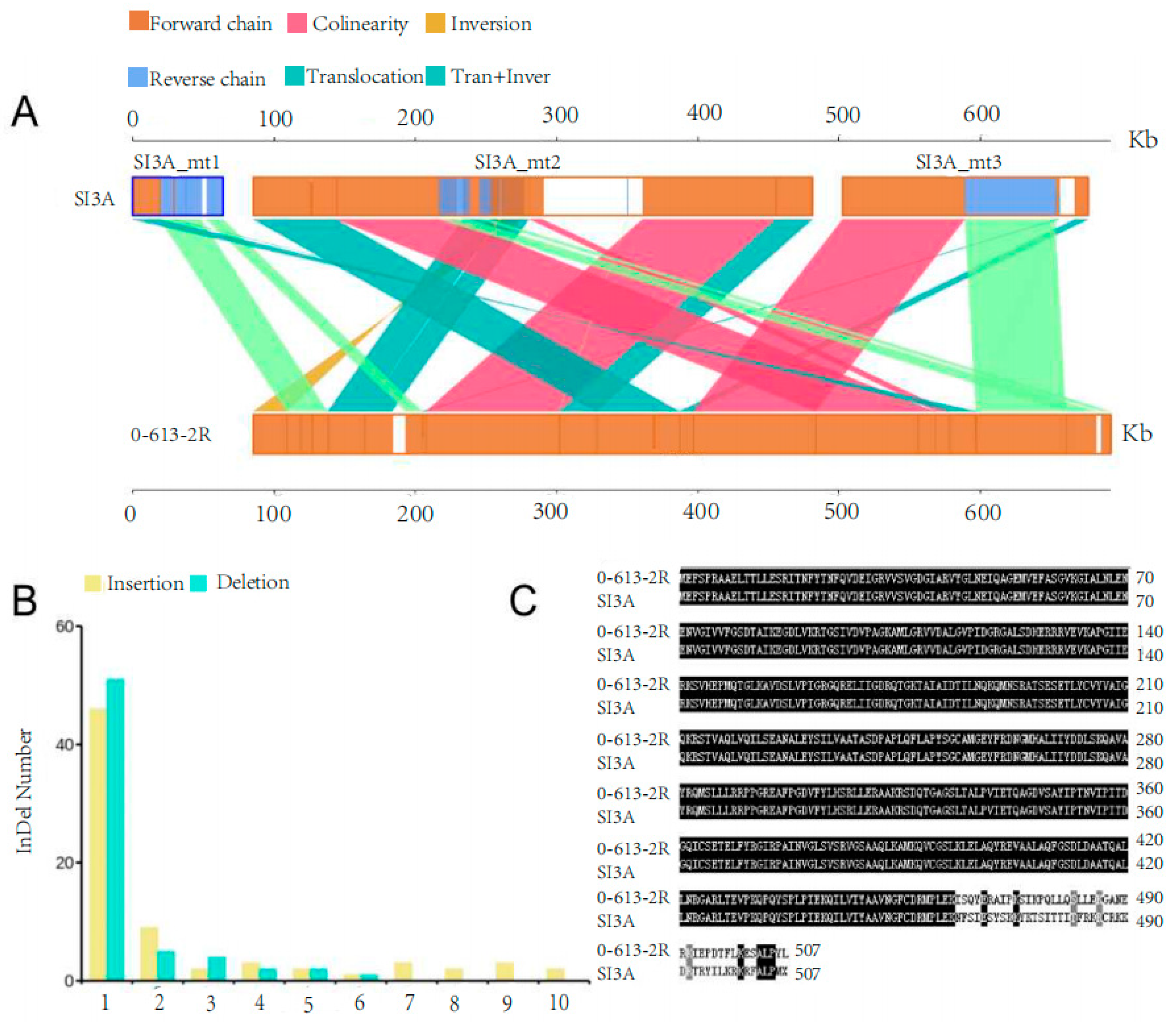
2.3. Comparative Analysis of the SI3A and 0-613-2R Mitochondrial Genomes
2.3.1. Repeat Sequences
2.3.2. Structural Variation, SNPs, and InDels
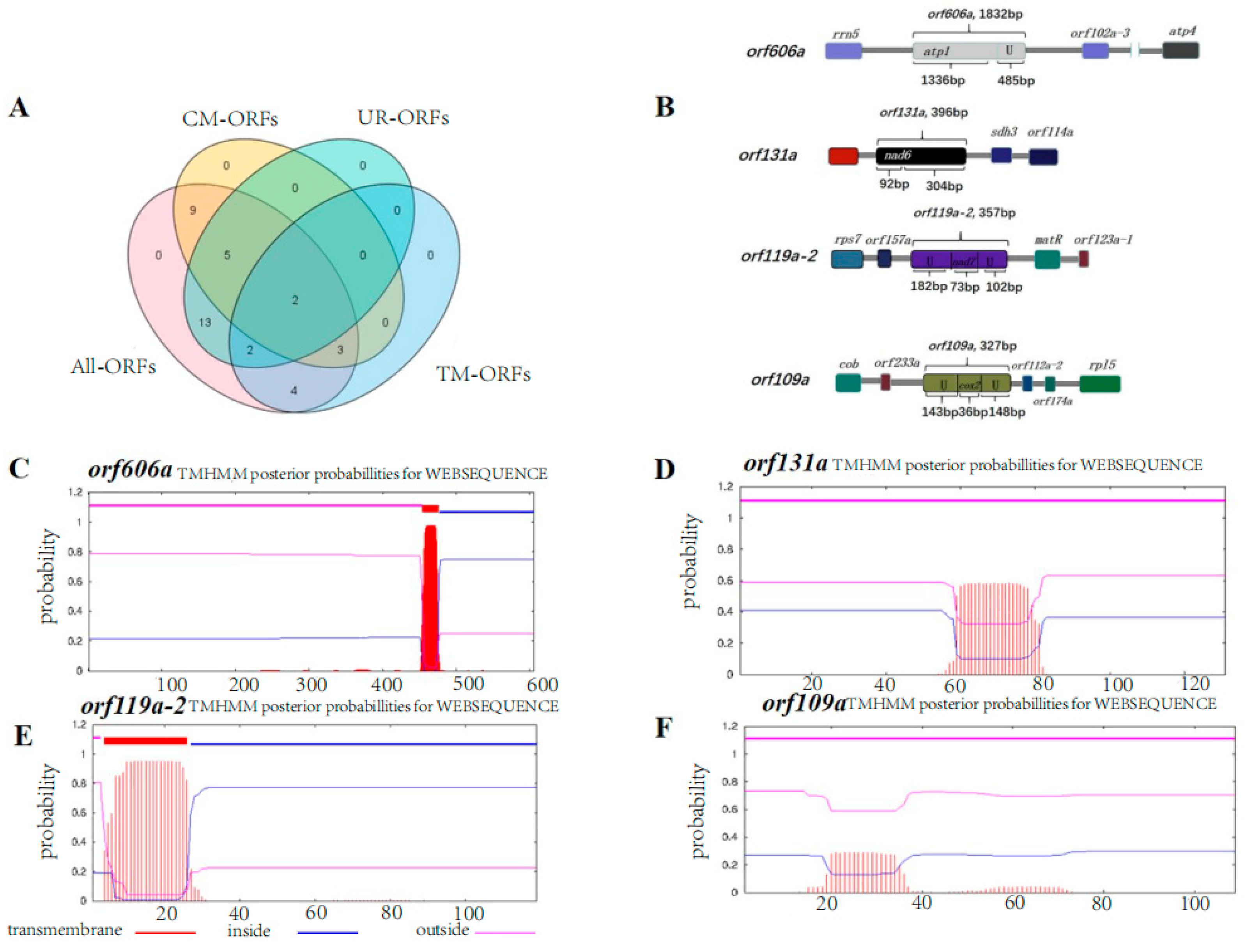
2.4. Unique ORFs
2.5. Selection of Candidate Genes Resulting in Male Sterility of SI3A
3. Discussion
3.1. Characteristics of the SI3A and 0-613-2R Mitochondrial Genomes
3.2. orf606a and nad4 May Be CMS Candidate Genes for the CMS-D2 Line SI3A
4. Materials and Methods
4.1. Plant Materials and Phenotypic Analysis
4.2. Library Construction, Sequencing, and Assembly of Mitochondrial Genomes
4.3. Mitochondrial Genome Annotation and Identification of ORFs
4.4. Differential Analysis of the SI3A and 0-613-2R Mitochondrial Genomes
4.5. RNA Extraction and Transcriptome Sequencing
4.6. Full-Length Transcriptome Sequencing
4.7. RT-PCR and RT-qPCR Analysis of Candidate ORFs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.; Dey, S.S.; Bhatia, R.; Kumar, R.; Behera, T.K. Current understanding of male sterility systems in vegetable Brassicas and their exploitation in hybrid breeding. Plant Reprod. 2019, 32, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dey, S.S.; Bhatia, R.; Kumar, R.; Sharma, K.; Behera, T.K. Heterosis and combining ability in cytoplasmic male sterile and doubled haploid based Brassica oleracea progenies and prediction of heterosis using microsatellites. PLoS ONE 2019, 14, e0210772. [Google Scholar] [CrossRef]
- Wang, P.; Lu, Q.; Ai, Y.; Wang, Y.; Li, T.; Wu, L.; Liu, J.; Cheng, Q.; Sun, L.; Shen, H. Candidate gene selection for cytoplasmic male sterility in pepper (Capsicum annuum L.) through whole mitochondrial genome sequencing. Int. J. Mol. Sci. 2019, 20, 578. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.R.; Isshiki, S. Cytoplasmic male sterility in eggplant. Hortic. J. 2016, 85, 1–7. [Google Scholar] [CrossRef]
- Kuwabara, K.; Arimura, S.-I.; Shirasawa, K.; Ariizumi, T. orf137 triggers cytoplasmic male sterility in tomato. Plant Physiol. 2022, 189, 465–468. [Google Scholar] [CrossRef]
- Palumbo, F.; Vitulo, N.; Vannozzi, A.; Magon, G.; Barcaccia, G. The mitochondrial genome assembly of fennel (Foeniculum vulgare) reveals two different atp6 gene sequences in cytoplasmic male sterile. Int. J. Mol. Sci. 2020, 21, 4664. [Google Scholar] [CrossRef]
- Linke, B.; Alessandro, M.S.; Galmarini, C.R.; Nothnagel, T. Carrot floral development and reproductive biology. In The Carrot Genome; Simon, P., Lorizzo, M., Grzebelus, D., Baranski, R., Eds.; Springer: New York, NY, USA, 2019; Volume 126, pp. 415–423. [Google Scholar]
- Wu, J.Y.; Zhang, M.; Zhang, B.B.; Zhang, X.X.; Guo, L.P.; Qi, T.X.; Wang, H.L.; Zhang, J.F.; Xing, C.Z. Genome-wide comparative transcriptome analysis of CMS-D2 and its maintainer and restorer lines in upland cotton. BMC Genom. 2017, 18, 454. [Google Scholar] [CrossRef]
