Fluorescence Quenching of Tyrosine-Ag Nanoclusters by Metal Ions: Analytical and Physicochemical Assessment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Synthesis Protocol of the Blue-Emitting Tyr-AgNCs
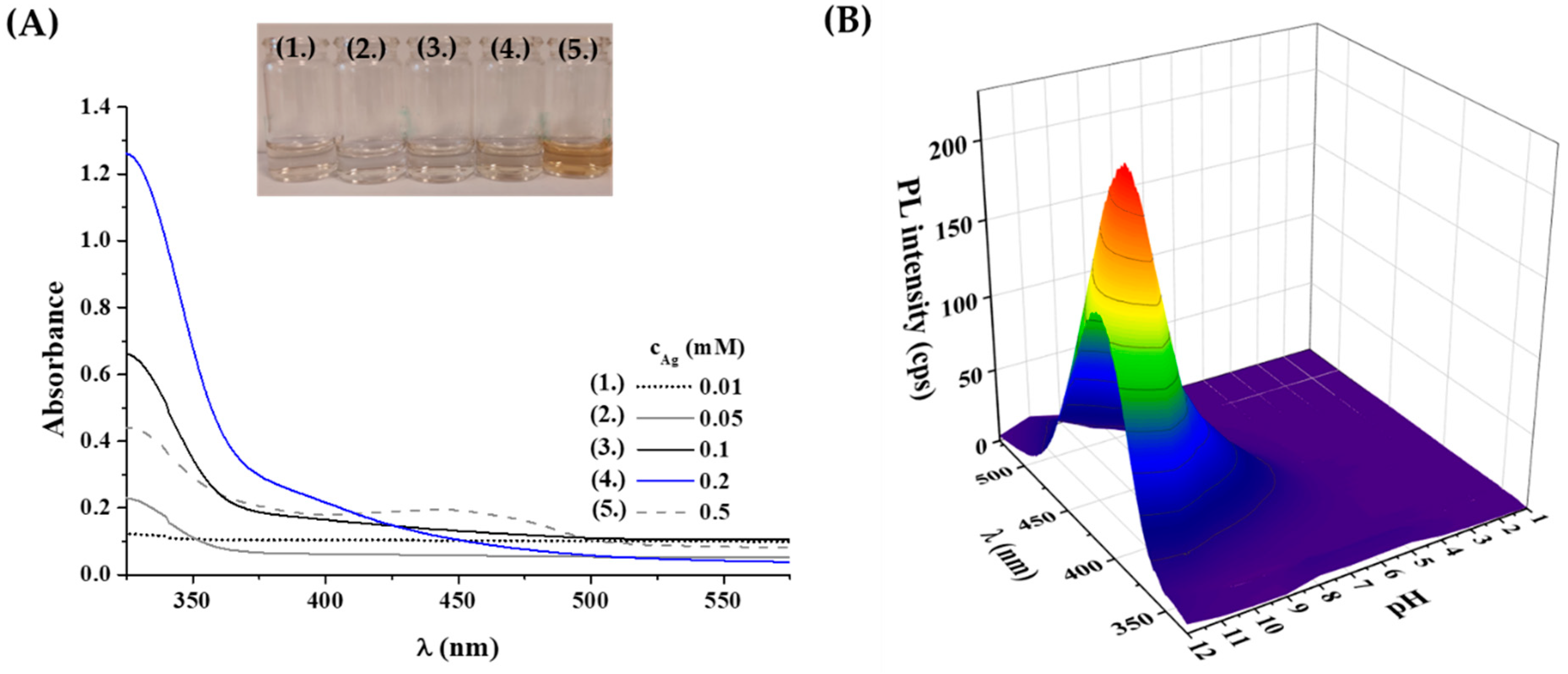
2.2. Optical and Structural Analysis of Tyr-AgNCs
2.3. Interaction between the Blue-Emitting NCs and Different Metal Ions
2.3.1. Analytical Evaluation of the Data
2.3.2. Physicochemical Interpretation of Interactions
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Blue-Emitting Tyr-AgNCs
3.3. Characterization Methods
3.4. Measurement of the Interactions between Metal Ions and Tyr-AgNCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boken, J.; Khurana, P.; Thatai, S.; Kumar, D.; Prasad, S. Plasmonic nanoparticles and their analytical applications: A review. Appl. Spectrosc. Rev. 2017, 52, 774–820. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Taylor, M.G.; Mascareno, A.; Mpourmpakis, G. Size-, Shape-, and Composition-Dependent Model for Metal Nanoparticle Stability Prediction. Nano Lett. 2018, 18, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Vigderman, L.; Khanal, B.P.; Zubarev, E.R. Functional gold nanorods: Synthesis, self-assembly, and sensing applications. Adv. Mater. 2012, 24, 4811–4841. [Google Scholar] [CrossRef] [PubMed]
- Suman, T.Y.; Radhika Rajasree, S.R.; Ramkumar, R.; Rajthilak, C.; Perumal, P. The Green synthesis of gold nanoparticles using an aqueous root extract of Morinda citrifolia L. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2014, 118, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhang, Z.; Luo, C.; Qiao, X. One-Pot Green Synthesis of Ag-Decorated SnO2 Microsphere: An Efficient and Reusable Catalyst for Reduction of 4-Nitrophenol. Nanoscale Res. Lett. 2017, 12, 435. [Google Scholar] [CrossRef]
- Nie, F.; Ga, L.; Ai, J. One-Pot Synthesis of Nucleoside-Templated Fluorescent Silver Nanoparticles and Gold Nanoparticles. ACS Omega 2019, 4, 7643–7649. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Gold nanoparticles and gold nanoparticle-conjugates for delivery of therapeutic molecules. Progress and challenges. J. Mater. Chem. B 2014, 2, 4204–4220. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.P.; Wu, T.H.; Yang, C.H.; Lin, C.W.; Chen, C.Y. Gold nanoparticle-based colorimetric strategies for chemical and biological sensing applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, H.; Zhang, S.; Xu, J. The synthesis of metal nanoclusters and their applications in bio-sensing and imaging. Methods Appl. Fluoresc. 2020, 8, 012001. [Google Scholar] [CrossRef] [PubMed]
