The Effect of Trehalose Coating for Magnetite Nanoparticles on Stability of Egg White Lysozyme
Abstract
:1. Introduction
2. Results and Discussion
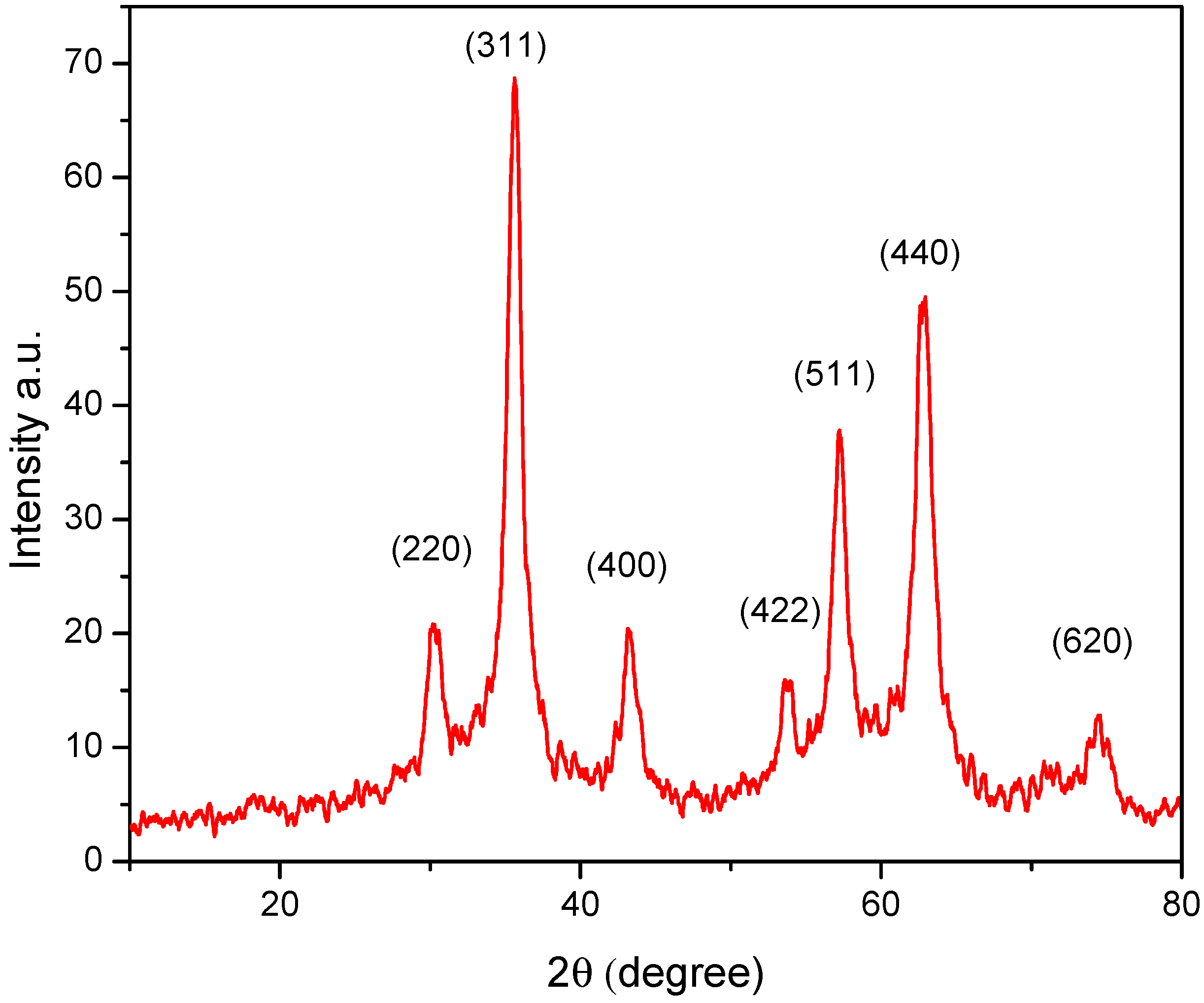
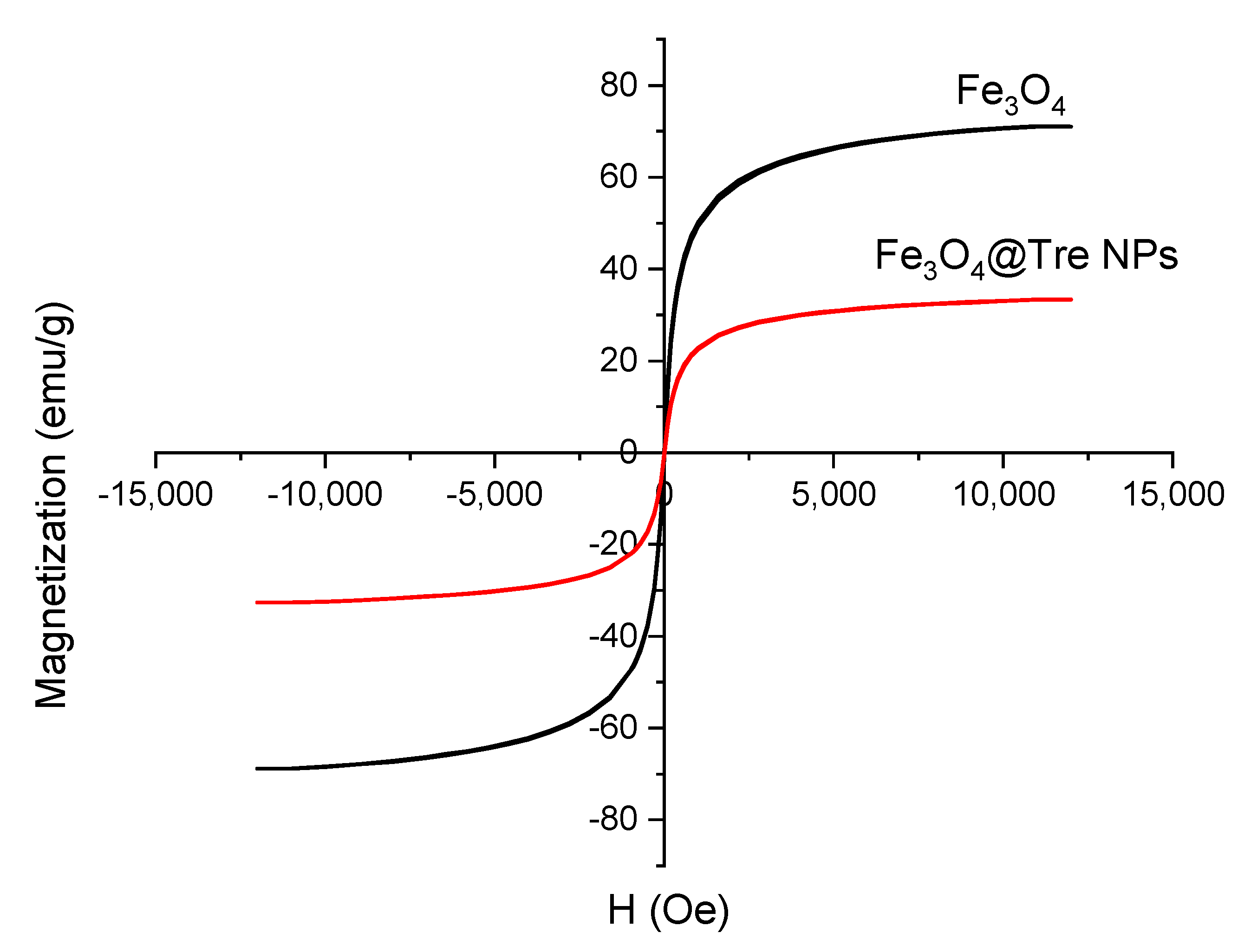
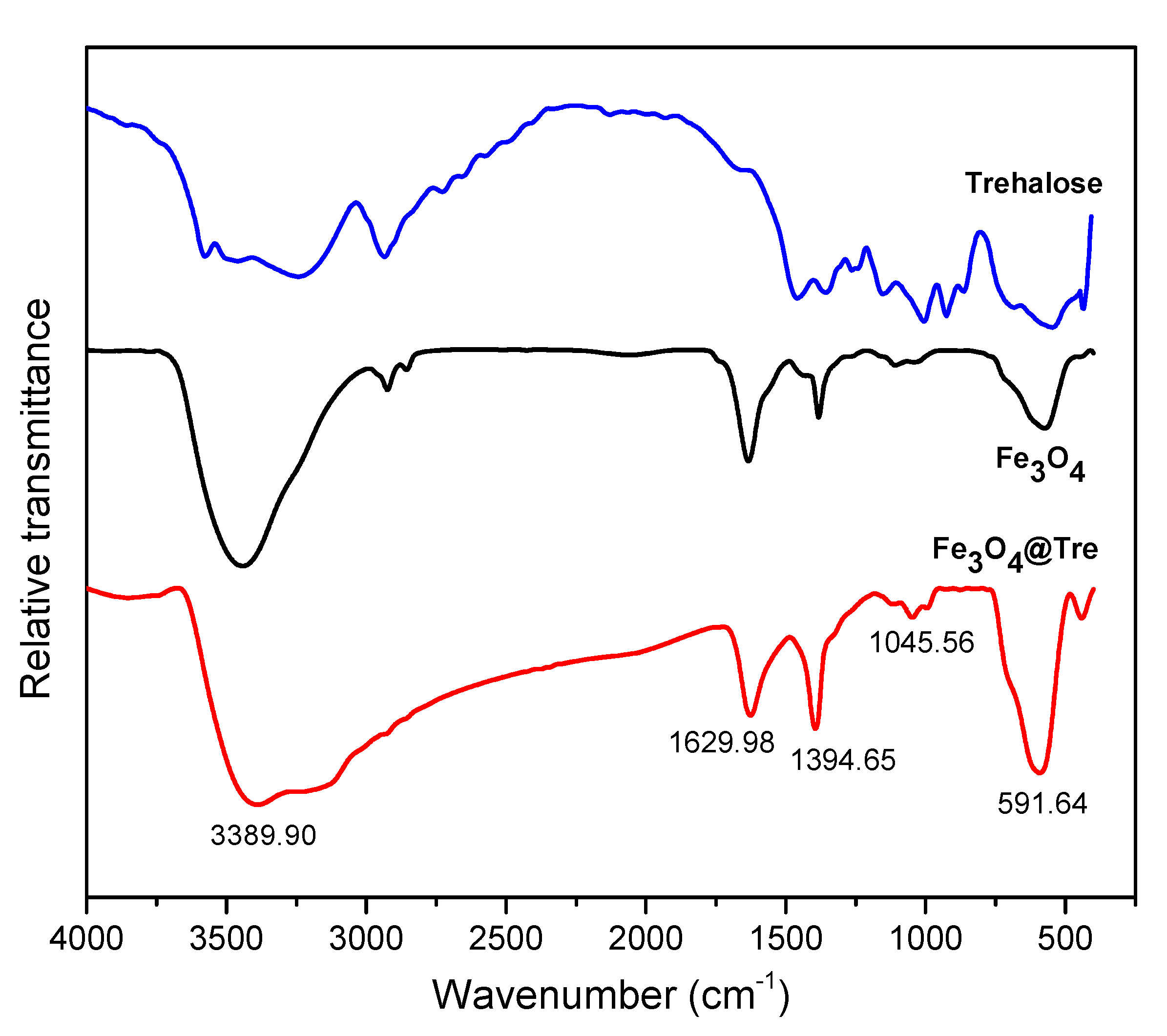
2.1. Characterization of Magnetic NPs
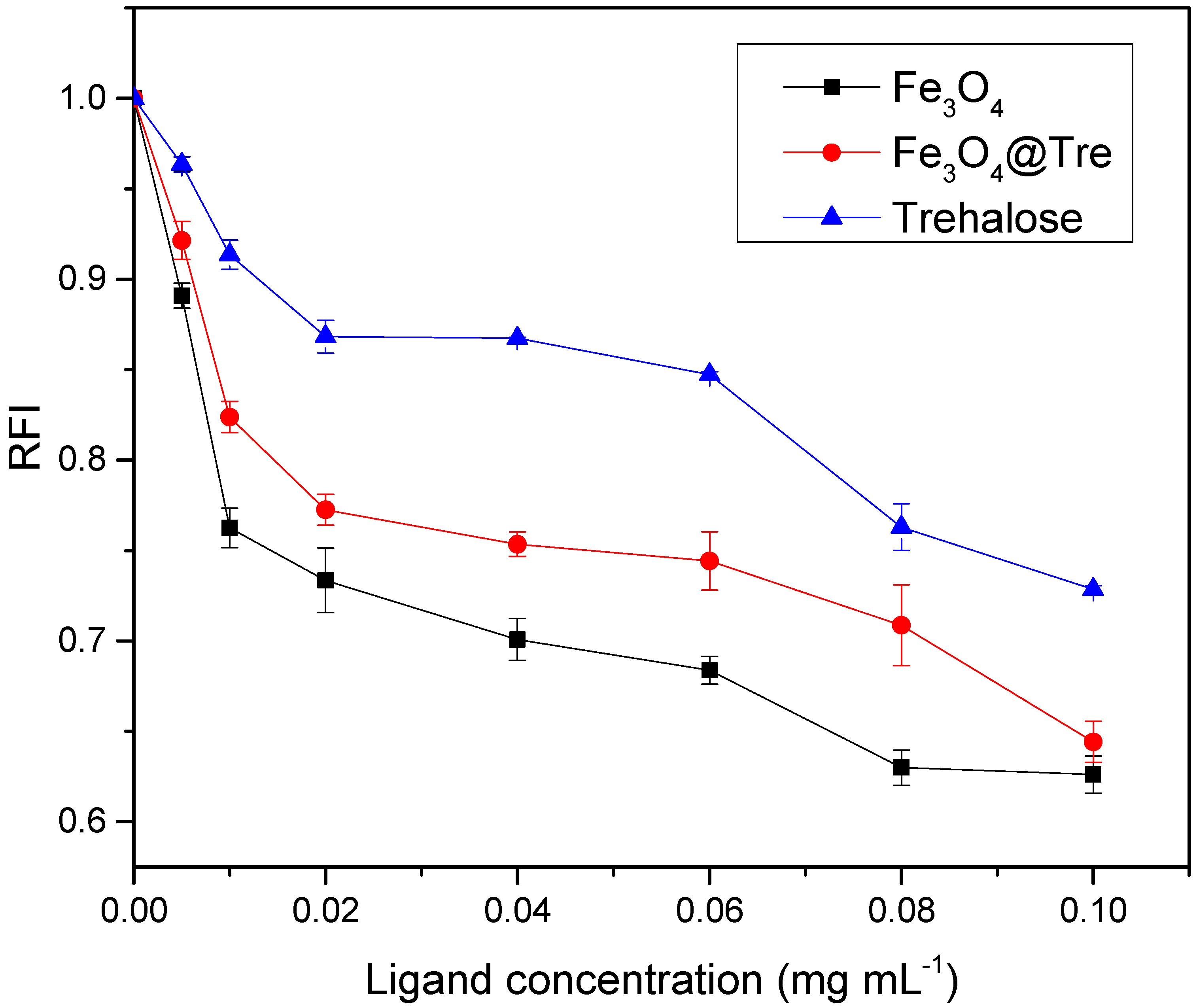
2.2. Fluorescence Measurement
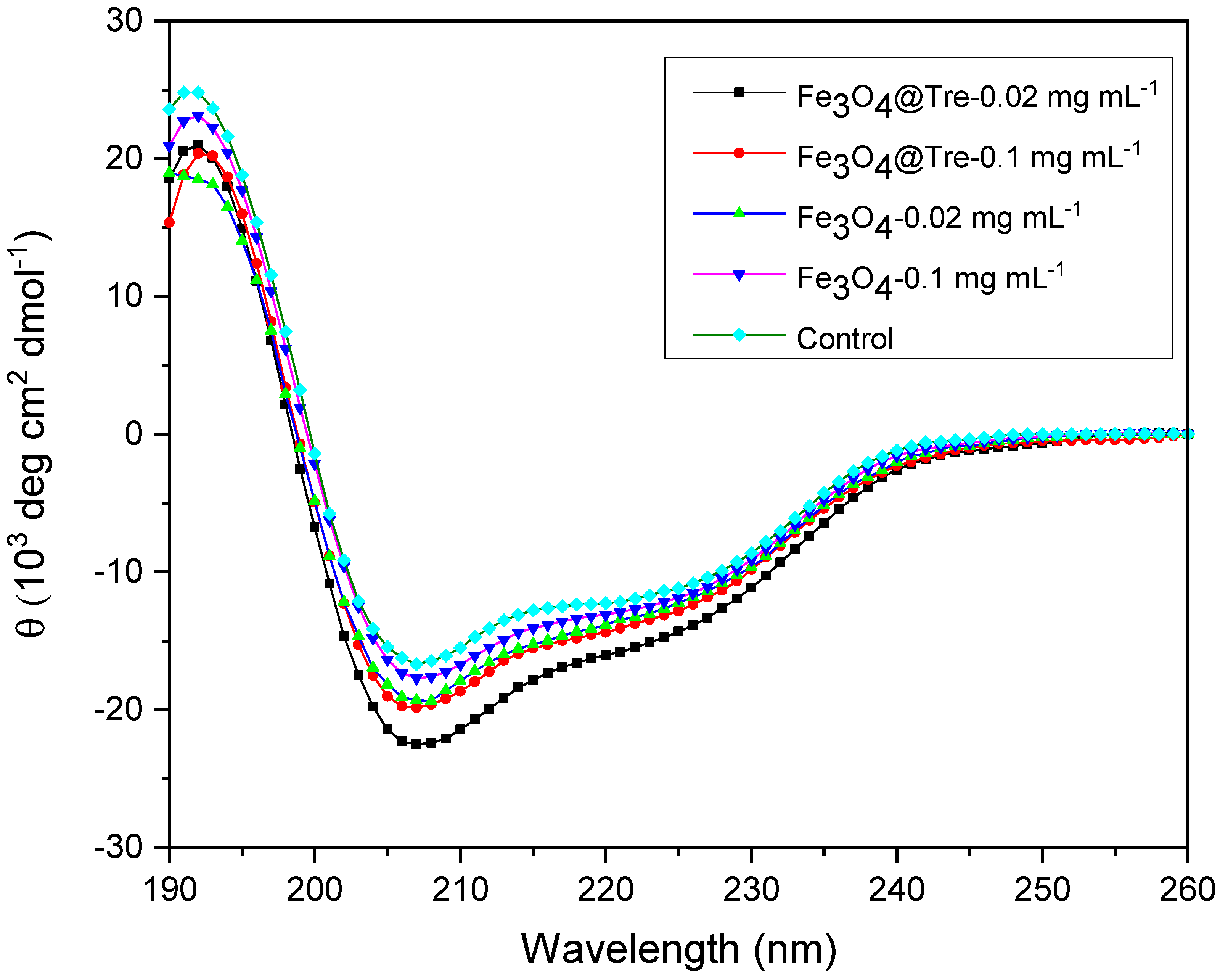
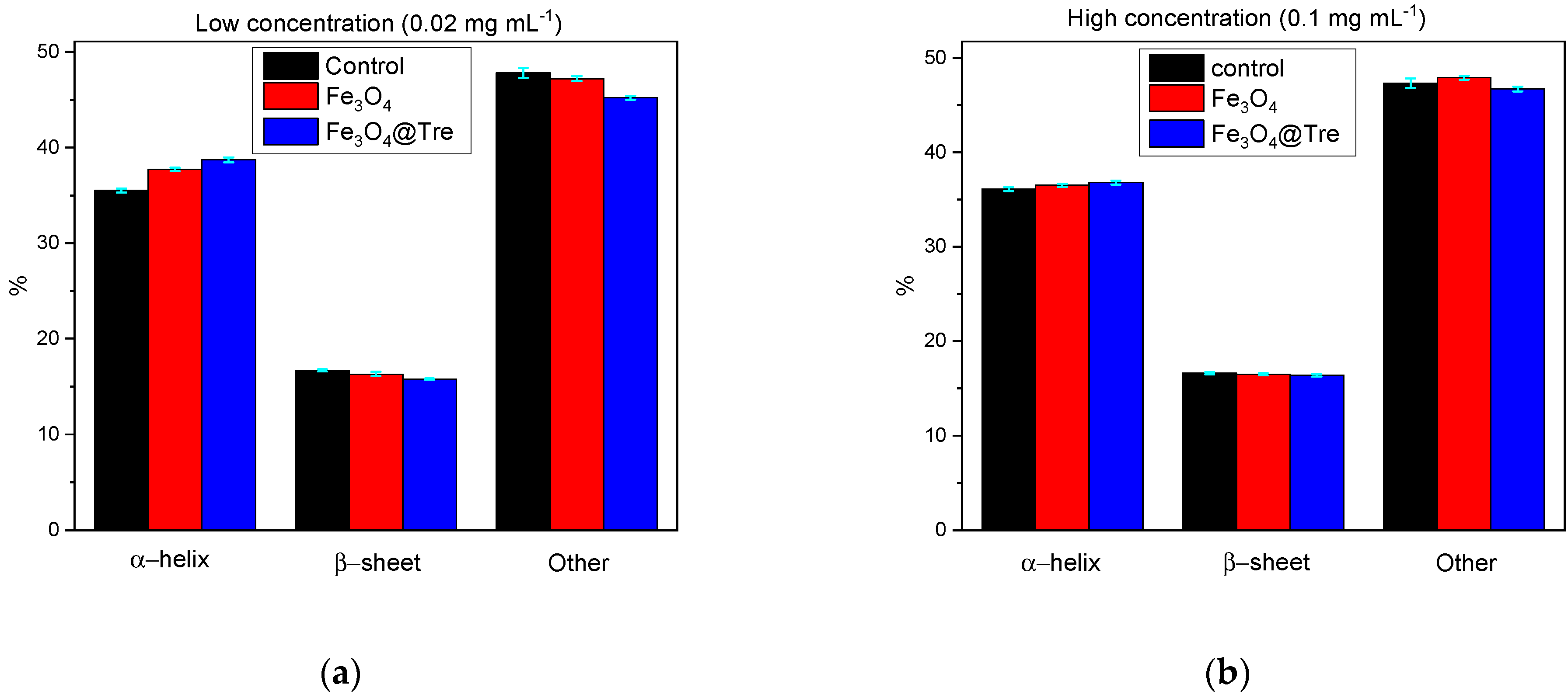
2.3. Circular Dichroism Spectroscopy
2.4. UV-Visible Investigation of Lysozyme Activity Limit
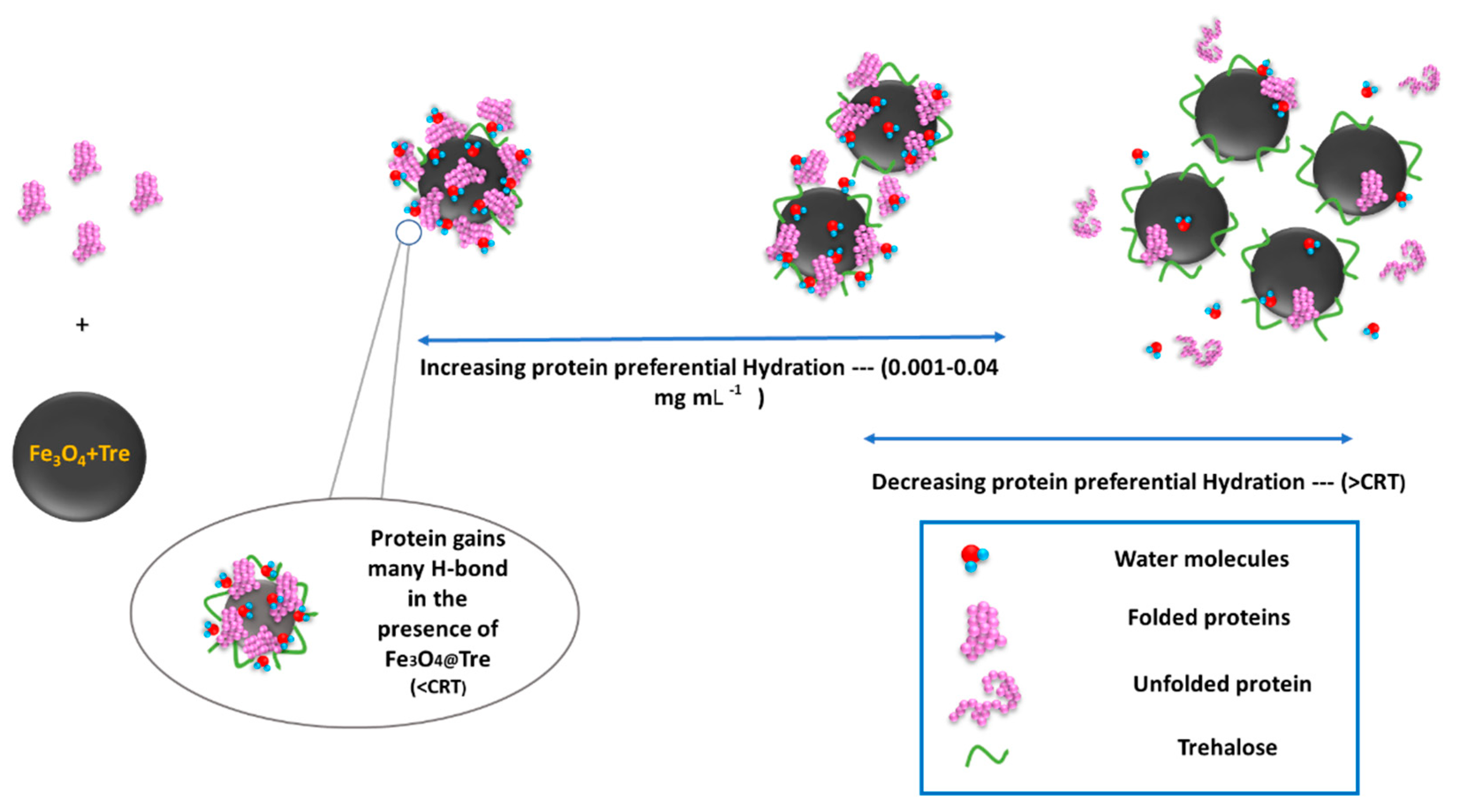
2.5. Protein Corona Formation
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Synthesis of Fe3O4 and Fe3O4@Tre NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Qiao, J.; Qi, L. Dual-functional polymer-modified magnetic nanoparticles for isolation of lysozyme. Anal. Chim. Acta 2018, 1035, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Leader, B.; Baca, Q.J.; Golan, D.E. Protein therapeutics: A summary and pharmacological classification. Nat. Rev. Drug Discov. 2008, 7, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Carrell, R.W.; Lomas, D.A. Conformational disease. Lancet 1997, 350, 134–138. [Google Scholar] [CrossRef]
- Agbas, A. Trends of protein aggregation in neurodegenerative diseases. In Neurochemical Basis of Brain Function and Dysfunction; IntechOpen: London, UK, 2018. [Google Scholar]
- Parveen, R.; Shamsi, T.N.; Fatima, S. Nanoparticles-protein interaction: Role in protein aggregation and clinical implications. Int. J. Biol. Macromol. 2016, 94, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Sirangelo, I.; Irace, G. Inhibition of aggregate formation as therapeutic target in protein misfolding diseases: Effect of tetracycline and trehalose. Expert Opin. Ther. Targets 2010, 14, 1311–1321. [Google Scholar] [CrossRef]
- Zaman, M.; Khan, A.N.; Wahiduzzaman; Zakariya, S.M.; Khan, R.H. Protein misfolding, aggregation and mechanism of amyloid cytotoxicity: An overview and therapeutic strategies to inhibit aggregation. Int. J. Biol. Macromol. 2019, 134, 1022–1037. [Google Scholar] [CrossRef]
- Clark, E.D.B. Protein refolding for industrial processes. Curr. Opin. Biotechnol. 2001, 12, 202–207. [Google Scholar] [CrossRef]
