Effects of Substitution Ratios of Zinc-Substituted Hydroxyapatite on Adsorption and Desorption Behaviors of Bone Morphogenetic Protein-2
Abstract
:1. Introduction
2. Results
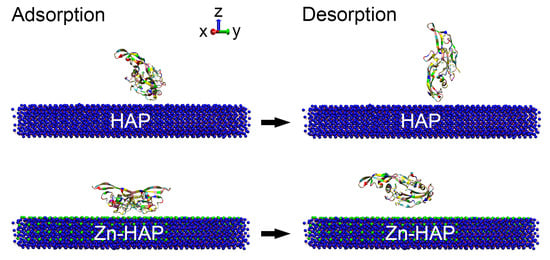
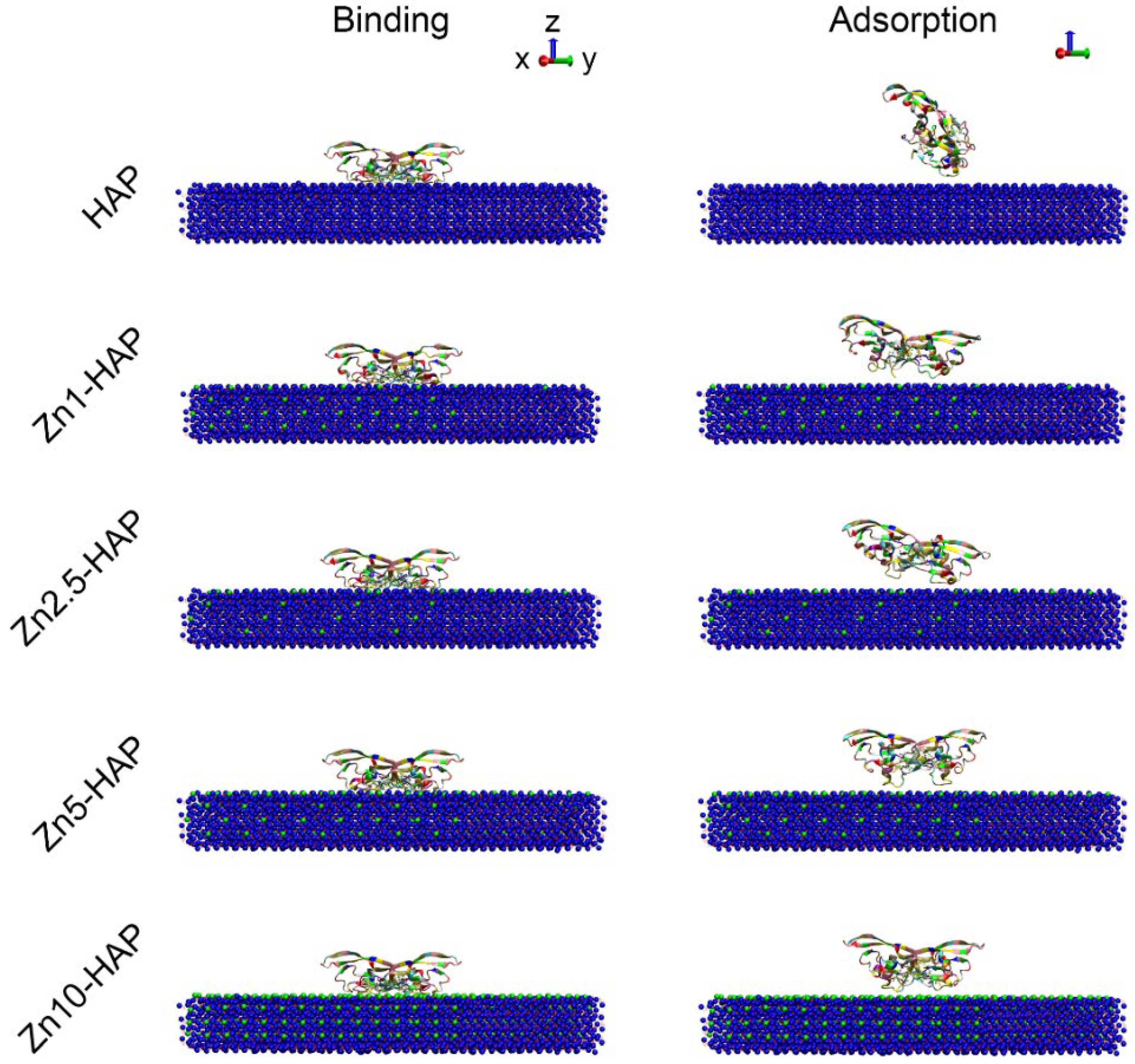
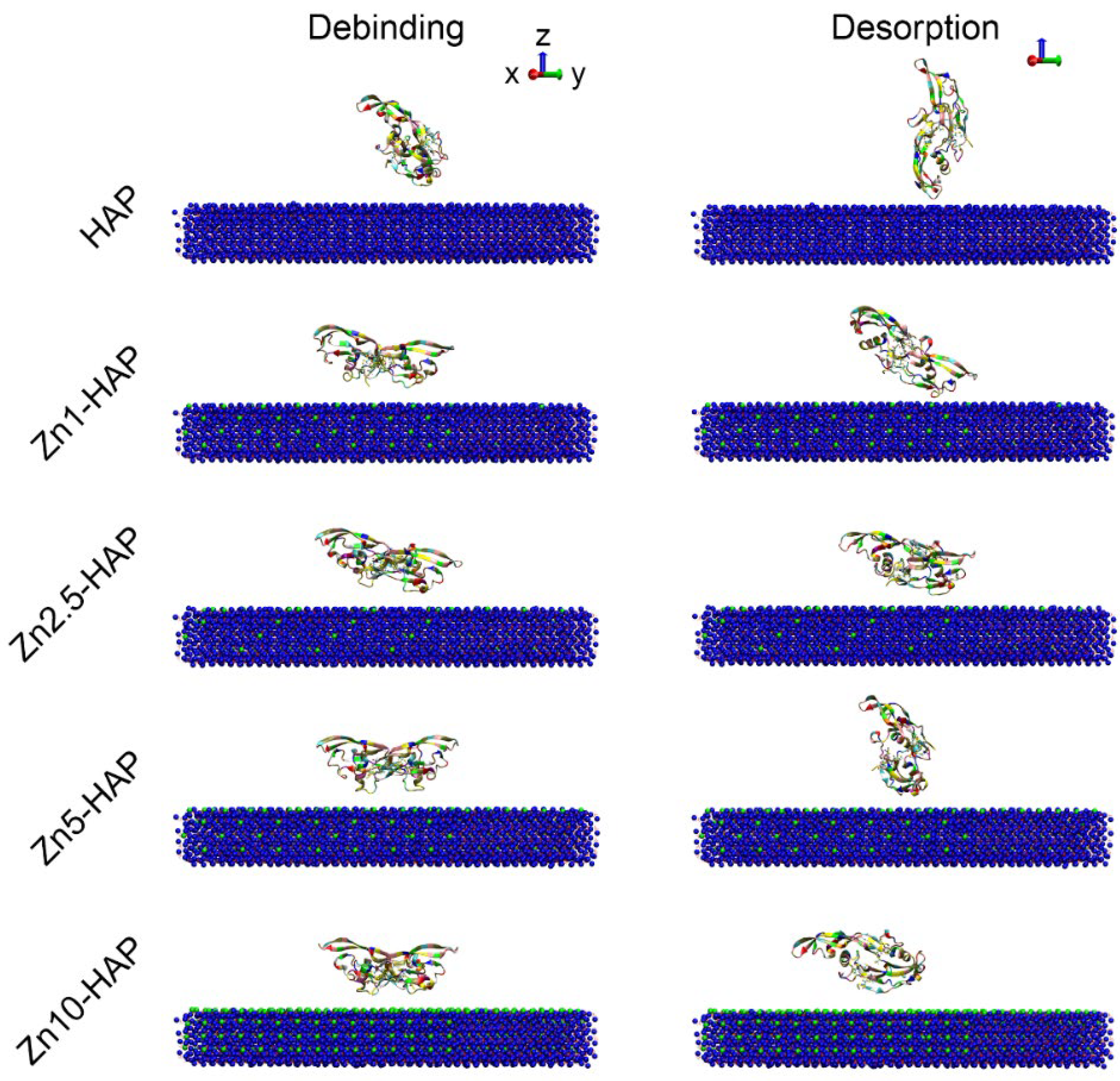
2.1. Interaction Energy
2.2. Structure States of BMP-2
2.3. Rg and RMSD
2.4. RMSF and Rf
2.5. Secondary Structures
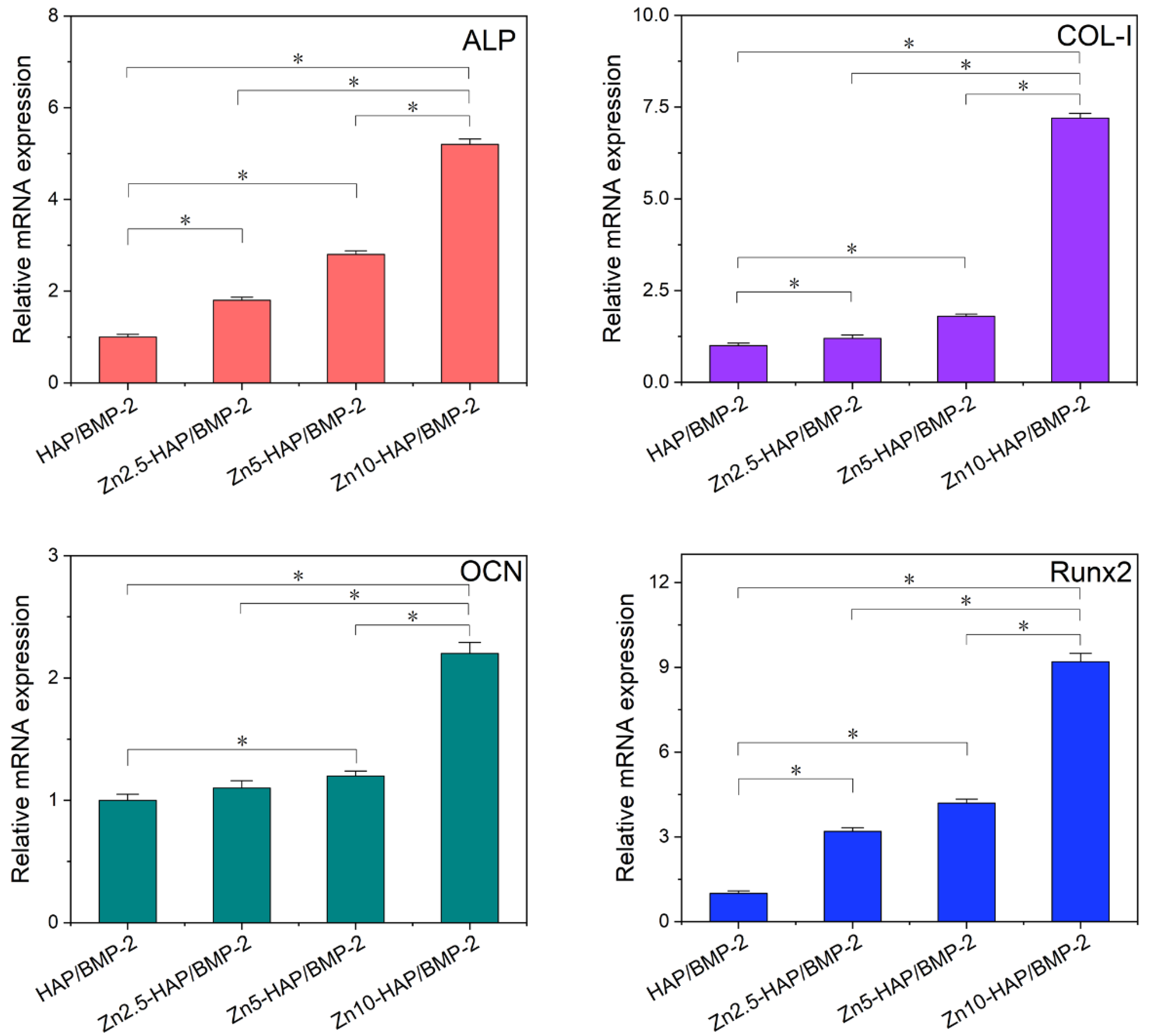
2.6. Osteogenic Activity
3. Discussion
4. Materials and Methods
4.1. Protein Model
4.2. Surface Models
4.3. System Setup
4.4. Computer Simulations
4.5. Data Collection
4.6. Experimental Studies
4.6.1. ALP Study
4.6.2. RT-PCR Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMPs | bone morphogenetic proteins |
| BMP-2 | bone morphogenetic protein-2 |
| Zn-HAP | zinc-substituted hydroxyapatite |
| MD | molecular dynamics |
| SMD | steered molecular dynamics |
| SPC | single point charge |
| PCV | constant velocity pulling |
| P’CV | constant velocity pushing |
| LJ-SR | short-range Lennard-Jones energies |
| Coul-SR | Coulombic potentials within R-coulomb |
| Rg | radius of gyration |
| RMSD | root mean square deviation |
| RMSF | root mean square fluctuation |
| Rf | RMSF of cysteine-knots |
| COM | center of mass |
| NOC | number of contacts |
| ALP | alkaline phosphatase |
| RT-PCR | reverse transcription-polymerase chain reaction |
References
- Othman, Z.; Cillero Pastor, B.; van Rijt, S.; Habibovic, P. Understanding interactions between biomaterials and biological systems using proteomics. Biomaterials 2018, 167, 191–204. [Google Scholar] [CrossRef]
- Molino, P.; Higgins, M.; Innis, P.; Kapsa, R.; Wallace, G. Fibronectin and Bovine Serum Albumin Adsorption and Conformational Dynamics on Inherently Conducting Polymers: A QCM-D Study. Langmuir 2012, 28, 8433–8445. [Google Scholar] [CrossRef]