- Ungor, D.; Dékány, I.; Csapó, E. Reduction of Tetrachloroaurate(III) Ions With Bioligands: Role of the Thiol and Amine Functional Groups on the Structure and Optical Features of Gold Nanohybrid Systems. Nanomaterials 2019, 9, 1229. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Sun, L.; Shen, T.; Gu, X.; Zhang, S.; Liu, J. Synthesis of fluorescent gold nanoclusters directed by bovine serum albumin and application for nitrite detection. J. Fluoresc. 2013, 23, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.; Geckeler, K.E. Synthesis of highly fluorescent gold nanoclusters using egg white proteins. Colloids Surf. B Biointerfaces 2014, 115, 46–50. [Google Scholar] [CrossRef]
- Han, S.; Zhu, S.; Liu, Z.; Hu, L.; Parveen, S.; Xu, G. Oligonucleotide-stabilized fluorescent silver nanoclusters for turn-on detection of melamine. Biosens. Bioelectron. 2012, 36, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.I.; Choi, S.; Hsiang, J.C.; Antoku, Y.; Vosch, T.; Bongiorno, A.; Tzeng, Y.L.; Dickson, R.M. Oligonucleotide-stabilized Ag nanocluster fluorophores. J. Am. Chem. Soc. 2008, 130, 5038–5039. [Google Scholar] [CrossRef]
- Ungor, D.; Csapó, E.; Kismárton, B.; Juhász, Á.; Dékány, I. Nucleotide-directed syntheses of gold nanohybrid systems with structure-dependent optical features: Selective fluorescence sensing of Fe3+ ions. Colloids Surf. B Biointerfaces 2017, 155, 135–141. [Google Scholar] [CrossRef]
- Lopez, A.; Liu, J. Light-Activated Metal-Coordinated Supramolecular Complexes with Charge-Directed Self-Assembly. J. Phys. Chem. C 2013, 117, 3653–3661. [Google Scholar] [CrossRef]
- Vanegas, J.P.; Zaballos-García, E.; González-Béjar, M.; Londoño-Larrea, P.; Pérez-Prieto, J. Adenosine monophosphate-capped gold(I) nanoclusters: Synthesis and lanthanide ion-induced enhancement of their luminescence. RSC Adv. 2016, 6, 17678–17682. [Google Scholar] [CrossRef]
- Csapó, E.; Ungor, D.; Juhász, Á.; Tóth, G.K.; Dékány, I. Gold nanohybrid systems with tunable fluorescent feature: Interaction of cysteine and cysteine-containing peptides with gold in two- and three-dimensional systems. Colloids Surf. A Physicochem. Eng. Asp. 2016, 511, 264–271. [Google Scholar] [CrossRef]
- Csapó, E.; Ungor, D.; Kele, Z.; Baranyai, P.; Deák, A.; Juhász, Á.; Janovák, L.; Dékány, I. Influence of pH and aurate/amino acid ratios on the tuneable optical features of gold nanoparticles and nanoclusters. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 601–608. [Google Scholar] [CrossRef]