- Wang, W. Protein aggregation and its inhibition in biopharmaceutics. Int. J. Pharm. 2005, 289, 1–30. [Google Scholar] [CrossRef]
- Vallejo, L.F.; Rinas, U. Strategies for the recovery of active proteins through refolding of bacterial inclusion body proteins. Microb. Cell Factories 2004, 3, 11. [Google Scholar] [CrossRef]
- Eiberle, M.K.; Jungbauer, A. Technical refolding of proteins: Do we have freedom to operate? Biotechnol. J. 2010, 5, 547–559. [Google Scholar] [CrossRef] [Green Version]
- Olsson, C.; Swenson, J. The role of disaccharides for protein–protein interactions—A SANS study. Mol. Phys. 2019, 117, 3408–3416. [Google Scholar] [CrossRef]
- van Oss, C.J. Hydrophobicity and hydrophilicity of biosurfaces. Curr. Opin. Colloid Interface Sci. 1997, 2, 503–512. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Bellova, A.; Bystrenova, E.; Koneracka, M.; Kopcansky, P.; Valle, F.; Tomasovicova, N.; Timko, M.; Bagelova, J.; Biscarini, F.; Gazova, Z. Effect of Fe3O4 magnetic nanoparticles on lysozyme amyloid aggregation. Nanotechnology 2010, 21, 065103. [Google Scholar] [CrossRef] [PubMed]
- Loizou, K.; Mourdikoudis, S.; Sergides, A.; Besenhard, M.O.; Sarafidis, C.; Higashimine, K.; Kalogirou, O.; Maenosono, S.; Thanh, N.T.K.; Gavriilidis, A. Rapid Millifluidic Synthesis of Stable High Magnetic Moment FexCy Nanoparticles for Hyperthermia. ACS Appl. Mater. Interfaces 2020, 12, 28520–28531. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Kalhor, H.R.; Laurent, S.; Lynch, I. Protein fibrillation and nanoparticle interactions: Opportunities and challenges. Nanoscale 2013, 5, 2570–2588. [Google Scholar] [CrossRef]
- Zhang, M.; Mao, X.; Yu, Y.; Wang, C.-X.; Yang, Y. Nanomaterials for Reducing Amyloid Cytotoxicity. Adv. Mater. 2013, 25, 3780–3801. [Google Scholar] [CrossRef]
- Mosayebi, J.; Kiyasatfar, M.; Laurent, S. Synthesis, functionalization, and design of magnetic nanoparticles for theranostic applications. Adv. Healthc. Mater. 2017, 6, 1700306. [Google Scholar] [CrossRef]
- Hamad-Schifferli, K.; Bergese, P. Nanomaterial Interfaces in Biology: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2013. [Google Scholar]