- Thyparambil, A.A.; Wei, Y.; Latour, R.A. Experimental characterization of adsorbed protein orientation, conformation, and bioactivity. Biointerphases 2015, 10, 019002. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, X.; Gao, L.; Jing, L.; Zhou, Q.; Chang, J. The role of the micro-pattern and nano-topography of hydroxyapatite bioceramics on stimulating osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018, 73, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Ozboyaci, M.; Kokh, D.B.; Corni, S.; Wade, R.C. Modeling and simulation of protein–surface interactions: Achievements and challenges. Q. Rev. Biophys. 2016, 49, e4. [Google Scholar] [CrossRef] [PubMed]
- Suliman, S.; Xing, Z.; Wu, X.; Xue, Y.; Pedersen, T.O.; Sun, Y.; Døskeland, A.P.; Nickel, J.; Waag, T.; Lygre, H.; et al. Release and bioactivity of bone morphogenetic protein-2 are affected by scaffold binding techniques in vitro and in vivo. J. Control. Release 2014, 197, 148–157. [Google Scholar] [CrossRef]
- Huang, B.; Wu, Z.; Ding, S.; Yuan, Y.; Liu, C. Localization and promotion of recombinant human bone morphogenetic protein-2 bioactivity on extracellular matrix mimetic chondroitin sulfate-functionalized calcium phosphate cement scaffolds. Acta Biomater. 2018, 71, 184–199. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, Y.; Liu, C. Biomaterial-guided immobilization and osteoactivity of bone morphogenetic protein-2. Appl. Mater. Today 2020, 19, 100599. [Google Scholar] [CrossRef]
- James, A.W.; Lachaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Begam, H.; Nandi, S.K.; Kundu, B.; Chanda, A. Strategies for delivering bone morphogenetic protein for bone healing. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 70, 856–869. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, Y.; Ding, S.; Li, J.; Ren, J.; Feng, B.; Li, T.; Gu, Y.; Liu, C. Nanostructured hydroxyapatite surfaces-mediated adsorption alters recognition of BMP receptor IA and bioactivity of bone morphogenetic protein-2. Acta Biomater. 2015, 27, 275–285. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, Y.; Li, T.; Ding, S.; Zhang, W.; Gu, Y.; Liu, C. Facilitated receptor-recognition and enhanced bioactivity of bone morphogenetic protein-2 on magnesium-substituted hydroxyapatite surface. Sci. Rep. 2016, 6, 24323. [Google Scholar] [CrossRef] [PubMed]
- Tabisz, B.; Schmitz, W.; Schmitz, M.; Luehmann, T.; Heusler, E.; Rybak, J.-C.; Meinel, L.; Fiebig, J.E.; Mueller, T.D.; Nickel, J. Site-Directed Immobilization of BMP-2: Two Approaches for the Production of Innovative Osteoinductive Scaffolds. Biomacromolecules 2017, 18, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Marquetti, I.; Desai, S. Nanoscale Topographical Effects on the Adsorption Behavior of Bone Morphogenetic Protein-2 on Graphite. Int. J. Mol. Sci. 2022, 23, 2432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, M.-H.; Wang, K.-F.; Liu, Y.; Zhang, H.-P.; Lu, X.; Zhang, X.-D. Computer simulation of biomolecule–biomaterial interactions at surfaces and interfaces. Biomed. Mater. 2015, 10, 032001. [Google Scholar] [CrossRef]
- Hao, L.; Li, T.; Wang, L.; Shi, X.; Fan, Y.; Du, C.; Wang, Y. Mechanistic insights into the adsorption and bioactivity of fibronectin on surfaces with varying chemistries by a combination of experimental strategies and molecular simulations. Bioact. Mater. 2021, 6, 3125–3135. [Google Scholar] [CrossRef]
- Dong, X.-L.; Qi, W.; Tao, W.; Ma, L.-Y.; Fu, C.-X. The dynamic behaviours of protein BMP-2 on hydroxyapatite nanoparticles. Mol. Simul. 2011, 37, 1097–1104. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Q.; Wu, T.; Pan, H. Understanding Adsorption-Desorption Dynamics of BMP-2 on Hydroxyapatite (001) Surface. Biophys. J. 2007, 93, 750–759. [Google Scholar] [CrossRef]