- Gibson, D.W.; Beer, M.; Barrnett, R.J. Gold (III) Complexes of Adenine Nucleotides. Biochemistry 1971, 10, 3669–3679. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.H.; Smith, W.E. Complexes of L-cysteine and D-penicillamine with gold (I) and (III). Proc. R. Soc. Med. 1977, 70 (Suppl. S3), 41–42. [Google Scholar] [CrossRef]
- Martin, R.B. Nucleoside sites for transition metal ion binding. Acc. Chem. Res. 1985, 18, 32–38. [Google Scholar] [CrossRef]
- Ghose, R. Metal Complexation with Adenine and Thymine. Synth. React. Inorg. Met. Chem. 1992, 22, 379–392. [Google Scholar] [CrossRef]
- Fazary, A.E.; Ju, Y.H.; Rajhi, A.Q.; Alshihri, A.S.; Alfaifi, M.Y.; Alshehri, M.A.; Saleh, K.A.; Elbehairi, S.E.I.; Fawy, K.F.; Abd-Rabboh, H.S.M. Bioactivities of Novel Metal Complexes Involving B Vitamins and Glycine. Open Chem. 2016, 14, 287–298. [Google Scholar] [CrossRef]
- Londoño-Lemos, M.E.; Martínez-Bulit, P.; López-Sandoval, H.; Gracia-Mora, I.; Sánchez-Bartez, F.; Castro-Jiménez, T.; Duarte-Hernández, A.M.; Flores-Parra, A.; Contreras, R.; Barba-Behrens, N. Transition metal coordination compounds of an antiobesity serotoninergic ligand: Spectroscopic characterization and adipogenesis activity. Transit. Met. Chem. 2017, 42, 587–596. [Google Scholar] [CrossRef]
- Esarte Palomero, O.; Cunningham, A.L.; Davies, B.W.; Jones, R.A. Antibacterial thiamine inspired silver (I) and gold (I) N-heterocyclic carbene compounds. Inorg. Chim. Acta 2021, 517, 120152. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Harriman, A.; Simic, M.G. Electron-transfer reactions of tryptophan and tyrosine derivatives. J. Phys. Chem. 1986, 90, 1935–1939. [Google Scholar] [CrossRef]
- Navaratnam, S.; Parsons, B.J. Reduction potential of histidine free radicals: A pulse radiolysis study. J. Chem. Soc.—Faraday Trans. 1998, 94, 2577–2581. [Google Scholar] [CrossRef]
- Yang, X.; Luo, Y.; Zhuo, Y.; Feng, Y.; Zhu, S. Novel synthesis of gold nanoclusters templated with l-tyrosine for selective analyzing tyrosinase. Anal. Chim. Acta 2014, 840, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Qi, L.; Qiao, J.; Ma, H. One-pot synthesis of tyrosine-stabilized fluorescent gold nanoclusters and their application as turn-on sensors for Al3+ ions and turn-off sensors for Fe3+ ions. Anal. Methods 2014, 6, 6445–6451. [Google Scholar] [CrossRef]
- Bai, Y.; Shu, T.; Su, L.; Zhang, X. Fluorescent Gold Nanoclusters for Biosensor and Bioimaging Application. Crystals 2020, 10, 357. [Google Scholar] [CrossRef]