- Singh, M.; Brown, G.G. Suppression of cytoplasmic male sterility by nuclear genes alters expression of a novel mitochondrial gene region. Plant Cell 1991, 3, 1349–1362. [Google Scholar] [CrossRef]
- Peng, X.J.; Wang, K.; Hu, C.F.; Zhu, Y.L.; Wang, T.; Yang, J.; Tong, J.P.; Li, S.Q.; Zhu, Y.G. The mitochondrial gene orfH79 plays a critical role in impairing both male gametophyte development and root growth in CMS-Honglian rice. BMC Plant Biol. 2010, 10, 125. [Google Scholar] [CrossRef]
- Luo, D.P.; Xu, H.; Liu, Z.L.; Guo, J.X.; Li, H.Y.; Chen, L.T.; Fang, C.; Zhang, Q.Y.; Bai, M.; Yao, N.; et al. A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat. Genet. 2013, 45, 573–577. [Google Scholar] [CrossRef]
- Yang, H.L.; Xue, Y.D.; Li, B.; Lin, Y.N.; Li, H.C.; Guo, Z.Y.; Li, W.H.; Fu, Z.Y.; Ding, D.; Tang, J. The chimeric gene atp6c confers cytoplasmic male sterility in maize by impairing the assembly of the mitochondrial ATP synthase complex. Mol. Plant 2022, 15, 872–886. [Google Scholar] [CrossRef]
- Ashutosh; Kumar, P.; Dinesh Kumar, V.; Sharma, P.C.; Prakash, S.; Bhat, S.R. A novel orf108 co-transcribed with the atpA gene is associated with cytoplasmic male sterility in Brassica juncea carrying Moricandia arvensis cytoplasm. Plant Cell Physiol. 2008, 49, 284–289. [Google Scholar] [CrossRef]
- Kojima, H.; Kazama, T.; Fujii, S.; Toriyama, K. Cytoplasmic male sterility-associated ORF79 is toxic to plant regeneration when expressed with mitochondrial targeting sequence of ATPase γ subunit. Plant Biotechnol. 2010, 27, 111–114. [Google Scholar] [CrossRef]
- Kazama, T.; Itabashi, E.; Fujii, S.; Nakamura, T.; Toriyama, K. Mitochondrial ORF79 levels determine pollen abortion in cytoplasmic male sterile rice. Plant J. 2016, 85, 707–716. [Google Scholar] [CrossRef]
- Jing, B.; Heng, S.P.; Tong, D.; Wan, Z.J.; Fu, T.D.; Tu, J.X.; Ma, C.Z.; Yi, B.; Wen, J.; Shen, J. A male sterility-associated cytotoxic protein ORF288 in Brassica juncea causes aborted pollen development. J. Exp. Bot. 2012, 63, 1285–1295. [Google Scholar] [CrossRef]
- Moneger, F.; Smart, C.J.; Leaver, C.J. Nuclear restoration of cytoplasmic male sterility in sunflower is associated with the tissue-specific regulation of a novel mitochondrial gene. EMBO J. 1994, 13, 8–17. [Google Scholar] [CrossRef]
- Xiao, S.L.; Zang, J.; Pei, Y.R.; Liu, J.; Liu, J.; Song, W.; Shi, Z.; Su, A.G.; Zhao, J.R.; Chen, H. Activation of mitochondrial orf355 gene expression by a nuclear-encoded DREB transcription factor causes cytoplasmic male sterility in maize. Mol. Plant 2020, 13, 1270–1283. [Google Scholar] [CrossRef]
- Mell, P.H. Experiments in Crossing for the Purpose of Improving the Cotton Fiber; Alabama Agricultural Experiment Station (AAES) Report; Agricultural Experiment Station of the Agricultural and Mechanical College: Auburn, AL, USA, 1894; Volume 6. [Google Scholar]
