Subfunctionalization of D27 Isomerase Genes in Saffron
Abstract
:1. Introduction
2. Results
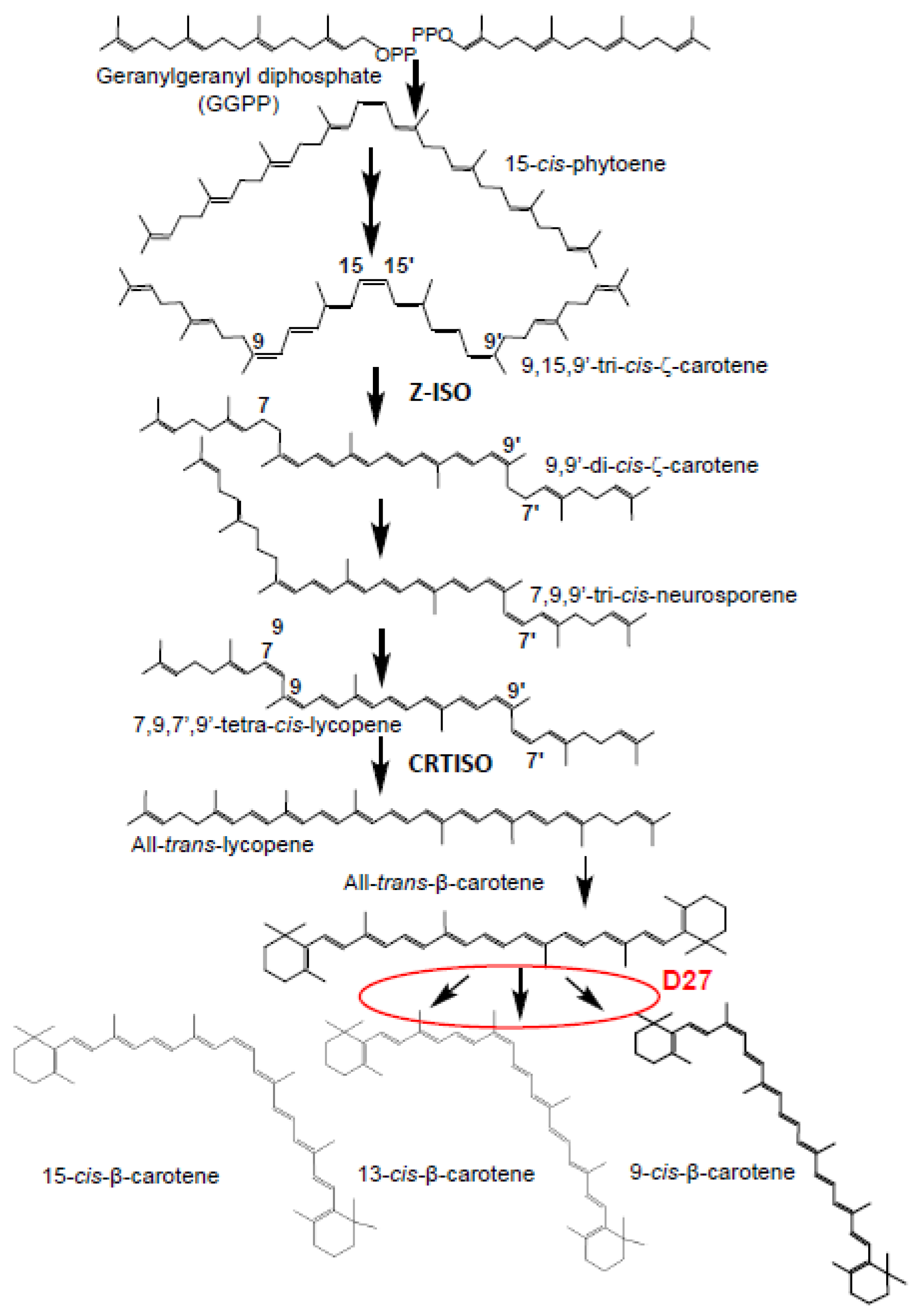
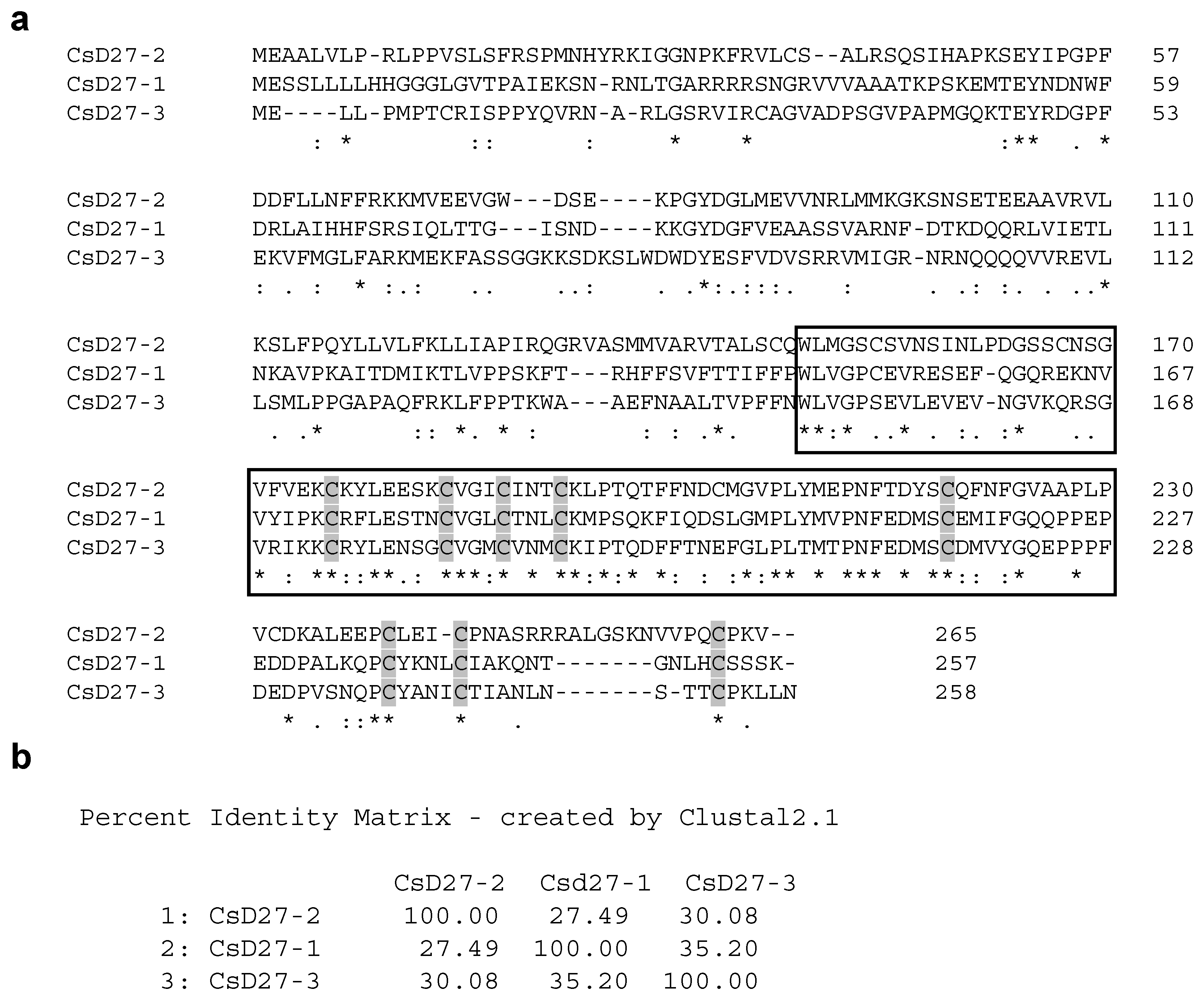
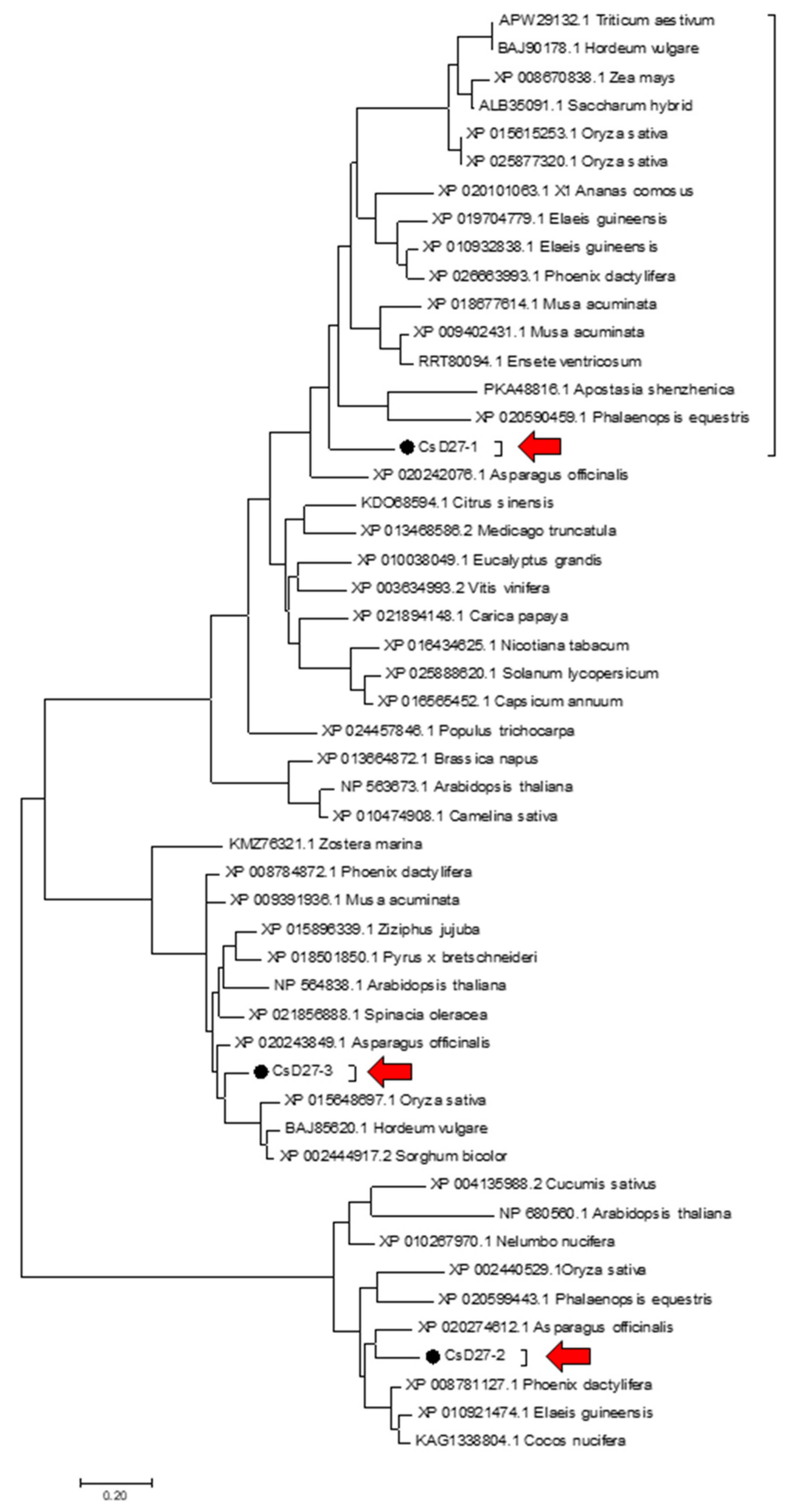
2.1. Isolation and Identification of SL Biosynthetic Gene D27 in Saffron
