1. Introduction
Globally, multiple myeloma (MM) is the most frequent Plasma cell malignancy (PCM), the second most frequent haematological malignancy and represents 1% of all cancers [
1,
2]. Despite important therapeutic advances, that significantly improve life expectancy, most patients relapse, with an estimated mean death rate of 3.4 per 100,000 people per year [
3].
The diagnosis of overt myeloma is based on the presence of at least 10% of clonal bone marrow plasma cell (BMPC) and serum M-protein or urinary monoclonal component, together with the presence of myeloma-related organ dysfunction, including increased calcium level, renal dysfunction, anaemia, and bone lesions (CRAB criteria), or the recently defined as SLIM CRAB criteria, that include clonal BMPC infiltration >60%, involved/uninvolved free light chain (FLC) ratio >100 (involved FLC level must be >100 mg/L) and >1 focal lesion (FL) on Magnetic Resonance Imaging (MRI) larger than 5 mm [
4,
5]. Recommended diagnostic tests [
6] include routine laboratory tests, BM examination and imaging techniques, such as whole-body X-ray (WBXR), MRI, computed tomography (CT) and molecular imaging using Positron emission tomography (PET). In 2014, the International Myeloma Working Group (IMWG) established the diagnosis of bone lesions [
5] according to the presence of >1 lytic lesion in CT or PET/CT, regardless of whether they were detected on WBXR, or >1 unequivocal FL >5 mm in MRI.
PET/CT with radiolabelled glucose analog [
18F]-2′-deoxy-2′-fluoro-D-glucose (FDG), is a reliable non-invasive imaging technique with global sensitivity of 90% and specificity ranging from 70 to 100% for staging MM [
7], and has demonstrated prognostic impact not only on staging, but also on response assessment [
8,
9,
10].
The studies of Bartel et al. [
8] found that the presence of ≥4 FL was associated to shorter overall survival (OS) and progression free survival (PFS). Subsequently, Zamagni et al. [
9] demonstrated poor prognosis associated with ≥4 FL, maximum Standardized Uptake Values (SUV
max) >4.2 and the presence of EMD, the latter with prognostic value on PFS and OS, as recently confirmed by Moreau et al. [
10].
Given that metabolism is considered a hallmark of cancer and glycolysis is one of the main metabolic pathways that are deregulated [
11], new metabolic biomarkers, such as total lesion glycolysis (TLG) and total metabolically active tumor lesion volume (TMTV) have been investigated [
12,
13]. However, the diagnostic value of FDG decreases when only diffuse BM infiltration or only small lytic skeletal lesions (smaller than 10 mm) are present [
14,
15,
16].
Methionine, labeled with C-11 (MET), is an amino acid PET tracer used mainly in oncological diseases of the central nervous system. The rationale for its use in MM is related to the evidence that radiolabeled amino acids show rapid metabolic absorption and incorporation into newly synthesized immunoglobulins. MET appears to be the best radiopharmaceutical currently, apart from FDG, for evaluating MM, with higher sensitivity in detecting focal lesions and extramedullary disease and better correlation with tumor burden [
17,
18]. Preliminary data suggest that it may be superior to
18F-FDG-based imaging, even if the literature is very limited, and, therefore, insufficient for the use of this tracer in clinical practice. [
19,
20,
21,
22]. On the other hand, despite the fact that recent publications show a good correlation with other well-known myeloma prognostic factors [
23], the prognostic value of MET parameters has not yet been clarified.
The purposes of the present study were to compare MET and FDG PET/CT in the evaluation of active disease at staging and re-staging MM, to analyze the correlation between the BM uptake patterns and BM infiltration, and to evaluate the prognostic impact of FDG and MET PET/CT in patients with myeloma.
3. Discussion
FDG PET/CT represents one of the most valuable tools to evaluate disease burden in patients with MM and has demonstrated prognostic impact, not only in staging but also in response assessment [
8,
9,
10]. However, there is evidence that more sensitive and specific tracers are required. This prospective study was conducted to evaluate the outcome of FDG and MET PET/CT in patients with MM. To the best of our knowledge, our study is the first to evaluate the prognostic value of tumor burden biomarkers by MET PET/CT in patients with MM.