- Marquetti, I.; Desai, S. Molecular modeling the adsorption behavior of bone morphogenetic protein-2 on hydrophobic and hydrophilic substrates. Chem. Phys. Lett. 2018, 706, 285–294. [Google Scholar] [CrossRef]
- Utesch, T.; Daminelli, G.; Mroginski, M.A. Molecular Dynamics Simulations of the Adsorption of Bone Morphogenetic Protein-2 on Surfaces with Medical Relevance. Langmuir 2011, 27, 13144–13153. [Google Scholar] [CrossRef]
- Hung, S.-W.; Hsiao, P.-Y.; Chieng, C.-C. Dynamic information for cardiotoxin protein desorption from a methyl-terminated self-assembled monolayer using steered molecular dynamics simulation. J. Chem. Phys. 2011, 134, 194705. [Google Scholar] [CrossRef]
- Lecot, S.; Chevolot, Y.; Phaner-Goutorbe, M.; Yeromonahos, C. Impact of Silane Monolayers on the Adsorption of Streptavidin on Silica and Its Subsequent Interactions with Biotin: Molecular Dynamics and Steered Molecular Dynamics Simulations. J. Phys. Chem. B 2020, 124, 6786–6796. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Tian, Y.; Zhang, W.; Ma, Y.; Yuan, Y.; Liu, C. Strontium doping promotes bioactivity of rhBMP-2 upon calcium phosphate cement via elevated recognition and expression of BMPR-IA. Colloids Surf. B Biointerfaces 2017, 159, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Begam, H.; Nandi, S.K.; Chanda, A.; Kundu, B. Effect of bone morphogenetic protein on Zn-HAp and Zn-HAp/collagen composite: A systematic in vivo study. Res. Vet. Sci. 2017, 115, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, H.; Gao, Y.; Li, J.; Lin, K.; Liu, B.; Lei, X.; Cheng, P.; Zhang, S.; Wang, Y.; et al. Zinc Silicate/Nano-Hydroxyapatite/Collagen Scaffolds Promote Angiogenesis and Bone Regeneration via the p38 MAPK Pathway in Activated Monocytes. ACS Appl. Mater. Inter. 