Leukotriene A4 Hydrolase and Hepatocyte Growth Factor Are Risk Factors of Sudden Cardiac Death Due to First-Ever Myocardial Infarction
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Plasma Proteins and SCD Risk
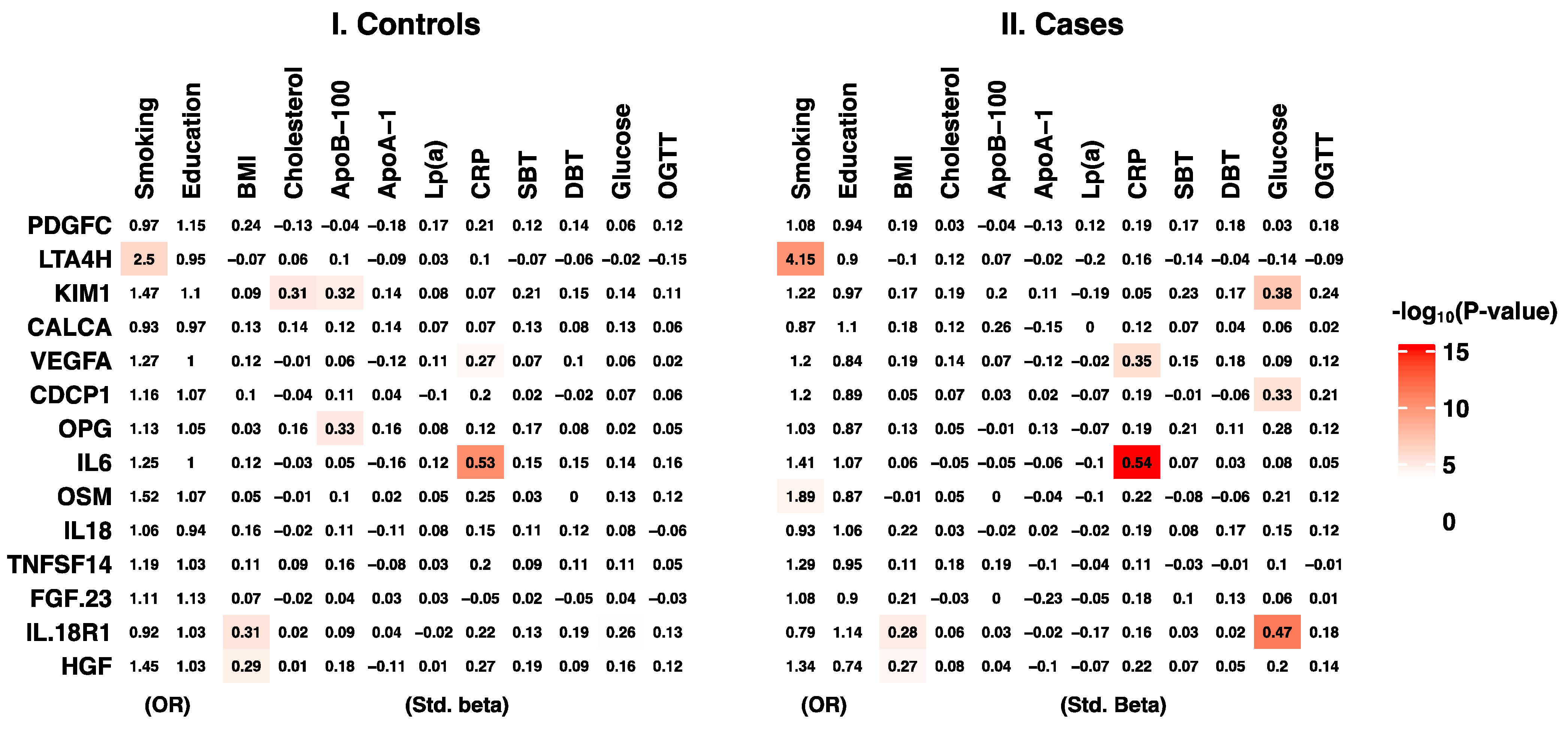
2.3. Plasma Proteins and Traditional Cardiovascular Disease Risk Factors
3. Discussion
4. Materials and Method
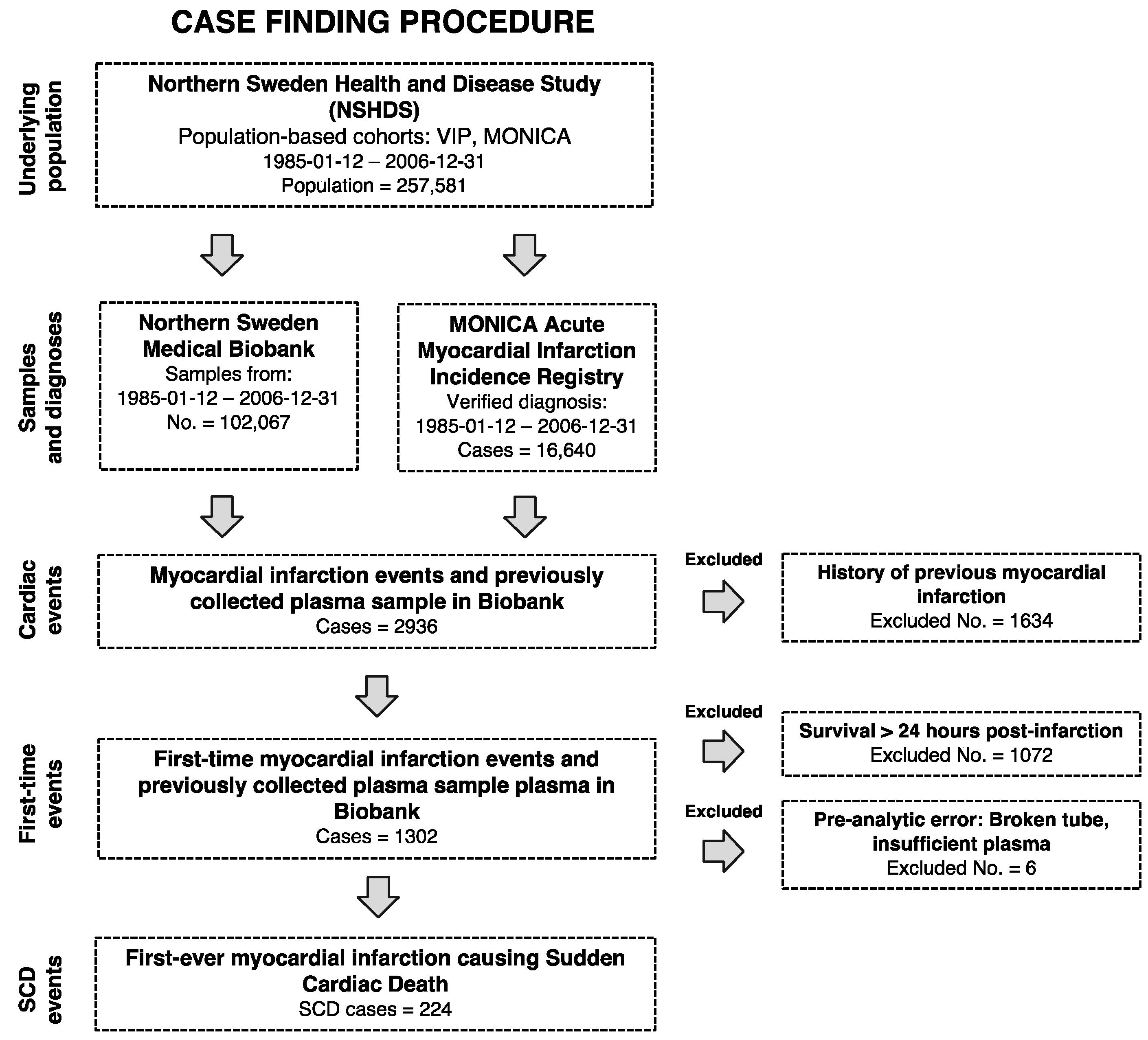
4.1. Study Population
4.2. Baseline Variables
4.3. Blood Sample Collection and Storage
4.4. Proximity Extension Assay
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myerburg, R.J.; Goldberger, J.J. Sudden cardiac arrest risk assessment: Population science and the individual risk mandate. JAMA Cardiol. 2017, 2, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Nichol, G.; Thomas, E.; Callaway, C.W.; Hedges, J.; Powell, J.L.; Aufderheide, T.P.; Rea, T.; Lowe, R.; Brown, T.; Dreyer, J.; et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA 2008, 300, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Myerburg, R.J.; Junttila, M.J. Sudden cardiac death caused by coronary heart disease. Circulation 2012, 125, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death a report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 2018, 72, e91–e220. [Google Scholar] [CrossRef]
- Tomaniak, M.; Katagiri, Y.; Modolo, R.; Silva, R.d.; Khamis, R.Y.; Bourantas, C.V.; Torii, R.; Wentzel, J.J.; Gijsen, F.J.H.; von Soest, G.; et al. Vulnerable plaques and patients: State-of-the-art. Eur. Heart J. 2020, 41, 2997–3004. [Google Scholar] [CrossRef]
- Adabag, A.S.; Peterson, G.; Apple, F.S.; Titus, J.; King, R.; Luepker, R.V. Etiology of sudden death in the community: Results of anatomical, metabolic, and genetic evaluation. Am. Heart J. 2010, 159, 33–39. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Fuster, V. From detecting the vulnerable plaque to managing the vulnerable patient JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 74, 1582–1593. [Google Scholar] [CrossRef]
- Smith, J.G.; Gerszten, R.E. Emerging affinity-based proteomic technologies for large-scale plasma profiling in cardiovascular disease. Circulation 2017, 135, 1651–1664. [Google Scholar] [CrossRef]
- Anderson, N.L.; Anderson, N.G. The human plasma proteome history, character, and diagnostic prospects. Mol. Cell. Proteom. 2002, 1, 845–867. [Google Scholar] [CrossRef]