- Li, C.; Yu, H.R.; Li, C.; Zhao, T.L.; Dong, Y.T.; Deng, X.L.; Hu, J.H.; Zhang, Y.; Zhang, F.; Li, J. QTL mapping and heterosis analysis for fiber quality traits across multiple genetic populations and environments in upland cotton. Front. Plant Sci. 2018, 9, 1364. [Google Scholar] [CrossRef]
- Munir, S.; Hussain, S.B.; Manzoor, H.; Quereshi, M.K.; Zubair, M.; Nouman, W.; Shehzad, A.N.; Rasul, S.; Manzoor, S.A. Heterosis and correlation in interspecific and intraspecific hybrids of cotton. Genet. Mol. Res. 2016, 15, gmr8083. [Google Scholar] [CrossRef]
- Zaidi, S.S.A.; Naqvi, R.Z.; Asif, M.; Strickler, S.; Shakir, S.; Shafiq, M.; Khan, A.M.; Amin, I.; Mishra, B.; Mukhtar, M.S.; et al. Molecular insight into cotton leaf curl geminivirus disease resistance in cultivated cotton (Gossypium hirsutum). Plant Biotechnol. J. 2020, 18, 691–706. [Google Scholar] [CrossRef]
- Zhang, T.Z.; Jing, S.R. Theory and Practice of Selection and Breeding of Male Sterile Hybrids in Cotton; China Agriculture Press: Beijing, China, 1998. [Google Scholar]
- Wang, X.D.; Zhang, T.Z.; Pan, J.J. Cytological observation of microsporogenesis and RAPD analysis of mitochondrial DNAs for cytoplasmic male sterile cotton lines. Sci. Agric. Sin. 1998, 31, 70–75. [Google Scholar]
- Feng, C.D.; Guo, J.H.; Nie, Y.C.; Wu, Z.B.; Zhang, X.L.; Zhang, J.F.; Stewart, J.M. Cytoplasmic-nuclear male sterility in cotton: Comparative RFLP analysis of mitochondrial DNA. In Proceedings of the 2000 Proceedings Beltwide Cotton Conferences, San Antonio, TX, USA, 4–8 January 2000. [Google Scholar]
- Wang, X.D. Analyses of mitochondrial protein and DNA from cytoplasmic male sterile cotton. Acta Agron. Sin. 2000, 26, 35–39. [Google Scholar]
- Li, S.S.; Chen, Z.W.; Zhao, N.; Wang, Y.M.; Nie, H.S.; Hua, J.P. The comparison of four mitochondrial genomes reveals cytoplasmic male sterility candidate genes in cotton. BMC Genom. 2018, 19, 775. [Google Scholar] [CrossRef]
- Khan, A.; Kong, X.; Liao, X.; Zheng, J.; You, J.; Li, M.; Hussain, R.M.; Raza, H.; Zhou, R. Mitochondrial gene expression analysis reveals aberrant transcription of cox3 in Gossypium barbadense CMS line H276A. Dev. Genes Evol. 2022, 232, 15–23. [Google Scholar] [CrossRef]
- You, J.Y.; Li, M.; Li, H.W.; Bai, Y.L.; Zhu, X.; Kong, X.J.; Chen, X.Y.; Zhou, R.Y. Integrated methylome and transcriptome analysis widen the knowledge of cytoplasmic male sterility in cotton (Gossypium barbadense L.). Front. Plant Sci. 2022, 13, 77098. [Google Scholar] [CrossRef]
- Heng, S.P.; Wei, C.; Jing, B.; Wan, Z.J.; Wen, J.; Yi, B.; Ma, C.Z.; Tu, J.X.; Fu, T.D.; Shen, J. Comparative analysis of mitochondrial genomes between the hau cytoplasmic male sterility (CMS) line and its iso-nuclear maintainer line in Brassica juncea to reveal the origin of the CMS-associated gene orf288. BMC Genom. 2014, 15, 322. [Google Scholar] [CrossRef]