- Sauvage, F.; Schymkowitz, J.; Rousseau, F.; Schmidt, B.Z.; Remaut, K.; Braeckmans, K.; De Smedt, S.C. Nanomaterials to avoid and destroy protein aggregates. Nano Today 2020, 31, 100837. [Google Scholar] [CrossRef]
- Silva, V.; Andrade, P.; Silva, M.P.C.; Valladares, L.D.L.S.; Aguiar, J.A. Synthesis and characterization of Fe3O4 nanoparticles coated with fucan polysaccharides. J. Magn. Magn. Mater. 2013, 343, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Baniukevic, J.; Boyaci, I.H.; Bozkurt, A.G.; Tamer, U.; Ramanavicius, A.; Ramanaviciene, A. Magnetic gold nanoparticles in SERS-based sandwich immunoassay for antigen detection by well oriented antibodies. Biosens. Bioelectron. 2013, 43, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Izmitli, A.; Schebor, C.; McGovern, M.P.; Reddy, A.S.; Abbott, N.L.; De Pablo, J.J. Effect of trehalose on the interaction of Alzheimer’s Aβ-peptide and anionic lipid monolayers. Biochim. Biophys. Acta Biomembr. 2011, 1808, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Samuel, D.; Ganesh, G.; Yang, P.-W.; Chang, M.-M.; Wang, S.-L.; Hwang, K.-C.; Yu, C.; Jayaraman, G.; Kumar, T.K.S.; Trivedi, V.D.; et al. Proline inhibits aggregation during protein refolding. Protein Sci. 2008, 9, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Anbarasu, M.; Anandan, M.; Chinnasamy, E.; Gopinath, V.; Balamurugan, K. Synthesis and characterization of polyethylene glycol (PEG) coated Fe3O4 nanoparticles by chemical co-precipitation method for biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 536–539. [Google Scholar] [CrossRef]
- Dias, A.M.G.C.; Hussain, A.; Marcos, A.S.; Roque, A.C.A. A biotechnological perspective on the application of iron oxide magnetic colloids modified with polysaccharides. Biotechnol. Adv. 2011, 29, 142–155. [Google Scholar] [CrossRef]
- Debnath, K.; Pradhan, N.; Singh, B.K.; Jana, N.R.; Jana, N.R. Poly(trehalose) Nanoparticles Prevent Amyloid Aggregation and Suppress Polyglutamine Aggregation in a Huntington’s Disease Model Mouse. ACS Appl. Mater. Interfaces 2017, 9, 24126–24139. [Google Scholar] [CrossRef]
- Meng, F.-G.; Park, Y.-D.; Zhou, H.-M. Role of proline, glycerol, and heparin as protein folding aids during refolding of rabbit muscle creatine kinase. Int. J. Biochem. Cell Biol. 2001, 33, 701–709. [Google Scholar] [CrossRef]