- Uhlén, M.; Karlsson, M.J.; Hober, A.; Svensson, A.-S.; Scheffel, J.; Kotol, D.; Zhong, W.; Tebani, A.; Strandberg, L.; Edfors, F.; et al. The human secretome. Sci. Signal. 2019, 12, eaaz0274. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, M.; Lin, S.; Jian, R.; Li, X.; Chan, J.; Dong, G.; Fang, H.; Robinson, A.E.; Consortium, G.; et al. A quantitative proteome map of the human body. Cell 2020, 183, 269–283.e19. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Libby, P.; Tabas, I. Inflammation and plaque vulnerability. J. Intern. Med. 2015, 278, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Gabrielsen, A.; Agardh, H.E.; Wan, M.; Wetterholm, A.; Wong, C.-H.; Hedin, U.; Swedenborg, J.; Hansson, G.K.; Samuelsson, B.; et al. Expression of 5-lipoxygenase and leukotriene A4 hydrolase in human atherosclerotic lesions correlates with symptoms of plaque instability. Proc. Natl. Acad. Sci. USA 2006, 103, 8161–8166. [Google Scholar] [CrossRef]
- Snelgrove, R.J.; Jackson, P.L.; Hardison, M.T.; Noerager, B.D.; Kinloch, A.; Gaggar, A.; Shastry, S.; Rowe, S.M.; Shim, Y.M.; Hussell, T.; et al. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science 2010, 330, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Paige, M.; Wang, K.; Burdick, M.; Park, S.; Cha, J.; Jeffery, E.; Sherman, N.; Shim, Y.M. Role of leukotriene A4 hydrolase aminopeptidase in the pathogenesis of emphysema. J. Immunol. 2014, 192, 5059–5068. [Google Scholar] [CrossRef] [PubMed]
- Bielinski, S.J.; Berardi, C.; Decker, P.A.; Larson, N.B.; Bell, E.J.; Pankow, J.S.; Sale, M.M.; Tang, W.; Hanson, N.Q.; Wassel, C.L.; et al. Hepatocyte growth factor demonstrates racial heterogeneity as a biomarker for coronary heart disease. Heart 2017, 103, 1185. [Google Scholar] [CrossRef] [PubMed]
- Lamblin, N.; Susen, S.; Dagorn, J.; Mouquet, F.; Jude, B.; Belle, E.V.; Bauters, C.; de Groote, P. Prognostic significance of circulating levels of angiogenic cytokines in patients with congestive heart failure. Am. Heart J. 2005, 150, 137–143. [Google Scholar] [CrossRef]
- Snelgrove, R.; Kheradmand, F. Leukotriene A4 hydrolase: The Janus enzyme shows its ugly side in smokers. Am. J. Respir. Crit. Care Med. 2014, 190, 5–7. [Google Scholar] [CrossRef]