- He, T.T.; Ding, X.L.; Zhang, H.; Li, Y.W.; Chen, L.F.; Wang, T.L.; Yang, L.S.; Nie, Z.X.; Song, Q.J.; Gai, J.; et al. Comparative analysis of mitochondrial genomes of soybean cytoplasmic male-sterile lines and their maintainer lines. Funct. Integr. Genom. 2021, 21, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cui, P.; Zhan, K.; Lin, Q.; Zhuo, G.; Guo, X.; Ding, F.; Yang, W.; Liu, D.; Hu, S.; et al. Comparative analysis of mitochondrial genomes between a wheat K-type cytoplasmic male sterility (CMS) line and its maintainer line. BMC Genom. 2011, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Unseld, M.; Marienfeld, J.R.; Brandt, P.; Brennicke, A. The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides. Nat. Genet 1997, 15, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, W.C.; Huang, Q.; Qin, X.J.; Yu, C.C.; Wang, L.L.; Li, S.Q.; Zhu, R.S.; Zhu, Y.G. Mitochondria and cytoplasmic male sterility in plants. Mitochondrion 2014, 19, 282–288. [Google Scholar] [CrossRef]
- Toriyama, K. Molecular basis of cytoplasmic male sterility and fertility restoration in rice. Plant Biotechnol. 2021, 38, 285–295. [Google Scholar] [CrossRef]
- Ivanov, M.K.; Dymshits, G.M. Cytoplasmic male sterility and restoration of pollen fertility in higher plants. Genetika 2007, 43, 451–468. [Google Scholar] [CrossRef]
- Sloan, D.B.; Alverson, A.J.; Štorchová, H.; Palmer, J.D.; Taylor, D.R. Extensive loss of translational genes in the structurally dynamic mitochondrial genome of the angiosperm Silene latifolia. BMC Evol. Biol. 2010, 10, 274. [Google Scholar] [CrossRef]
- Arrieta-Montiel, M.P.; Mackenzie, S.A. Plant mitochondrial genomes and recombination. In Plant Mitochondrial; Kempken, F., Ed.; Springer: New York, NY, USA, 2011; Volume 1, pp. 65–82. [Google Scholar]
- Tang, H.W.; Zheng, X.M.; Li, C.L.; Xie, X.R.; Chen, Y.L.; Chen, L.T.; Zhao, X.C.; Zheng, H.Q.; Zhou, J.J.; Ye, S.; et al. Multi-step formation, evolution, and functionalization of new cytoplasmic male sterility genes in the plant mitochondrial genomes. Cell Res. 2016, 27, 130–146. [Google Scholar] [CrossRef]
- Bentolila, S.; Stefanov, S. A reevaluation of rice mitochondrial evolution based on the complete sequence of male-fertile and male-sterile mitochondrial genomes. Plant Physiol. 2012, 158, 996–1017. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Han, Y.; Zhang, M.; Zhang, X.X.; Guo, L.P.; Qi, T.X.; Li, Y.Q.; Feng, J.J.; Wang, H.L.; Tang, H.; et al. The cotton mitochondrial chimeric gene orf610a causes male sterility by disturbing the dynamic balance of ATP synthesis and ROS burst. Crop. J. 2022. [Google Scholar] [CrossRef]
- Kuwabara, K.; Harada, I.; Matsuzawa, Y.; Ariizumi, T.; Shirasawa, K. Organelle genome assembly uncovers the dynamic genome reorganization and cytoplasmic male sterility associated genes in tomato. Hortic Res. 2021, 8, 250–261. [Google Scholar] [CrossRef]
- Alverson, A.J.; Rice, D.W.; Dickinson, S.; Barry, K.; Palmer, J.D. Origins and recombination of the bacterial-sized multichromosomal mitochondrial genome of cucumber. Plant Cell 2011, 23, 2499–2513. [Google Scholar] [CrossRef]