- Zhuang, Q.Q.; He, S.B.; Huang, K.Y.; Peng, H.P.; Chen, C.M.; Deng, H.H.; Xia, X.H.; Chen, W.; Hong, G.L. Decisive role of pH in synthesis of high purity fluorescent BSA-Au20 nanoclusters. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2020, 239, 118520. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, J.; Jiang, H.; Wang, X. Gold nanoclusters for theranostic applications. Coord. Chem. Rev. 2021, 431, 213689. [Google Scholar] [CrossRef]
- Ungor, D.; Szilágyi, I.; Csapó, E. Yellow-emitting Au/Ag bimetallic nanoclusters with high photostability for detection of folic acid. J. Mol. Liq. 2021, 338, 116695. [Google Scholar] [CrossRef]
- Ungor, D.; Horváth, K.; Dékány, I.; Csapó, E. Red-emitting gold nanoclusters for rapid fluorescence sensing of tryptophan metabolites. Sens. Actuators B Chem. 2019, 288, 728–733. [Google Scholar] [CrossRef]
- Jouyban, A.; Samadi, A.; Khoubnasabjafari, M. A new “turn-on” fluorescent sensor based on gold quantum dots and silver nanoparticles for lamotrigine detection in plasma. Talanta 2017, 172, 126–132. [Google Scholar] [CrossRef]
- Ju, C.; Gong, X.; Song, W.; Zhao, Y.; Li, R. Turn-on fluorescent probe for Cd2+ detection by gold nanoclusters/graphene oxide nanocomplex. Micro Nano Lett. 2018, 13, 804–806. [Google Scholar] [CrossRef]
- Pan, T.; Zhou, T.; Tu, Y.; Yan, J. Turn-on fluorescence measurement of acid phosphatase activity through an aggregation-induced emission of thiolate-protected gold nanoclusters. Talanta 2021, 227, 122197. [Google Scholar] [CrossRef]
- Warren, J.J.; Winkler, J.R.; Gray, H.B. Redox properties of tyrosine and related molecules. FEBS Lett. 2012, 586, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Csapó, E.; Patakfalvi, R.; Hornok, V.; Tóth, L.T.; Sipos, Á.; Szalai, A.; Csete, M.; Dékány, I. Effect of pH on stability and plasmonic properties of cysteine-functionalized silver nanoparticle dispersion. Colloids Surf. B Biointerfaces 2012, 98, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Canel, E.; Gültepe, A.; Doğan, A.; Kilıç, E. The Determination of Protonation Constants of Some Amino Acids and Their Esters by Potentiometry in Different Media. J. Solut. Chem. 2006, 35, 5–19. [Google Scholar] [CrossRef]
- Roy, A.; Seidel, R.; Kumar, G.; Bradforth, S.E. Exploring Redox Properties of Aromatic Amino Acids in Water: Contrasting Single Photon vs. Resonant Multiphoton Ionization in Aqueous Solutions. J. Phys. Chem. B 2018, 122, 3723–3733. [Google Scholar] [CrossRef]
- An, Y.; Ren, Y.; Bick, M.; Dudek, A.; Hong-Wang Waworuntu, E.; Tang, J.; Chen, J.; Chang, B. Highly fluorescent copper nanoclusters for sensing and bioimaging. Biosens. Bioelectron. 2020, 154, 112078. [Google Scholar] [CrossRef]
- Yeh, H.-C.; Sharma, J.; Han, J.J.; Martinez, J.S.; Werner, J.H. Nanocluster Beacon (NCB): A DNA-Silver Nanocluster Probe that Fluoresces upon Hybridization. Biophys. J. 2011, 100, 484a–485a. [Google Scholar] [CrossRef]