2020, 12, 16058–16075. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, S.; Xiao, W.; Cui, X.; Huang, W.; Rahaman, M.N.; Zhang, C.; Wang, D. Three-dimensional zinc incorporated borosilicate bioactive glass scaffolds for rodent critical-sized calvarial defects repair and regeneration. Colloids Surf. B Biointerfaces 2015, 130, 149–156. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Cheng, M.; Wang, Q.; Yeung, K.W.K.; Chu, P.K.; Zhang, X. Zinc-Modified Sulfonated Polyetheretherketone Surface with Immunomodulatory Function for Guiding Cell Fate and Bone Regeneration. Adv. Sci. 2018, 5, 1800749. [Google Scholar] [CrossRef]
- Gu, H.; Xue, Z.; Wang, M.; Yang, M.; Wang, K.; Xu, D. Effect of Hydroxyapatite Surface on BMP-2 Biological Properties by Docking and Molecular Simulation Approaches. J. Phys. Chem. B 2019, 123, 3372–3382. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, M.; Li, P.; Wang, K.; Fang, L.; Ren, F.; Lu, G.; Lu, X. The interaction of Chitosan and BMP-2 tuned by deacetylation degree and pH value. J. Biomed. Mater. Res. Part A 2018, 107, 769–779. [Google Scholar] [CrossRef]
- Huang, B.; He, M.; Zhang, K.; Sun, S.; Lin, Z.; Chen, D.; Li, T.; Chen, X. Rotation-induced secondary structure losses and bioactivity changes of bone morphogenetic protein-2 on strontium-substituted hydroxyapatite surfaces. Appl. Surf. Sci. 2020, 511, 145623. [Google Scholar] [CrossRef]
- Huang, B.; Lou, Y.; Li, T.; Lin, Z.; Sun, S.; Yuan, Y.; Liu, C.; Gu, Y. Molecular dynamics simulations of adsorption and desorption of bone morphogenetic protein-2 on textured hydroxyapatite surfaces. Acta Biomater. 2018, 80, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-J.; Heinz, H. Accurate Force Field Parameters and pH Resolved Surface Models for Hydroxyapatite to Understand Structure, Mechanics, Hydration, and Biological Interfaces. J. Phys. Chem. C 2016, 120, 4975–4992. [Google Scholar] [CrossRef] [Green Version]