- Samuelsson, B. Leukotrienes: Mediators of immediate hypersensitivity reactions and inflammation. Science 1983, 220, 568–575. [Google Scholar] [CrossRef]
- Shimizu, T.; Rådmark, O.; Samuelsson, B. Enzyme with dual lipoxygenase activities catalyzes leukotriene A4 synthesis from arachidonic acid. Proc. Natl. Acad. Sci. USA 1984, 81, 689–693. [Google Scholar] [CrossRef]
- Rådmark, O.; Shimizu, T.; Jörnvall, H.; Samuelsson, B. Leukotriene A4 hydrolase in human leukocytes. Purification and properties. J. Biol. Chem. 1984, 259, 12339–12345. [Google Scholar] [CrossRef]
- Fitzpatrick, F.; Haeggström, J.; Granström, E.; Samuelsson, B. Metabolism of leukotriene A4 by an enzyme in blood plasma: A possible leukotactic mechanism. Proc. Natl. Acad. Sci. USA 1983, 80, 5425–5429. [Google Scholar] [CrossRef] [PubMed]
- Corlin, L.; Liu, C.; Lin, H.; Leone, D.; Yang, Q.; Ngo, D.; Levy, D.; Cupples, L.A.; Gerszten, R.E.; Larson, M.G.; et al. Proteomic signatures of lifestyle risk factors for cardiovascular disease: A cross-sectional analysis of the plasma proteome in the framingham heart study. J. Am. Heart Assoc. 2020, 10, e018020. [Google Scholar] [CrossRef] [PubMed]
- Low, C.M.; Akthar, S.; Patel, D.F.; Löser, S.; Wong, C.-T.; Jackson, P.L.; Blalock, J.E.; Hare, S.A.; Lloyd, C.M.; Snelgrove, R.J. The development of novel LTA4H modulators to selectively target LTB4 generation. Sci. Rep. 2017, 7, 44449. [Google Scholar] [CrossRef]
- Boros, P.; Miller, C.M. Hepatocyte growth factor: A multifunctional cytokine. Lancet 1995, 345, 293–295. [Google Scholar] [CrossRef]
- Bottaro, D.P.; Rubin, J.S.; Faletto, D.L.; Chan, A.M.; Kmiecik, T.E.; Woude, G.F.V.; Aaronson, S.A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 1991, 251, 802–804. [Google Scholar] [CrossRef]
- Folkersen, L.; Gustafsson, S.; Wang, Q.; Hansen, D.H.; Hedman, Å.K.; Schork, A.; Page, K.; Zhernakova, D.V.; Wu, Y.; Peters, J.; et al. Genomic and drug target evaluation of 90 cardiovascular proteins in 30,931 individuals. Nat. Metab. 2020, 2, 1135–1148. [Google Scholar] [CrossRef]
- Andersson, J.; Wennberg, P.; Lundblad, D.; Escher, S.A.; Jansson, J.-H. Diabetes mellitus, high BMI and low education level predict sudden cardiac death within 24 hours of incident myocardial infarction. Eur. J. Prev. Cardiol. 2016, 23, 1814–1820. [Google Scholar] [CrossRef]