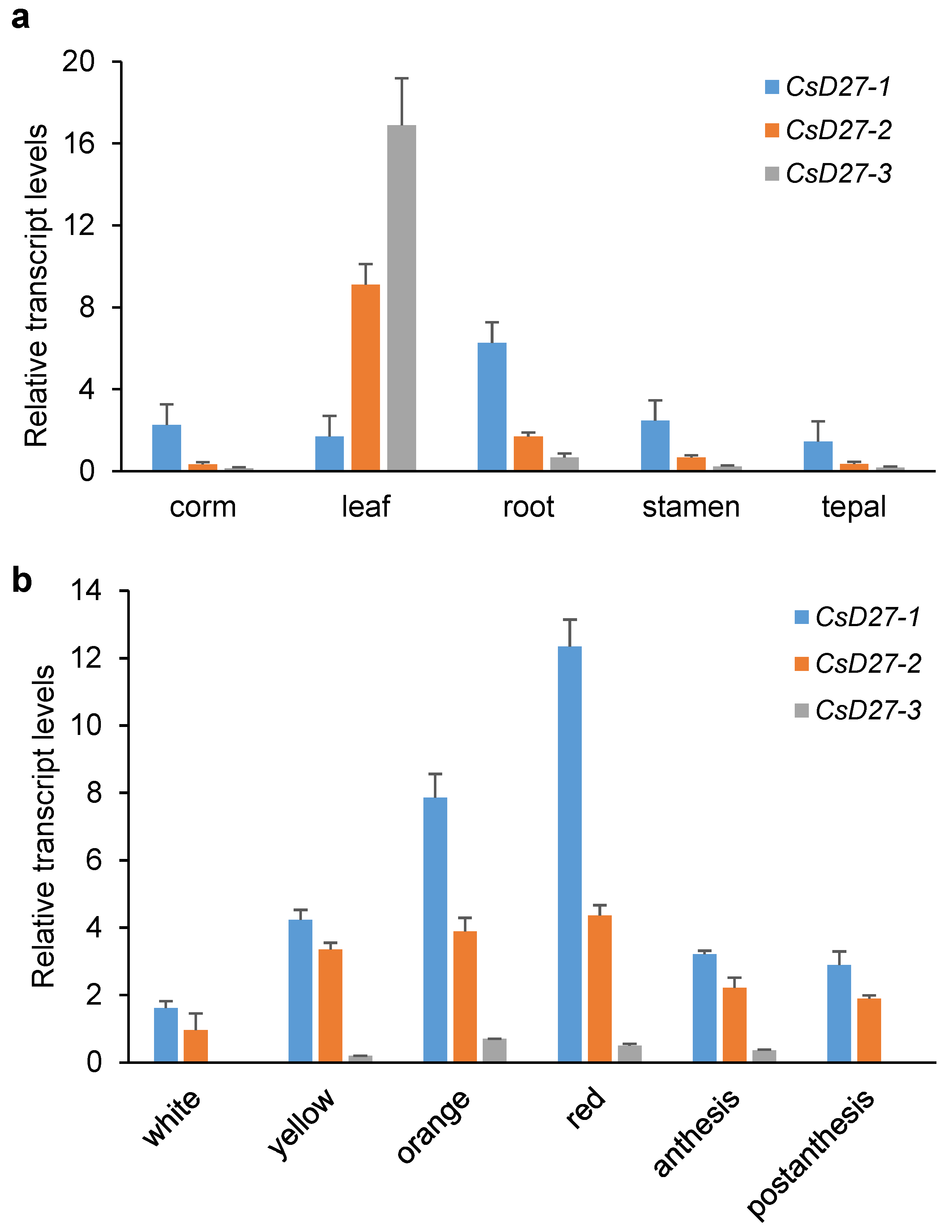
2.2. Expression Levels in Vegetative Tissues and Saffron Stigmas
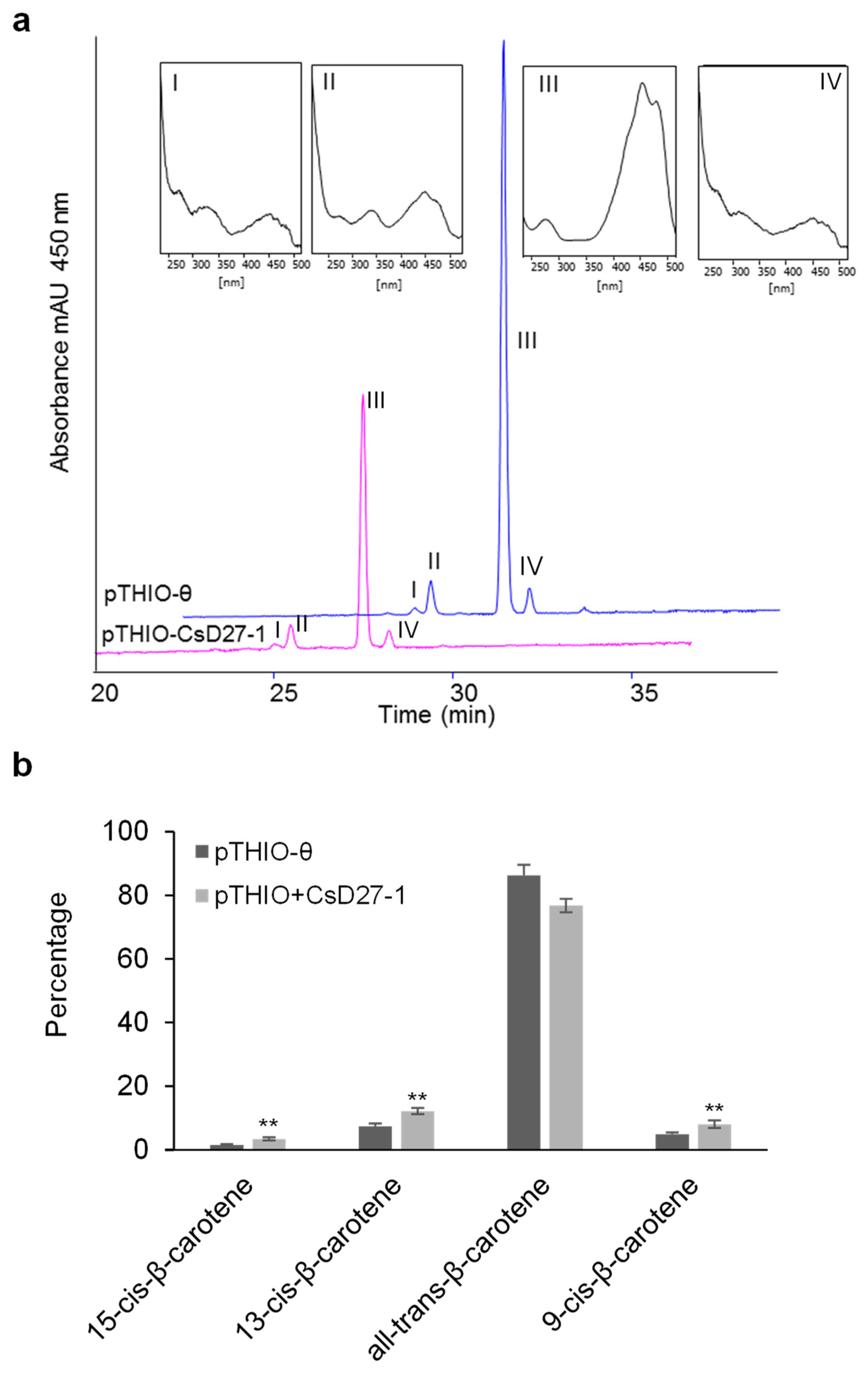
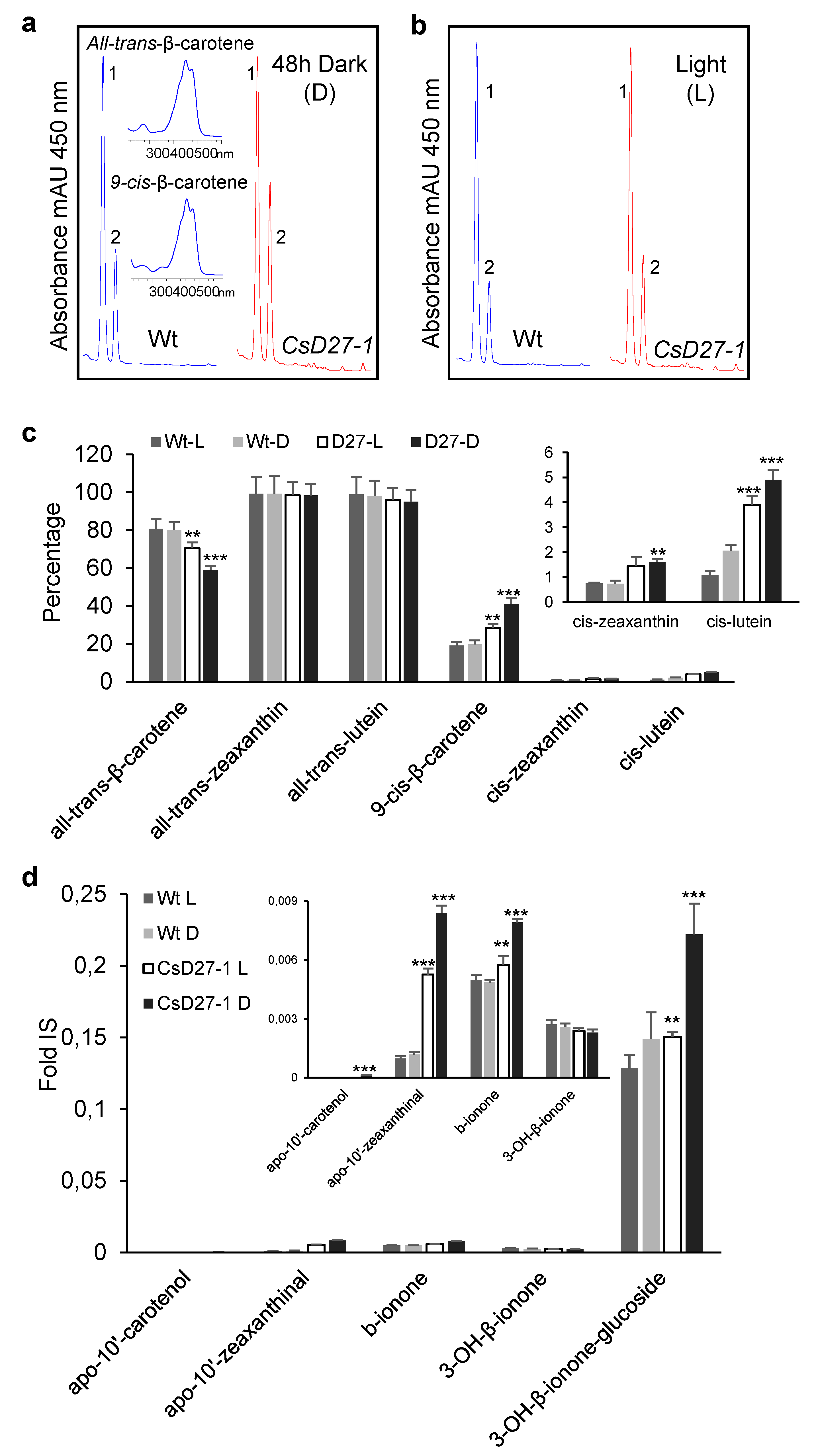
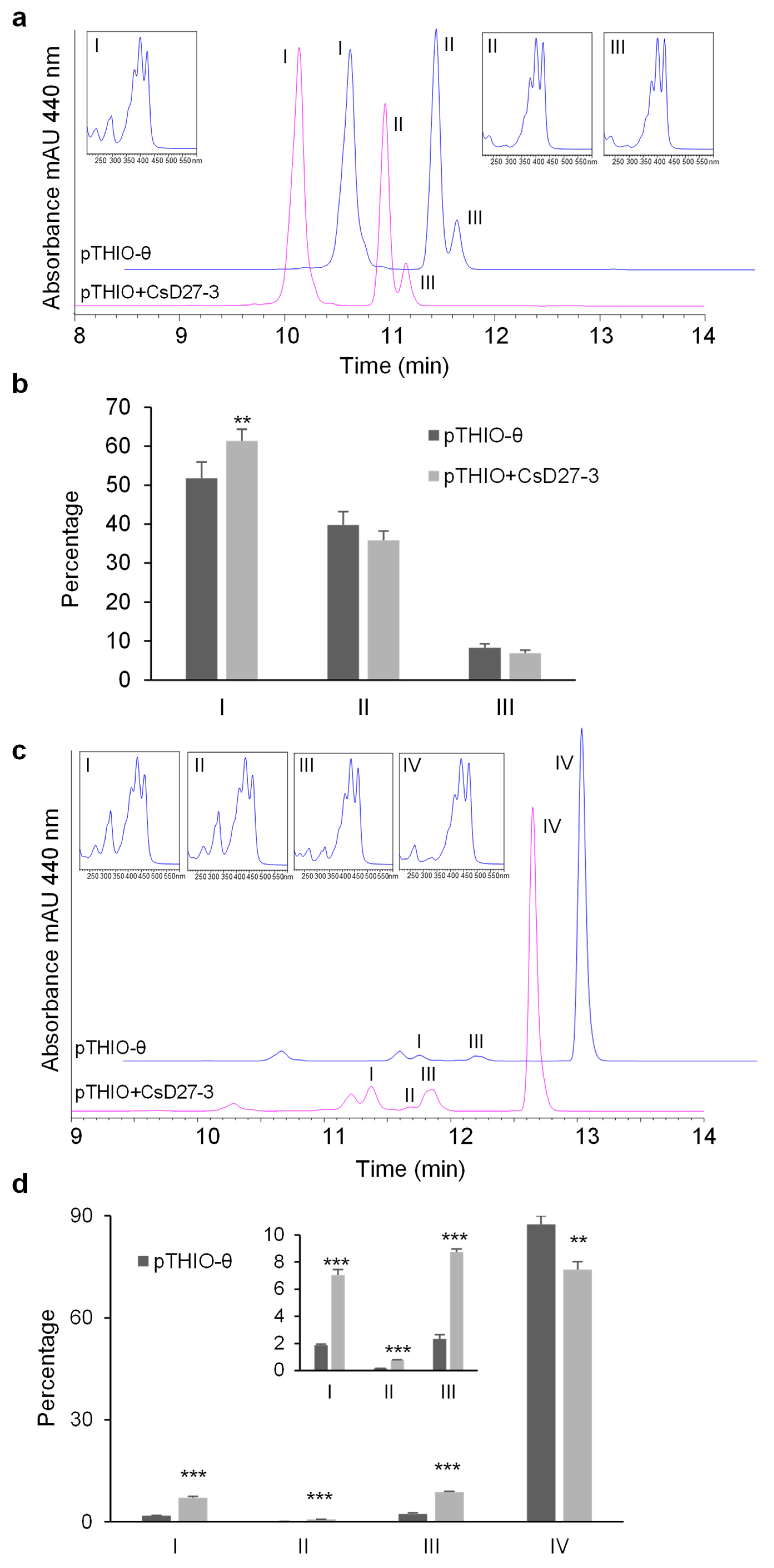