- Pradhan, N.; Jana, N.R.; Jana, N.R. Inhibition of protein aggregation by iron oxide nanoparticles conjugated with glutamine-and proline-based osmolytes. ACS Appl. Nano Mater. 2018, 1, 1094–1103. [Google Scholar] [CrossRef]
- Bednarikova, Z.; Marek, J.; Demjen, E.; Dutz, S.; Mocanu, M.; Wu, J.W.; Wang, S.S.-S.; Gazova, Z. Effect of nanoparticles coated with different modifications of dextran on lysozyme amyloid aggregation. J. Magn. Magn. Mater. 2019, 473, 1–6. [Google Scholar] [CrossRef]
- Arakawa, T.; Timasheff, S.N. Stabilization of protein structure by sugars. Biochemistry 1982, 21, 6536–6544. [Google Scholar] [CrossRef]
- Jakob, U.; Gaestel, M.; Engel, K.; Buchner, J. Small heat shock proteins are molecular chaperones. J. Biol. Chem. 1993, 268, 1517–1520. [Google Scholar] [CrossRef]
- Malferrari, M.; Savitsky, A.; Lubitz, W.; Mobius, K.; Venturoli, G. Protein immobilization capabilities of sucrose and trehalose glasses: The effect of protein/sugar concentration unraveled by high-field EPR. J. Phys. Chem. Lett. 2016, 7, 4871–4877. [Google Scholar] [CrossRef] [PubMed]
- Wiemken, A. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Van Leeuwenhoek 1990, 58, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.; Jansson, H.; Swenson, J. The Role of Trehalose for the Stabilization of Proteins. J. Phys. Chem. B 2016, 120, 4723–4731. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Silveira, C.; Durán, M.; Martinez, D.S.T. Silver nanoparticle protein corona and toxicity: A mini-review. J. Nanobiotechnology 2015, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Gupta, A.; Verma, N.C.; Nandi, C.K. Kinetics of protein adsorption on gold nanoparticle with variable protein structure and nanoparticle size. J. Chem. Phys. 2015, 143, 164709. [Google Scholar] [CrossRef]
- Varkhede, N.; Peters, B.-H.; Wei, Y.; Middaugh, C.R.; Schöneich, C.; Forrest, M.L. Effect of Iron Oxide Nanoparticles on the Oxidation and Secondary Structure of Growth Hormone. J. Pharm. Sci. 2019, 108, 3372–3381. [Google Scholar] [CrossRef]