- Panduru, N.M.; Sandholm, N.; Forsblom, C.; Saraheimo, M.; Dahlström, E.H.; Thorn, L.M.; Gordin, D.; Tolonen, N.; Wadén, J.; Harjutsalo, V.; et al. Kidney injury molecule-1 and the loss of kidney function in diabetic nephropathy: A likely causal link in patients with type 1 diabetes. Diabetes Care 2015, 38, 1130–1137. [Google Scholar] [CrossRef]
- Schulz, C.-A.; Engström, G.; Nilsson, J.; Almgren, P.; Petkovic, M.; Christensson, A.; Nilsson, P.M.; Melander, O.; Orho-Melander, M. Plasma kidney injury molecule-1 (p-KIM-1) levels and deterioration of kidney function over 16 years. Nephrol. Dial. Transplant. 2020, 35, 265–273. [Google Scholar] [CrossRef]
- Vignoli, A.; Tenori, L.; Giusti, B.; Valente, S.; Carrabba, N.; Balzi, D.; Barchielli, A.; Marchionni, N.; Gensini, G.F.; Marcucci, R.; et al. Differential network analysis reveals metabolic determinants associated with mortality in acute myocardial infarction patients and suggests potential mechanisms underlying different clinical scores used to predict death. J. Proteome Res. 2020, 19, 949–961. [Google Scholar] [CrossRef]
- Messner, T.; Lundberg, V. Trends in sudden cardiac death in the northern Sweden MONICA area 1985–99. J. Intern. Med. 2003, 253, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Wellens, H.J.J.; Schwartz, P.J.; Lindemans, F.W.; Buxton, A.E.; Goldberger, J.J.; Hohnloser, S.H.; Huikuri, H.V.; Kääb, S.; Rovere, M.T.L.; Malik, M.; et al. Risk stratification for sudden cardiac death: Current status and challenges for the future. Eur. Heart J. 2014, 35, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Patton, K.K.; Sotoodehnia, N.; DeFilippi, C.; Siscovick, D.S.; Gottdiener, J.S.; Kronmal, R.A. N-terminal pro-B-type natriuretic peptide is associated with sudden cardiac death risk: The cardiovascular health study. Heart Rhythm 2011, 8, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.A.; Gottdiener, J.S.; Bartz, T.M.; Sotoodehnia, N.; deFilippi, C.; Dickfeld, T.; Deo, R.; Siscovick, D.; Stein, P.K.; Lloyd-Jones, D. Cardiomyocyte injury assessed by a highly sensitive troponin assay and sudden cardiac death in the community: The cardiovascular health study. J. Am. Coll. Cardiol. 