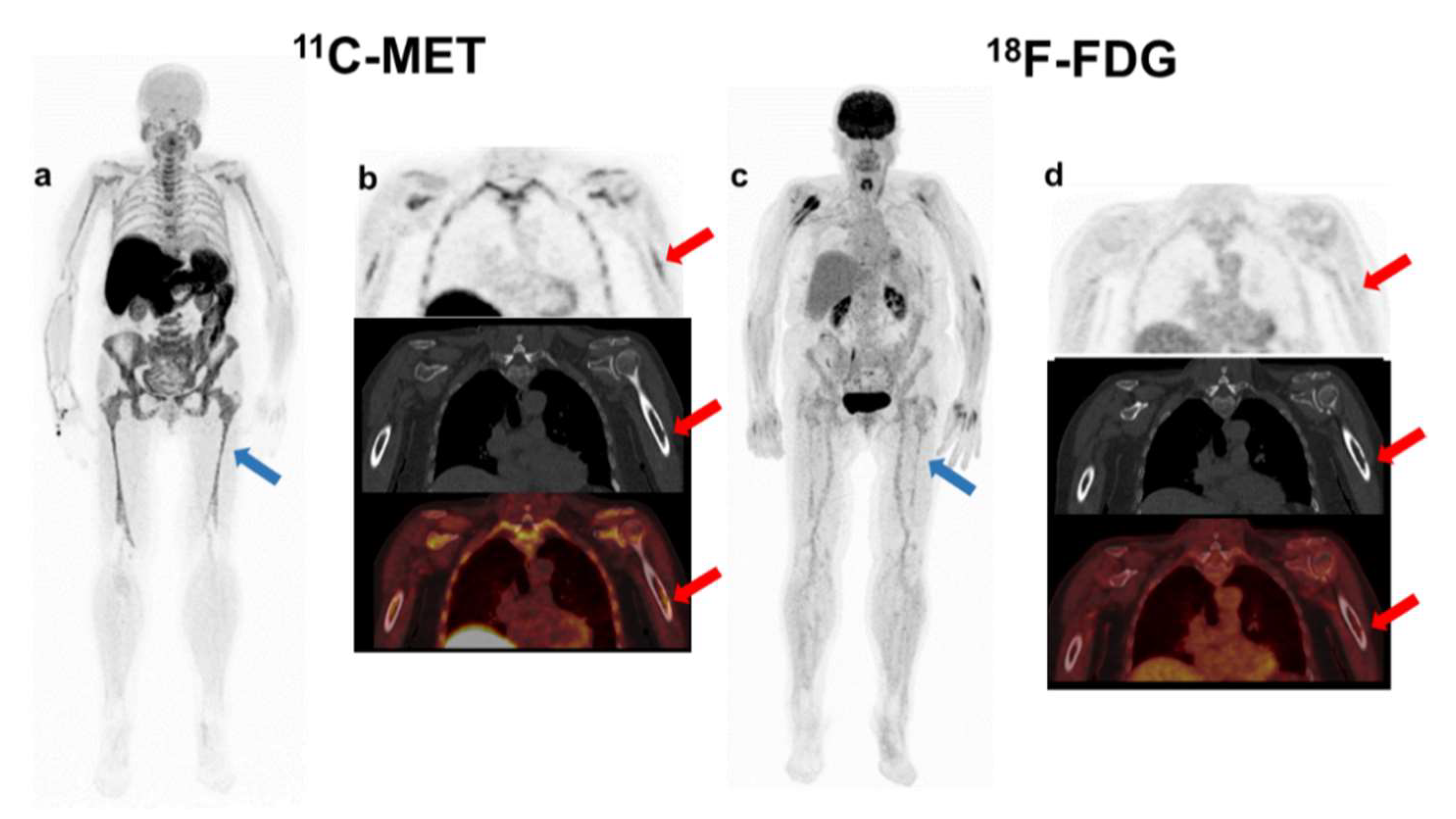
In the present study, we found that MET had higher detectability than
18F-FDG, with negative FDG PET/CT uptake and detectable disease in MET PET/CT in six patients (27%). These results are comparable to previous publications [
19,
20,
21,
22] that reveal a not negligible 10–15% of MM patients without FDG avidity [
24]. Two out of six patients with active disease on MET PET/CT without correlation with FDG PET/CT corresponded to patients with high-risk MM. In fact, these patients with a normal pattern on FDG PET/CT and active disease on MET PET/CT showed worse evolution than those patients that presented negative results on the dual tracer study, progressing at 9 and 42 months after staging, respectively.
In parallel to previous publications that demonstrate a higher sensitivity of MET PET/CT, in the present study, this tracer suggested higher detectability than FDG PET/CT, with a greater number of focal lesions in 63% of patients and more extramedullary lesions detected in 25% of patients, located in lungs, pleura, kidneys, etc. Unfortunately, the biological implications of these discrepancies remain unexplained by the absence of biopsies to analyze their molecular nature. In the literature, only one publication [
22] confirms the higher specificity of MET by biopsy, although the authors recognised the small sample size and the lack of study of the underlying biological factors.
The fact that the infiltration rate of BMPC is positively correlated only with PET biomarkers upon suing MET PET/CT, could indicate that this tracer better reflects total tumor burden, as recently postulated by Lapa et al. [
21]. These authors found that MET was positive in patients with non-secretor MM, and SUV
mean and SUV
peak demonstrated superior grade of correlation with the BM infiltration than FDG, suggesting that the aminoacidic tracer uptake could represent the tumor anabolism and then, better reflect the true total tumor burden.