- Kurtz-Chalot, A.; Villiers, C.; Pourchez, J.; Boudard, D.; Martini, M.; Marche, P.N.; Cottier, M.; Forest, V. Impact of silica nanoparticle surface chemistry on protein corona formation and consequential interactions with biological cells. Mater. Sci. Eng. C 2017, 75, 16–24. [Google Scholar] [CrossRef]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time Evolution of the Nanoparticle Protein Corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [Green Version]
- Milani, S.; Bombelli, F.B.; Pitek, A.S.; Dawson, K.A.; Rädler, J. Reversible versus Irreversible Binding of Transferrin to Polystyrene Nanoparticles: Soft and Hard Corona. ACS Nano 2012, 6, 2532–2541. [Google Scholar] [CrossRef] [PubMed]
- Fattah, R.; Rashedi, H.; Yazdian, F.; Mousavi, S.B.; Fazeli, A. Promising insights into the kosmotropic effect of magnetic nanoparticles on proteins: The pivotal role of protein corona formation. Biotechnol. Prog. 2020, 36, e3051. [Google Scholar] [CrossRef] [PubMed]
- Marichal, L.; Klein, G.; Armengaud, J.; Boulard, Y.; Chédin, S.; Labarre, J.; Pin, S.; Renault, J.-P.; Aude, J.-C. Protein Corona Composition of Silica Nanoparticles in Complex Media: Nanoparticle Size does not Matter. Nanomaterials 2020, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, B. Water Dynamics in the Hydration Layer around Proteins and Micelles. Chem. Rev. 2005, 105, 3197–3219. [Google Scholar] [CrossRef]
- Hunt, N.T.; Kattner, L.; Shanks, R.P.; Wynne, K. The Dynamics of Water−Protein Interaction Studied by Ultrafast Optical Kerr-Effect Spectroscopy. J. Am. Chem. Soc. 2007, 129, 3168–3172. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Erdőssy, J.; Horvath, V. Temperature-Responsive Magnetic Nanoparticles for Bioanalysis of Lysozyme in Urine Samples. Nanomaterials 2021, 11, 3015. [Google Scholar] [CrossRef]
- Trusova, V. Modulation of physiological and pathological activities of lysozyme by biological membranes. Cell. Mol. Biol. Lett. 2012, 17, 349–375. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Acaite, J.; Ramanavicius, A. Chronic caffeine intake affects lysozyme activity and immune cells in mice. J. Pharm. Pharmacol. 2004, 56, 671–676. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Jin, M.; Han, Q.; Liu, S.; Chen, X.; Han, Y. Enhancing the Thermo-Stability and Anti-Bacterium Activity of Lysozyme by Immobilization on Chitosan Nanoparticles. Int. J. Mol. Sci. 2020, 21, 1635. [Google Scholar] [CrossRef]