2013, 62, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Svensson, D.; Rentoft, M.; Dahlin, A.M.; Lundholm, E.; Olason, P.I.; Sjödin, A.; Nylander, C.; Melin, B.S.; Trygg, J.; Johansson, E. A whole-genome sequenced control population in northern Sweden reveals subregional genetic differences. PLoS ONE 2020, 15, e0237721. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Edfors, F.; Gummesson, A.; Bergström, G.; Fagerberg, L.; Uhlén, M. Next generation plasma proteome profiling to monitor health and disease. Nat. Commun. 2021, 12, 2493. [Google Scholar] [CrossRef]
- Tebani, A.; Gummesson, A.; Zhong, W.; Koistinen, I.S.; Lakshmikanth, T.; Olsson, L.M.; Boulund, F.; Neiman, M.; Stenlund, H.; Hellström, C.; et al. Integration of molecular profiles in a longitudinal wellness profiling cohort. Nat. Commun. 2020, 11, 4487. [Google Scholar] [CrossRef]
- Norberg, M.; Wall, S.; Boman, K.; Weinehall, L. The Västerbotten intervention programme: Background, design and implications. Glob. Health Action 2010, 3, 4643. [Google Scholar] [CrossRef]
- Stegmayr, B.; Lundberg, V.; Asplund, K. The events registration and survey procedures in the northern Sweden MONICA Project. Scand. J. Public Health 2003, 31, 9–17. [Google Scholar] [CrossRef]
- Tunstall-Pedoe, H.; Kuulasmaa, K.; Amouyel, P.; Arveiler, D.; Rajakangas, A.M.; Pajak, A. Myocardial infarction and coronary deaths in the World Health Organization MONICA project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation 1994, 90, 583–612. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, M.; Eriksson, A.; Tran, B.; Assarsson, E.; Fredriksson, S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011, 39, e102. [Google Scholar] [CrossRef] [PubMed]
- Van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Tchetgen, E.J.T. A general regression framework for a secondary outcome in case-control studies. Biostatistics 2014, 15, 117–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
| NOBS | Cases | Nobs | Cases | Controls | |
|---|---|---|---|---|---|
| Age at blood sampling (years) | 224 | 59.8 (IQR: 50.1–60.1; range: 29.8–69.9) | 224 | 59.9 (IQR: 50.2–60.2; range: 30.2–72.9) | 224 |
| Age at event (years) | 224 | 64.6 (IQR: 58.7–68.7; range: 40.6–74.9) | 224 | ||
| Age at blood sampling (years) | 224 | 59.8 (IQR: 50.1–60.1; range: 29.8–69.9) | 224 | 59.9 (IQR: 50.2–60.2; range: 30.2–72.9) | 224 |
| Sex | 224 | 224 | 224 | ||
| Male | 178 (79.5%) | 178 (79.5%) | |||