| Surfaces | Interaction Energy | COM Distance | SMD Time | NOC |
|---|---|---|---|---|
| HAP | −257 ± 60 | 2.86 ± 0.04 | 190 ± 10 | 48.2 ± 10.1 |
| Zn1-HAP | −236 ± 23 | 2.75 ± 0.04 | 215 ± 10 | 68.4 ± 8.2 |
| Zn2.5-HAP | −383 ± 76 | 2.68 ± 0.04 | 225 ± 10 | 83.2 ± 9.9 |
| Zn5-HAP | −291 ± 36 | 2.86 ± 0.04 | 190 ± 10 | 38.2 ± 4.7 |
| Zn10-HAP | −397 ± 98 | 2.58± 0.04 | 240 ± 10 | 105.6 ± 5.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Li, M.; Mo, H.; Chen, C.; Chen, K. Effects of Substitution Ratios of Zinc-Substituted Hydroxyapatite on Adsorption and Desorption Behaviors of Bone Morphogenetic Protein-2. Int. J. Mol. Sci. 2022, 23, 10144. https://doi.org/10.3390/ijms231710144
Huang B, Li M, Mo H, Chen C, Chen K. Effects of Substitution Ratios of Zinc-Substituted Hydroxyapatite on Adsorption and Desorption Behaviors of Bone Morphogenetic Protein-2. International Journal of Molecular Sciences. 2022; 23(17):10144. https://doi.org/10.3390/ijms231710144
Chicago/Turabian StyleHuang, Baolin, Manchun Li, Hailing Mo, Chuang Chen, and Kun Chen. 2022. "Effects of Substitution Ratios of Zinc-Substituted Hydroxyapatite on Adsorption and Desorption Behaviors of Bone Morphogenetic Protein-2" International Journal of Molecular Sciences 23, no. 17: 10144. https://doi.org/10.3390/ijms231710144
APA StyleHuang, B., Li, M., Mo, H., Chen, C., & Chen, K. (2022). Effects of Substitution Ratios of Zinc-Substituted Hydroxyapatite on Adsorption and Desorption Behaviors of Bone Morphogenetic Protein-2. International Journal of Molecular Sciences, 23(17), 10144. https://doi.org/10.3390/ijms231710144