- Ghosh, S.; Pandey, N.K.; Roy, A.S.; Tripathy, D.R.; Dinda, A.K.; Dasgupta, S. Prolonged Glycation of Hen Egg White Lysozyme Generates Non Amyloidal Structures. PLoS ONE 2013, 8, e74336. [Google Scholar]
- Liu, K.; Lai, C.; Lee, Y.; Wang, S.; Chen, R.P.-Y.; Jan, J.; Liu, H.; Wang, S.S.-S. Curcumin’s pre-incubation temperature affects its inhibitory potency toward amyloid fibrillation and fibril-induced cytotoxicity of lysozyme. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Atale, S.S.; Dyawanapelly, S.; Jagtap, D.D.; Jain, R.; Dandekar, P. Understanding the nano-bio interactions using real-time surface plasmon resonance tool. Int. J. Biol. Macromol. 2018, 123, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.; Chantrell, R.; Chubykalo-Fesenko, O. Surface and interface effects in magnetic core–shell nanoparticles. MRS Bull. 2013, 38, 909–914. [Google Scholar] [CrossRef]
- Shirkhanzadeh, M. Microneedles coated with porous calcium phosphate ceramics: Effective vehicles for transdermal delivery of solid trehalose. J. Mater. Sci. Mater. Electron. 2005, 16, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Imoto, T.; Forster, L.S.; Rupley, J.A.; Tanaka, F. Fluorescence of Lysozyme: Emissions from Tryptophan Residues 62 and 108 and Energy Migration. Proc. Natl. Acad. Sci. 1972, 69, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Calzolai, L.; Franchini, F.; Gilliland, D.; Rossi, F. Protein-nanoparticle interaction: Identification of the ubiquitin—Gold nanoparticle interaction site. Nano Lett. 2010, 10, 3101–3105. [Google Scholar] [CrossRef]
- Kashanian, F.; Habibi-Rezaei, M.; Bagherpour, A.R.; Seyedarabi, A.; Moosavi-Movahedi, A.A. Magnetic nanoparticles as double-edged swords: Concentration-dependent ordering or disordering effects on lysozyme. RSC Adv. 2017, 7, 54813–54822. [Google Scholar] [CrossRef]
- Masters, B.R. Principles of fluorescence spectroscopy. J. Biomed. Opt. 2008, 13, 029901. [Google Scholar]
- Yu, W.-B.; Jiang, T.; Lan, D.-M.; Lu, J.-H.; Yue, Z.-Y.; Wang, J.; Zhou, P. Trehalose inhibits fibrillation of A53T mutant alpha-synuclein and disaggregates existing fibrils. Arch. Biochem. Biophys. 2012, 523, 144–150. [Google Scholar] [CrossRef]