| Female | 46 (20.5%) | 46 (20.5%) | |||
| Serum total cholesterol (mmol/L) | 216 | 6.43 ± 1.32 | 214 | 6.09 ± 1.14 | 216 |
| Apolipoprotein B-100 (g/L) | 170 | 1.32 ± 0.292 | 173 | 1.17 ± 0.254 | 170 |
| Apolipoprotein A-1 (g/L) | 169 | 1.36 ± 0.211 | 173 | 1.42 ± 0.255 | 169 |
| C-reactive protein (mg/L) | 168 | 1.7 (IQR: 0.788–3.39; range: 0–61.8) | 169 | 0.9 (IQR: 0.47–2.01; range: 0.03–47.9) | 168 |
| Lipoprotein(a) (μmol/L) | 169 | 17.7 (IQR: 7.1–69.3; range: 0.9–398) | 173 | 13.7 (IQR: 6.7–33.1; range: 1.9–301) | 169 |
| Body mass index (kg/m2) | 223 | 27.1 (IQR: 25.2–29.7; range: 19.9–44.8) | 219 | 25.6 (IQR: 23.2–27.8; range: 17.9–40.7) | 223 |
| Systolic blood pressure (mmHg) | 220 | 142 ± 20 | 220 | 133 ± 18 | 220 |
| Diastolic blood pressure (mmHg) | 220 | 86.7 ± 10.9 | 220 | 82.3 ± 10.2 | 220 |
| Glucose (mmol/L) | 201 | 5.5 (IQR: 5.05–6.2; range: 3.9–26.9) | 204 | 5.4 (IQR: 5.09–5.9; range: 2.9–12.5) | 201 |
| Current smoker | 218 | 213 | 218 | ||
| Yes | 82 (37.6%) | 50 (23.5%) | |||
| No | 136 (62.4%) | 163 (76.5%) | |||
| Diabetes | 223 | 218 | 223 | ||
| Yes | 23 (10.3%) | 5 (2.3%) | |||
| No | 200 (89.7%) | 213 (97.7%) | |||
| Secondary education | 209 | 212 | 209 | ||
| Yes | 92 (44%) | 125 (59%) | |||
| No | 117 (56%) | 87 (41%) | |||
| Time to death 1 | 224 | 224 | |||
| <1 h | 108 (48.2%) | ||||
| <24 h | 116 (51.8%) | ||||
| Blood pressure lowering | 216 | 204 | 216 | ||
| Yes | 65 (30.1%) | 25 (12.3%) | |||
| No | 151 (69.9%) | 179 (87.7%) | |||
| ASA or nitroglycerin | 216 | 204 | 216 | ||
| Yes | 24 (11.1%) | 10 (4.9%) | |||
| No | 192 (88.9%) | 194 (95.1%) | |||
| Lipid-lowering drug | 177 | 173 | 177 | ||
| Yes | 19 (10.7%) | 1 (0.6%) | |||
| No | 158 (89.3%) | 172 (99.4%) |
| Protein | Gene | Molecular Function 1 | Predicted Location 2 | Enhanced Tissue Expression 3 | OR (95% CI) 4 |
|---|---|---|---|---|---|
| Hepatocyte growth factor | HGF (DFNB39, F-TCF, HGFB, HPTA, SF) | Growth factor, serine protease homolog | Secreted | RNA: Placenta Protein: Tibial and coronary arteries | 2.27 (1.39–3.71) |
| Leukotriene A4 hydrolase | LTA4H | Hydrolase, metalloprotease, protease | Intracellular | RNA: Low tissue specificity Protein: Lung | 1.67 (1.09–2.55) |