- Shan, P.; Yang, J.; Zang, Z.; Zhao, Q.; Cheng, Y.; Li, L.; Yang, X.; Yu, X.; Lu, Z.; Zhang, X. Effects of silver nanoclusters on the spectral properties for fluorescein isothiocyanate with restrained photobleaching. Appl. Surf. Sci. 2021, 548, 149287. [Google Scholar] [CrossRef]
- Yang, T.Q.; Peng, B.; Shan, B.Q.; Zong, Y.X.; Jiang, J.G.; Wu, P.; Zhang, K. Origin of the photoluminescence of metal nanoclusters: From metal-centered emission to ligand-centered emission. Nanomaterials 2020, 10, 261. [Google Scholar] [CrossRef]
- Ferraria, A.M.; Carapeto, A.P.; Botelho Do Rego, A.M. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Selvakannan, P.; Swami, A.; Srisathiyanarayanan, D.; Shirude, P.S.; Pasricha, R.; Mandale, A.B.; Sastry, M. Synthesis of Aqueous Au Core−Ag Shell Nanoparticles Using Tyrosine as a pH-Dependent Reducing Agent and Assembling Phase-Transferred Silver Nanoparticles at the Air−Water Interface. Langmuir 2004, 20, 7825–7836. [Google Scholar] [CrossRef]
- Bourke, S.L.; Kohn, J. Polymers derived from the amino acid l-tyrosine: Polycarbonates, polyarylates and copolymers with poly(ethylene glycol). Adv. Drug Deliv. Rev. 2003, 55, 447–466. [Google Scholar] [CrossRef]
- Mehta, R.; Kumari, R.; Das, P.; Bhowmick, A.K. Synthesis and characterization of a biocompatible monotyrosine-based polymer and its interaction with DNA. J. Mater. Chem. B 2014, 2, 6236–6248. [Google Scholar] [CrossRef] [PubMed]
- Loock, H.-P.P.; Wentzell, P.D. Detection limits of chemical sensors: Applications and misapplications. Sens. Actuators B Chem. 2012, 173, 157–163. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2010; Volume 1961, ISBN 978-0-387-31278-1 (Print), 978-0-387-46312-4 (Online). [Google Scholar]
- Pettit, L.D.; Swash, J.L.M. Formation constants of complexes of tyrosine. J. Chem. Soc. Dalt. Trans. 1982, 2, 485–486. [Google Scholar] [CrossRef]
- Juhász, Á.; Csapó, E.; Ungor, D.; Tóth, G.K.; Vécsei, L.; Dékány, I. Kinetic and Thermodynamic Evaluation of Kynurenic Acid Binding to GluR1270-300 Polypeptide by Surface Plasmon Resonance Experiments. J. Phys. Chem. B 2016, 120, 7844–7850. [Google Scholar] [CrossRef] [PubMed]
- Al-Omari, S. Separation of static and dynamic thermodynamic parameters for the interaction between pyropheophorbide methyl ester and copper. J. Porphyr. Phthalocyanines 2014, 18, 297–304. [Google Scholar] [CrossRef]





| Type of Metal Ion | Limit of Detection (μM) | Dynamic Range (μM) |
|---|---|---|
| Cu2+ | 2.07 ± 0.18 | 2.5–100 |