Regarding the prognostic value of PET/CT, the impact of the number of focal lesions in FDG PET/CT has also been confirmed in this study. Paschali et al. prospectively analyzed FDG PET/CT in patients with newly diagnosed and relapsed/refractory MM [
25]. The presence of ≥10 focal lesions negatively predicted for overall response. Moreover, we showed the prognostic impact of TLG and TMTV FDG, an observation that was coincidental with that reported by McDonald et al. [
13].
For the first time, MET PET/CT biomarkers and their prognostic implications have been assessed, being the first study to date that evaluates the prognostic implications of the volume-based parameters of dual tracers PET/CT in multiple myelomas. Our data indicate that TMTV MET and TLMU may have prognostic implications. In the case of MET PET/CT, the lack of prognostic relevance of the number of focal lesions and SUVmax values could suggest that higher cut-off points should be explored for MET, due to its higher sensitivity. This seems a logical argument, given that the semi-quantitative parameters of MET are usually superior to FDG PET/CT, not only in physiological circumstances, but also when there is tumor infiltration, either focal or diffuse. For this reason, the search for specific thresholds for MET PET/CT is mandatory to confirm whether this tracer has prognostic value.
Regarding the new tumor biomarkers, our study confirms the prognostic value of TMTV and TLG FDG PET/CT, as described by Mc Donald et al. [
13]. As regards MET PET/CT biomarkers with prognostic value, we cannot compare our results with other groups as it is the first time that the prognostic impact of this tracer has been proposed.
The new tumor biomarkers have advantages over the manual determination of SUV, since it is a more automatic process that provides not only the total tumor burden in PET/CT, but also the most important semi-quantitative parameters of the hottest focal lesion as SUV
max, SUV
mean, and SUV
peak. However, certain considerations are necessary before its extended use. As our group has postulated in a previous work, there is still a need to standardize the calculation of TMTV [
23]. However, the aim of the present study was to analyze the prognostic implications of the new tumor biomarkers, focusing especially on MET PET/CT, so it was out of the scope to describe procedural aspects, the object of study of the aforementioned publication.
4. Materials and Methods
4.1. Patients
From August 2015 to May 2019, a total of fifty-two consecutive patients, 44 with MM and 8 with Smoldering MM (SMM), referred for dual tracer FDG and MET PET/CT for staging (n = 26) and restaging (n = 26) were enrolled prospectively.
4.2. Methods FDG and MET- PET/CT Methodology and Evaluation
FDG and MET were synthesized in-house with an 18 MeV Cyclotron (Navarra; Cyclone 18/9, IBA Radio pharma Solutions, Louvain-la-Neuve, Belgium) [
26]. PET/CT was performed on a PET/CT scanner (Siemens Biograph mCT 64, Siemens, Knoxville, TX, USA) within a median interval of 1 day between FDG and MET scans (range, 0–15). Patients fasted at least 4 h before FDG (3 to 5 MBq/kg) and MET injection (6–10 MBq/kg). No adverse effects associated to radiotracer injection were observed. PET/CT scans were acquired after 60 min (FDG) or 20 min (MET), using non-contrast-enhanced CT with 120 kV Care Dose 4D and a quality reference of 80 mAs, including the skull to the proximal thighs and lower limbs. Consecutively, PET emission data were acquired in 3D-mode with an emission time of 2–3 min per bed position in the skull to mid-thighs and 1 min per bed in the lower limbs. For each acquisition, two different reconstructions were performed: a high-quality reconstruction optimized for lesion detection and tumour volume segmentation (PSF + TOF, Gaussian filter 2 mm) and a reconstruction optimized for quantification (OSEM with 2 iterations, 8 subsets and a Gaussian filter of 8 mm for studies before July 2016 and OSEM with 3 iterations, 21 subsets and a Gaussian filter of 5mm from July 2016 onwards, in compliance with EARL).
After anonymization, both FDG and MET PET/CT were visually and semi-quantitatively analyzed by two PET professionals (junior -MML- and senior expert with more than 20 years of experience-MJGV-) on a dedicated workstation with Syngo.via (Siemens) PET software applying the criteria to define FDG PET/CT positivity described by Moreau et al. [
10].
First, a visual inspection of scans was performed. BM involvement was defined as a homogenous FDG uptake in the axial and appendicular skeleton higher than the liver activity or as a heterogeneous uptake regardless of the intensity of uptake. FL was defined as the presence of areas of increased focal tracer uptake within the bones, more intense than the normal background uptake of BM, recorded according to the International Myeloma for PET Use criteria (IMPeTUs) [
27], and EMD was defined as FDG-avid tissue in soft tissue not contiguous to bone on CT examination. After treatment, the normalization of FDG PET/CT uptake was defined as residual uptake greater than or equal to liver activity in FL, BM, and EMD. All other PET/CT results were considered positive, excluding increased FDG uptake related to bone reconstruction or to the use of hematopoietic growth factors.