| Kidney injury molecule 1 (Hepatitis A virus cellular receptor 1) | KIM1 (CD365, HAVCR, HAVCR1, HAVCR-1, TIM-1, TIM1, TIMD1) | Host cell receptor for virus entry, receptor | Intracellular, membrane | RNA: Kidney | 2.11 (1.35–3.32) |
| Osteoprotegerin (TNF receptor superfamily member 11b) | OPG (OCIF, TR1, TNFRSF11B) | Receptor | Secreted | RNA: Thyroid, kidney Protein: Aorta, tibial and coronary arteries | 1.76 (1.17–2.66) |
| Fibroblast growth factor 23 | FGF23 | Growth factor | Secreted | RNA: Blood, heart muscle, liver, urinary bladder | 1.69 (1.07–2.65) |
| Oncostatin M | OSM (MGC20461) | Cytokine, mitogen | Intracellular, secreted | RNA: Blood, bone marrow | 1.57 (1.08–2.27) |
| CUB domain-containing protein 1 | CDCP1 (CD318, SIMA135) | Intracellular, membrane | RNA: Low tissue specificity | 1.56 (1.05–2.31) | |
| Platelet-derived growth factor C | PDGFC (fallotein, SCDGF) | Developmental protein, growth factor, mitogen | Secreted | RNA: Low tissue specificity Protein: Tibial artery and aorta | 1.66 (1.06–2.59) |
| Interleukin 18 | IL18 (IGIF, IL-18, IL-1g, IL1F4) | Cytokine | Intracellular, secreted | RNA: Skin, esophagus Protein: Skin, esophagus | 1.57 (1.05–2.35) |
| Vascular endothelial growth factor A | VEGFA (VEGF, VEGF-A, VPF) | Developmental protein, growth factor, heparin-binding, mitogen | Intracellular, secreted | RNA: Low tissue specificity | 1.66 (1.11–2.5) |
| Calcitonin-related polypeptide alpha | CALCA (CALC1) | Hormone | Secreted | RNA: Thyroid, parathyroid | 1.59 (1.02–2.47) |
| Interleukin 6 | IL6 (BSF2, HGF, HSF, IFNB2, IL-6) | Cytokine, growth factor | Intracellular, secreted | RNA: Adipose, lymphoid tissue | 1.54 (1.03–2.29) |
| TNF superfamily member 14 | TNFSF14 (CD258, HVEM-L, LIGHT, LTg) | Cytokine | Intracellular, membrane, secreted | RNA: Blood, liver | 1.5 (1.03–2.2) |
| Interleukin 18 receptor 1 | IL18R1 (CD218a, IL-1Rrp, IL1RRP) | Hydrolase, receptor | Intracellular, membrane | RNA: Lung | 1.54 (1.07–2.21) |
| Hepatocyte growth factor | HGF (DFNB39, F-TCF, HGFB, HPTA, SF) | Growth factor, serine protease homolog | Secreted | RNA: Placenta Protein: Tibial and coronary arteries | 2.27 (1.39–3.71) |
| Leukotriene A4 hydrolase | LTA4H | Hydrolase, metalloprotease, protease | Intracellular | RNA: Low tissue specificity Protein: Lung | 1.67 (1.09–2.55) |