- Mower, J.P.; Case, A.L.; Floro, E.R.; Willis, J.H. Evidence against equimolarity of large repeat arrangements and a predominant master circle structure of the mitochondrial genome from a monkeyflower (Mimulus guttatus) lineage with cryptic CMS. Genome Biol. Evol. 2012, 4, 670–686. [Google Scholar] [CrossRef]
- Tian, X.J.; Zheng, J.; Hu, S.N.; Yu, J. The rice mitochondrial genomes and their variations. Plant Physiol. 2006, 140, 401–410. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, Y.P.; Lee, J.; Choi, B.S.; Kim, S.; Yang, T.J. Complete mitochondrial genome sequence and identification of a candidate gene responsible for cytoplasmic male Sterility in Radish (Raphanus sativus L.) containing DCGMS cytoplasm. Theor. Appl. Genet. 2013, 126, 1763–1774. [Google Scholar] [CrossRef]
- Zabala, G.; Gabay-Laughnan, S.; Laughnan, J.R. The nuclear gene Rf3 affects the expression of the mitochondrial chimeric sequence R implicated in S-type male sterility in maize. Genetics 1997, 147, 847–860. [Google Scholar] [CrossRef]
- Sang, S.F.; Cheng, H.T.; Hao, M.Y.; Ding, B.L.; Mei, D.S.; Wang, H.; Wang, W.X.; Liu, J.; Fu, L.; Liu, K.; et al. Mitochondrial localization of ORF346 causes pollen abortion in alloplasmic male sterility. Crop. J. 2021, 9, 1320–1329. [Google Scholar] [CrossRef]
- Song, J.; Hedgcoth, C. A chimeric gene (orf256) is expressed as protein only in cytoplasmic male-sterile lines of wheat. Plant Mol. Biol. 1994, 26, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Dahan, J.; Mireau, H. The Rf and Rf-like PPR in higher plants, a fast-evolving subclass of PPR genes. RNA Biol. 2013, 10, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Bentolila, S.; Alfonso, A.A.; Hanson, M.R. A pentatricopeptide repeat-containing gene restores fertility to cytoplasmic male-sterile plants. Proc. Natl. Acad. Sci. USA 2002, 99, 10887–10892. [Google Scholar] [CrossRef]
- Brown, G.G.; Formanová, N.; Jin, H.; Wargachuk, R.; Dendy, C.; Patil, P.; Laforest, M.; Zhang, J.; Cheung, W.Y.; Landry, B.S. The radish Rfo restorer gene of ogura cytoplasmic male sterility encodes a protein with multiple pentatricopeptide repeats. Plant J. 2003, 35, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Ohta, S.; Murai, N.; Takakura, Y.; Kuraya, Y.; Suzuki, S.; Hiei, Y.; Imaseki, H.; Nitta, N. Map-based cloning of a fertility restorer gene, Rf-1, in rice (Oryza sativa L.). Plant J. 2004, 37, 315–325. [Google Scholar] [CrossRef]
- Klein, R.R.; Klein, P.E.; Mullet, J.E.; Minx, P.; Rooney, W.L.; Schertz, K.F. Fertility restorer locus Rf1 of sorghum (Sorghum bicolor L.) encodes a pentatricopeptide repeat protein not present in the colinear region of rice chromosome 12. Appl Genet. 2005, 111, 994–1012. [Google Scholar] [CrossRef]
- Barkan, A.; Small, I. Pentatricopeptide repeat proteins in plants. Annu Rev. Plant. Biol 2014, 65, 415–442. [Google Scholar] [CrossRef]