- Chen, C.-H.; Yao, T.; Zhang, Q.; He, Y.-M.; Xu, L.-H.; Zheng, M.; Zhou, G.-R.; Zhang, Y.; Yang, H.-J.; Zhou, P. Influence of trehalose on human islet amyloid polypeptide fibrillation and aggregation. RSC Adv. 2016, 6, 15240–15246. [Google Scholar] [CrossRef]
- Magazù, S.; Migliardo, F.; Ramirez-Cuesta, A. Inelastic neutron scattering study on bioprotectant systems. J. R. Soc. Interface 2005, 2, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Palchetti, S.; Digiacomo, L.; Pozzi, D.; Peruzzi, G.; Micarelli, E.; Mahmoudi, M.; Caracciolo, G. Nanoparticles-cell association predicted by protein corona fingerprints. Nanoscale 2016, 8, 12755–12763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, S.; Das, K.; Mehan, S.; Aswal, V.; Kohlbrecher, J. Structure and interaction among protein and nanoparticle mixture in solution: Effect of temperature. Chem. Phys. Lett. 2015, 641, 68–73. [Google Scholar] [CrossRef]
- Treuel, L.; Brandholt, S.; Maffre, P.; Wiegele, S.; Shang, L.; Nienhaus, G.U. Impact of Protein Modification on the Protein Corona on Nanoparticles and Nanoparticle–Cell Interactions. ACS Nano 2014, 8, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Kopac, T. Protein corona, understanding the nanoparticle–protein interactions and future perspectives: A critical review. Int. J. Biol. Macromol. 2021, 169, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Peigneux, A.; Glitscher, E.A.; Charbaji, R.; Weise, C.; Wedepohl, S.; Calderón, M.; Jimenez-Lopez, C.; Hedtrich, S. Protein corona formation and its influence on biomimetic magnetite nanoparticles. J. Mater. Chem. B 2020, 8, 4870–4882. [Google Scholar] [CrossRef] [PubMed]
- Siddhanta, S.; Barman, I.; Narayana, C. Revealing the trehalose mediated inhibition of protein aggregation through lysozyme–silver nanoparticle interaction. Soft Matter 2015, 11, 7241–7249. [Google Scholar] [CrossRef]
- Aghili, Z.; Taheri, S.; Zeinabad, H.A.; Pishkar, L.; Saboury, A.A.; Rahimi, A.; Falahati, M. Investigating the Interaction of Fe Nanoparticles with Lysozyme by Biophysical and Molecular Docking Studies. PLoS ONE 2016, 11, e0164878. [Google Scholar] [CrossRef]
- Mandal, S.; Debnath, K.; Jana, N.R.; Jana, N.R. Trehalose-Functionalized Gold Nanoparticle for Inhibiting Intracellular Protein Aggregation. Langmuir 2017, 33, 13996–14003. [Google Scholar] [CrossRef]