| Ni2+ | 49.12 ± 1.3 | 50–100 |
| Fe3+ | 48.28 ± 0.7 | 50–150 |
| Rh3+ | 66.65 ± 0.2 | 70–350 |
| Type of Metal Ion | T (K) | KSV (M−1) | R2 | ΔG i (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (kJ mol−1 K−1) | ΔG ii (kJ mol−1) | ΔCp (kJ mol−1) |
|---|---|---|---|---|---|---|---|---|
| Cu2+ | 298 | 306,100 ± 6180 | 0.995 | −31.31 ± 0.01 | −14.29 ± 8.46 | 0.056 ± 0.025 | −31.09 | −1.35 ± 1.17 |
| 308 | 239,050 ± 4330 | 0.996 | −31.66 ± 0.01 | −31.66 | ||||
| 318 | 203,750 ± 4650 | 0.994 | −32.44 ± 0.02 | −32.22 | ||||
| 328 | 228,260 ± 2830 | 0.998 | −33.64 ± 0.01 | −32.78 | ||||
| Ni2+ | 298 | 4120 ± 850 | 0.920 | −20.62 ± 0.06 | 3.72 ± 1.24 | 0.083 ± 0.030 | −21.01 | −0.07 ± 0.05 |
| 308 | 5260 ± 320 | 0.947 | −21.94 ± 0.02 | −21.84 | ||||
| 318 | 5300 ± 110 | 0.953 | −22.67 ± 0.01 | −22.67 | ||||
| 328 | 5700 ± 370 | 0.943 | −23.58 ± 0.02 | −23.50 | ||||
| Fe3+ | 298 | 147,960 ± 6250 | 0.984 | −29.36 ± 0.01 | −19.36 ± 6.50 | 0.034 ± 0.013 | −29.59 | 1.36 ± 0.90 |
| 308 | 113,610 ± 2570 | 0.995 | −29.93 ± 0.01 | −29.93 | ||||
| 318 | 90,140 ± 1850 | 0.996 | −30.06 ± 0.01 | −30.27 | ||||
| 328 | 49,710 ± 2680 | 0.975 | −29.75 ± 0.02 | −30.62 | ||||
| Rh3+ | 298 | 19,850 ± 950 | 0.982 | −24.52 ± 0.01 | −15.81 ± 8.02 | 0.029 ± 0.014 | −24.52 | 0.39 ± 0.05 |
| 308 | 15,030 ± 690 | 0.984 | −24.79 ± 0.01 | −24.81 | ||||
| 318 | 13,690 ± 520 | 0.989 | −25.01 ± 0.01 | −25.11 | ||||
| 328 | 10,010 ± 310 | 0.993 | −25.16 ± 0.01 | −25.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungor, D.; Bélteki, R.; Horváth, K.; Dömötör, O.; Csapó, E. Fluorescence Quenching of Tyrosine-Ag Nanoclusters by Metal Ions: Analytical and Physicochemical Assessment. Int. J. Mol. Sci. 2022, 23, 9775. https://doi.org/10.3390/ijms23179775
Ungor D, Bélteki R, Horváth K, Dömötör O, Csapó E. Fluorescence Quenching of Tyrosine-Ag Nanoclusters by Metal Ions: Analytical and Physicochemical Assessment. International Journal of Molecular Sciences. 2022; 23(17):9775. https://doi.org/10.3390/ijms23179775
Chicago/Turabian StyleUngor, Ditta, Rita Bélteki, Krisztián Horváth, Orsolya Dömötör, and Edit Csapó. 2022. "Fluorescence Quenching of Tyrosine-Ag Nanoclusters by Metal Ions: Analytical and Physicochemical Assessment" International Journal of Molecular Sciences 23, no. 17: 9775. https://doi.org/10.3390/ijms23179775
APA StyleUngor, D., Bélteki, R., Horváth, K., Dömötör, O., & Csapó, E. (2022). Fluorescence Quenching of Tyrosine-Ag Nanoclusters by Metal Ions: Analytical and Physicochemical Assessment. International Journal of Molecular Sciences, 23(17), 9775. https://doi.org/10.3390/ijms23179775