- Li, X.L.; Sun, M.D.; Liu, S.J.; Teng, Q.; Li, S.H.; Jiang, Y.S. Functions of PPR proteins in plant growth and development. Int. J. Mol. Sci. 2021, 22, 11274. [Google Scholar] [CrossRef]
- Huang, W.C.; Yu, C.C.; Hu, J.; Wang, L.L.; Dan, Z.W.; Zhou, W.; He, C.L.; Zeng, Y.F.; Yao, G.X.; Qi, J.Z.; et al. Pentatricopeptide-repeat family protein RF6 functions with hexokinase 6 to rescue rice cytoplasmic male sterility. Proc. Natl. Acad. Sci. USA 2015, 112, 14984–14989. [Google Scholar] [CrossRef]
- Falcon de Longevialle, A.F.; Meyer, E.H.; Andrés, C.; Taylor, N.L.; Lurin, C.; Millar, A.H.; Smalla, I.D. The pentatricopeptide repeat gene OTP43 is required for trans-splicing of the mitochondrial nad1 intron 1 in Arabidopsis thaliana. Plant Cell 2007, 19, 3256–3265. [Google Scholar] [CrossRef]
- Wang, C.D.; Blondel, L.; Quadrado, M.; Dargel-Graffin, C.; Mireau, H. Pentatricopeptide repeat protein MTSF3 ensures mitochondrial RNA stability and embryogenesis. Plant Physiol. 2022, 1–13. [Google Scholar] [CrossRef]
- Qin, X.E.; Tian, S.K.; Zhang, W.L.; Zheng, Q.; Wang, H.; Feng, Y.; Lin, Y.N.; Tang, J.H.; Wang, Y.; Yan, J.B.; et al. The main restorer Rf3 of maize S type cytoplasmic male sterility encodes a PPR protein that functions in reduction of the transcripts of orf355. Mol. Plant 2021, 14, 1961–1964. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Chen, C.; Wang, H.T.; Niu, E.; Zhao, P.Y.; Fang, S.; Zhu, G.Z.; Shang, X.G.; Guo, W.Z. Cotton fiber development requires the pentatricopeptide repeat protein GhIm for splicing of mitochondrial Nad7 mRNA. Genetics 2021, 217, 1–17. [Google Scholar] [CrossRef]
- Paterson, A.H.; Brubaker, C.L.; Wendel, J.F. A rapid method for extraction of cotton (Gossypium spp.) genomic DNA suitable for RFLP or PCR analysis. Plant Mol. Biol. Rep. 1993, 11, 122–127. [Google Scholar] [CrossRef]
- Hahn, C.; Bachmann, L.; Chevreux, B. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads—A baiting and iterative mapping approach. Nucleic Acids Res. 2013, 41, e129. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Alverson, A.J.; Wei, X.; Rice, D.W.; Stern, D.B.; Barry, K.; Palmer, J.D. Insights into the evolution of mitochondrial genome size from complete sequences of Citrullus lanatus and Cucurbita pepo (Cucurbitaceae). Mol. Biol. Evol. 2010, 27, 1436–1448. [Google Scholar] [CrossRef]
- Lagesen, K.; Hallin, P.; Rødland, E.A.; Stærfeldt, H.H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef]
- Kurtz, S.; Phillippy, A.; Delcher, A.L.; Smoot, M.; Shumway, M.; Antonescu, C.; Salzberg, S.L. Versatile and open software for comparing large genomes. Genome Biol. 2004, 5, 12. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H. Exploring single-sample SNP and InDel calling with whole-genome de novo assembly. Bioinformatics 2012, 28, 1838–1844. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Wu, T.D.; Nacu, S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics 2010, 26, 873–881. [Google Scholar] [CrossRef]