In MET PET/CT studies, FLs were defined as bone lesions with higher uptake than the surrounding activity in normal tissue or contralateral structures, as previously described [
22,
23], and BM involvement was defined as increased uptake in the BM with/without expansion to the distal part of large bones. Given the absence of standardized criteria for their interpretation, the same variables obtained for FDG PET/CT were collected for further comparisons. Since BM uptake is physiologically superior to
18F-FDG, tumor to background ratios (TBR) were calculated in quantification reconstruction (OSEM 2.8 or OSEM 3.21 depending on the acquisition date), liver uptake being the reference organ, as in IMPeTUs criteria.
For semi-quantitative analysis, tracer uptake was determined by drawing a volume of interest (VOI) within the medullary and extramedullary lesions. In addition, a volume of interest was placed on the iliac crest and lumbar spine (L2), as long as no evident lytic lesions were observed in order to correlate the results to the BM biopsy. Other regions included as reference organs were the right hepatic lobe and the lumen of the descendant aorta, avoiding areas of calcification in the vessel wall. The semi-quantitative parameters recorded were SUVmax and SUVpeak of FL, BM and reference organs. As in the TBR calculations, the reconstructions used for quantification were OSEM 2.8 or OSEM 3.21.
In addition, the FDG and MET PET/CT images were also subjected to a 3-dimensional volume of interest analysis of the axial and appendicular skeleton using “PET/CT Viewer Beth Israel for FIJI”, a free software available for reading and image post-processing. This software automatically delineates areas with high uptake. Then, the operator discards those physiological uptakes (bladder, brain, liver, etc.) and selects the threshold to refine the delimitation of each focus. In order to calculate the tumour volume, apart from the recommended European Association of Nuclear Medicine threshold of 41% of SUV
max [
28], other absolute thresholds (SUV > 2, SUV > 4) and relative thresholds (SUV > 30%, 41%, 60% of SUV
max) were used to segment the images, choosing the threshold that best fitted with the visually identified active lesions among those automatically generated. Parameters explored were MTV, defined as sum of voxels of the active bone lesions based on absolute SUV threshold or relative threshold, and TLG, calculated with the following formula: TLG = ∑ (SUV
mean × MTV). It was decided to use the same cut-off of MTV and TLG in FDG PET/CT as in previous studies [
13] when exploring the prognostic association. For MET PET/CT, the equivalent term for TLG was the total lesion methionine uptake (TLMU), defined as MTV multiplied by mean standardized uptake values (SUV
mean) within the boundary [
29]. The median cut-off values of MTV and TLMU were chosen on MET PET/CT to explore their prognostic relevance since there were no stablished cut-offs in the literature.
4.3. Statistical Analysis
Quantitative values were summarized as mean and standard deviation or median and range if appropriate. Concordance analysis were performed per patient and number of lesions, estimating the kappa concordance coefficient (κ) which was classified as poor agreement (≤0.20), fair agreement (0.21–0.40), moderate agreement (0.41–0.60), substantial agreement (0.61–0.80) and almost perfect agreement (0.81–1.00). Fisher’s exact test or chi square were used for comparisons of frequency data in independent subgroups. Pearson or Spearman correlation coefficients were used for bivariate correlations. Prognostic analyses of PFS were performed using log-rank test and Cox regression analysis. The median (p50) and the 75th percentile (p75) were used as cut-off values. All statistical tests were two-tailed and a p value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 22.0 (Inc. Chicago, IL, USA) and Stata version 14.0 (Stata Corp. 2015. Stata Statistical Software: Release 14. College Station, TX, USA: StataCorp LP).
5. Conclusions
Although this study has the important limitation of the number and heterogeneity of the sample of patients, so the conclusions should be interpreted as preliminary and require confirmation in a larger series of patients, the results postulate for the first time the prognostic value of MET PET/CT, while confirming the prognostic value of the new tumor biomarkers in FDG PET imaging.
MET detected tumor infiltration in 11% of patients with MM and negative FDG PET/CT, detecting a greater number of lesions in the majority of patients.
As MET showed correlation with bone marrow infiltration, it could suggest this tracer better reflects the tumor burden than 18F-FDG, despite the assessment, in cases with slight infiltration, possibly being limited due to the physiological medullary uptake.
According to the prognostic relevance of the FDG PET/CT biomarkers in relapsed patients, in the present work the prognostic value of TLG, TLMU, TMTV on FDG and MET PET, and >10 FL on FDG PET were confirmed.