- Alkudaisi, N.; Russell, B.A.; Jachimska, B.; Birch, D.J.S.; Chen, Y. Detecting lysozyme unfolding via the fluorescence of lysozyme encapsulated gold nanoclusters. J. Mater. Chem. B 2019, 7, 1167–1175. [Google Scholar] [CrossRef]
- Karmakar, S.; Sarkar, N.; Pandey, L.M. Proline functionalized gold nanoparticles modulates lysozyme fibrillation. Colloids Surf. B Biointerfaces 2018, 174, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Udayan, S.; Bindiya, E.; Bhat, S.G.; Nampoori, V.; Kailasnath, M. Optical characterization and tunable antibacterial properties of gold nanoparticles with common proteins. Anal. Biochem. 2020, 612, 113975. [Google Scholar] [CrossRef] [PubMed]
- Tomašovičová, N.; Hu, P.-S.; Zeng, C.-L.; Majorošová, J.; Zakutanská, K.; Kopčanský, P. Dual Size-Dependent Effect of Fe3O4 Magnetic Nanoparticles Upon Interaction with Lysozyme Amyloid Fibrils: Disintegration and Adsorption. Nanomaterials 2018, 9, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| NPs | Size (nm) | Zeta Potential (mV) |
|---|---|---|
| Fe3O4 | 41 | −32.6 |
| Fe3O4@Tre | 32 | −36.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lajmorak, A.; Seyyed Ebrahimi, S.A.; Yazdian, F.; Lalegani, Z.; Hamawandi, B. The Effect of Trehalose Coating for Magnetite Nanoparticles on Stability of Egg White Lysozyme. Int. J. Mol. Sci. 2022, 23, 9657. https://doi.org/10.3390/ijms23179657
Lajmorak A, Seyyed Ebrahimi SA, Yazdian F, Lalegani Z, Hamawandi B. The Effect of Trehalose Coating for Magnetite Nanoparticles on Stability of Egg White Lysozyme. International Journal of Molecular Sciences. 2022; 23(17):9657. https://doi.org/10.3390/ijms23179657
Chicago/Turabian StyleLajmorak, Asma, Seyyed Ali Seyyed Ebrahimi, Fatemeh Yazdian, Zahra Lalegani, and Bejan Hamawandi. 2022. "The Effect of Trehalose Coating for Magnetite Nanoparticles on Stability of Egg White Lysozyme" International Journal of Molecular Sciences 23, no. 17: 9657. https://doi.org/10.3390/ijms23179657
APA StyleLajmorak, A., Seyyed Ebrahimi, S. A., Yazdian, F., Lalegani, Z., & Hamawandi, B. (2022). The Effect of Trehalose Coating for Magnetite Nanoparticles on Stability of Egg White Lysozyme. International Journal of Molecular Sciences, 23(17), 9657. https://doi.org/10.3390/ijms23179657