- Wu, T.D.; Watanabe, C.K. GMAP: A genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics 2005, 21, 1859–1875. [Google Scholar] [CrossRef]
- Rao, X.Y.; Huang, X.L.; Zhou, Z.C.; Lin, X. An improvement of the 2ˆ(–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71. [Google Scholar]

| Genome Characteristics | 0613-2R mt | SI3A | ||
|---|---|---|---|---|
| SI3A mt1 | SI3A mt2 | SI3A mt3 | ||
| Genomic size(bp) | 607,367 | 63,927 | 396,206 | 174,074 |
| G + C content (%) | 44 | 46 | 44 | 45 |
| coding sequence (%) | 25.3% | 19.0% | 7.4% | 8.4% |
| ORF | 48 | 12 | 33 | 14 |
| Protein coding genes | 33 | 6 | 20 | 7 |
| tRNA genes | 27 | 2 | 18 | 5 |
| rRNA genes | 6 1 | 3 1 | 1 1 | 2 1 |
| Repeat content percent coverage of total genome | 16.2% | 0.8% | 8.8% | 9.2% |
| Large repeats: >1 kb(number) | 13 | 0 | 5 | 0 |
| Small repeats: <1 kb(number) | 427 | 11 | 169 | 34 |
| Copy1 | Copy2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Size (bp) | Start1 | Stop1 | Type | Size (bp) | Start2 | Stop2 | Identity (%) | Difference between Copies |
| AR1 | 10,637 | 120,585 | 131,221 | DR 1 | 10,636 | 359,345 | 369,980 | 99 | copy1 9 bp indel, copy2 11 bp indel. |
| AR2 | 435 | 237,794 | 238,228 | DR | 435 | 334,087 | 334,521 | 100 | |
| AR3 | 333 | 211,333 | 211,665 | IR 2 | 333 | 360,536 | 360,868 | 100 | |
| AR4 | 229 | 154,125 | 154,353 | DR | 229 | 254,009 | 254,237 | 100 | |
| AR5 | 203 | 237,590 | 237,792 | DR | 203 | 333,883 | 334,085 | 100 | |
| AR6 | 175 | 83 | 257 | IR | 175 | 281,969 | 282,143 | 100 | |
| AR7 | 154 | 56,027 | 56,180 | DR | 154 | 359,760 | 359,913 | 100 | |
| AR8 | 121 | 56,027 | 56,147 | DR | 121 | 121,000 | 121,120 | 100 | |
| AR9 | 113 | 40,192 | 40,304 | DR | 113 | 102,378 | 102,490 | 100 | |
| AR10 | 113 | 57,141 | 57,253 | DR | 113 | 212,119 | 212,231 | 100 | |
| AR11 | 107 | 201,247 | 201,353 | DR | 107 | 389,303 | 389,409 | 100 | |
| AR12 | 93 | 111,326 | 111,418 | DR | 93 | 331,353 | 331,445 | 100 | |
| AR13 | 78 | 75,293 | 75,370 | IR | 78 | 358,615 | 358,692 | 100 | |
| AR14 | 71 | 264,143 | 264,213 | IR | 71 | 360,450 | 360,520 | 100 | |
| AR15 | 66 | 75,146 | 75,211 | IR | 66 | 116,361 | 116,426 | 100 | |
| AR16 | 66 | 211,392 | 211,457 | IR | 66 | 297,562 | 297,627 | 100 | |
| AR17 | 66 | 297,562 | 297,627 | DR | 66 | 360,744 | 360,809 | 100 | |
| AR18 | 60 | 40,306 | 40,365 | DR | 60 | 102,492 | 102,551 | 100 | |
| AR19 | 57 | 7252 | 7308 | DR | 57 | 324,694 | 324,750 | 100 | |
| AR20 | 53 | 47,233 | 47,285 | IR | 53 | 170,558 | 170,610 | 100 | |
| Gene | Location | 0-613-2R<->SI3A (Nucleic Acid) | 0-613-2R<->SI3A (Amino Acid) | Mutation Type 1 | SNP Type |
|---|---|---|---|---|---|
| cox1 | 495,890 | C<->A | Ile<->Ile | S | transversion |
| cox1 | 496,358 | C<->A | Ile<->Ile | S | transversion |