| Kidney injury molecule 1 (Hepatitis A virus cellular receptor 1) | KIM1 (CD365, HAVCR, HAVCR1, HAVCR-1, TIM-1, TIM1, TIMD1) | Host cell receptor for virus entry, receptor | Intracellular, membrane | RNA: Kidney | 2.11 (1.35–3.32) |
| Osteoprotegerin (TNF receptor superfamily member 11b) | OPG (OCIF, TR1, TNFRSF11B) | Receptor | Secreted | RNA: Thyroid, kidney Protein: Aorta, tibial and coronary arteries | 1.76 (1.17–2.66) |
| Fibroblast growth factor 23 | FGF23 | Growth factor | Secreted | RNA: Blood, heart muscle, liver, urinary bladder | 1.69 (1.07–2.65) |
| Oncostatin M | OSM (MGC20461) | Cytokine, mitogen | Intracellular, secreted | RNA: Blood, bone marrow | 1.57 (1.08–2.27) |
| CUB domain-containing protein 1 | CDCP1 (CD318, SIMA135) | Intracellular, membrane | RNA: Low tissue specificity | 1.56 (1.05–2.31) | |
| Platelet-derived growth factor C | PDGFC (fallotein, SCDGF) | Developmental protein, growth factor, mitogen | Secreted | RNA: Low tissue specificity Protein: Tibial artery and aorta | 1.66 (1.06–2.59) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landfors, F.; Vikström, S.; Wennberg, P.; Jansson, J.-H.; Andersson, J.; Chorell, E. Leukotriene A4 Hydrolase and Hepatocyte Growth Factor Are Risk Factors of Sudden Cardiac Death Due to First-Ever Myocardial Infarction. Int. J. Mol. Sci. 2022, 23, 10251. https://doi.org/10.3390/ijms231810251
Landfors F, Vikström S, Wennberg P, Jansson J-H, Andersson J, Chorell E. Leukotriene A4 Hydrolase and Hepatocyte Growth Factor Are Risk Factors of Sudden Cardiac Death Due to First-Ever Myocardial Infarction. International Journal of Molecular Sciences. 2022; 23(18):10251. https://doi.org/10.3390/ijms231810251
Chicago/Turabian StyleLandfors, Fredrik, Simon Vikström, Patrik Wennberg, Jan-Håkan Jansson, Jonas Andersson, and Elin Chorell. 2022. "Leukotriene A4 Hydrolase and Hepatocyte Growth Factor Are Risk Factors of Sudden Cardiac Death Due to First-Ever Myocardial Infarction" International Journal of Molecular Sciences 23, no. 18: 10251. https://doi.org/10.3390/ijms231810251
APA StyleLandfors, F., Vikström, S., Wennberg, P., Jansson, J.-H., Andersson, J., & Chorell, E. (2022). Leukotriene A4 Hydrolase and Hepatocyte Growth Factor Are Risk Factors of Sudden Cardiac Death Due to First-Ever Myocardial Infarction. International Journal of Molecular Sciences, 23(18), 10251. https://doi.org/10.3390/ijms231810251