| cox3 | 494,152 | C<->A | Leu<->Ile | N | transversion |
| nad7 | 583,680 | A<->C | Ile<->Ile | S | transversion |
| atp4 | 307,826 | C<->T | Phe<->Phe | S | transition |
| atp8 | 205,705 | C<->A | Ser<->Arg | N | transversion |
| sdh3 | 317,242 | A<->C | Leu<->Phe | N | transversion |
| matR | 16,075 | C<->A | Cys<->Lys | N | transversion |
| rps4 | 426,548 | A<->C | Lys<->Cys | N | transversion |
| rpl2 | 458,973 | T<->G | Phe<->Leu | N | transversion |
| rpl2 | 459,220 | A<->C | IIe<->Leu | N | transversion |
| rpl5 | 457,988 | A<->C | Lys<->Cys | N | transversion |
| rpl10 | 171,493 | G<->A | Gln<->Lys | N | transition |
| rpl16 | 399,033 | A<->C | Val<->Val | S | transversion |
| Sample | 0-613-2R-1 | 0-613-2R-2 | 0-613-2R-3 | SI3A-1 | SI3A-2 | SI3A-3 |
|---|---|---|---|---|---|---|
| Total raw reads | 27,570,398 | 27,645,300 | 27,163,449 | 24,396,971 | 30,170,978 | 22,451,704 |
| Total clean reads | 26,157,634 | 26,560,996 | 26,094,847 | 23,354,551 | 28,705,908 | 21,508,933 |
| Clean reads Q20(%) | 97.38 | 97.4 | 97.22 | 97.33 | 97.13 | 97.38 |
| Clean reads Q30(%) | 92.59 | 92.66 | 92.25 | 92.44 | 92.08 | 92.59 |
| Total alignment with mt genome | 25,765 (0.10%) | 25,125 (0.09%) | 26,713 (0.10%) | 16,247 (0.07%) | 21,734 (0.08%) | 18,759 (0.09%) |
| Exact alignment with mt genome | 14,708 (0.06%) | 14,376 (0.05%) | 15,446 (0.06%) | 12,592 (0.05%) | 16,988 (0.06%) | 14,509 (0.07%) |
| Average (exact) | 0.06% | 0.06% | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, L.; Qi, G.; Li, X.; Yan, S.; Cao, Y.; Huang, C.; He, L.; Zhang, T.; Shang, H.; Hu, Y. Comparison of Mitochondrial Genomes between a Cytoplasmic Male-Sterile Line and Its Restorer Line for Identifying Candidate CMS Genes in Gossypium hirsutum. Int. J. Mol. Sci. 2022, 23, 9198. https://doi.org/10.3390/ijms23169198
Xuan L, Qi G, Li X, Yan S, Cao Y, Huang C, He L, Zhang T, Shang H, Hu Y. Comparison of Mitochondrial Genomes between a Cytoplasmic Male-Sterile Line and Its Restorer Line for Identifying Candidate CMS Genes in Gossypium hirsutum. International Journal of Molecular Sciences. 2022; 23(16):9198. https://doi.org/10.3390/ijms23169198
Chicago/Turabian StyleXuan, Lisha, Guoan Qi, Xiaoran Li, Sunyi Yan, Yiwen Cao, Chujun Huang, Lu He, Tianzhen Zhang, Haihong Shang, and Yan Hu. 2022. "Comparison of Mitochondrial Genomes between a Cytoplasmic Male-Sterile Line and Its Restorer Line for Identifying Candidate CMS Genes in Gossypium hirsutum" International Journal of Molecular Sciences 23, no. 16: 9198. https://doi.org/10.3390/ijms23169198
APA StyleXuan, L., Qi, G., Li, X., Yan, S., Cao, Y., Huang, C., He, L., Zhang, T., Shang, H., & Hu, Y. (2022). Comparison of Mitochondrial Genomes between a Cytoplasmic Male-Sterile Line and Its Restorer Line for Identifying Candidate CMS Genes in Gossypium hirsutum. International Journal of Molecular Sciences, 23(16), 9198. https://doi.org/10.3390/ijms23169198