2.3. Activity Assays of CsD27 in E. coli and N. benthamiana
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Isolation of cDNA Sequences Encoding D27 Enzyme in Saffron
4.3. Phylogenetic Analysis
4.4. DNA Sequencing and Analysis of the DNA and Protein Sequences
4.5. Expression Analysis
4.6. Carotenoid Extraction and HPLC Analysis
4.7. D27 Activity in E. coli Cells
4.8. Transient Expression of CsD27-1 in Nicotiana benthamiana
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Melen-dez-Martinez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carote-noids: Metabolism, biotech-nology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of Witchweed (Striga lutea Lour.): Isolation and Properties of a Potent Stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jibran, R.; Wang, Y.; Dong, L.; Flokova, K.; Esfandiari, A.; McLachlan, A.R.G.; Heiser, A.; Sutherland-Smith, A.J.; Brummell, D.A.; et al. Strigolactones regulate sepal senescence in Arabidopsis. J. Exp. Bot. 2021, 72, 5462–5477. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, H.; Kyozuka, J. Spatial regulation of strigolactone function. J. Exp. Bot. 2018, 69, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, Q.; Liu, Y.; Cheng, P.; Cheng, H.; Liu, W.; Xing, X.; Guan, Z.; Fang, W.; Chen, S.; et al. Strigolac-tone represses the synthesis of melatonin, thereby inducing floral transition in Arabidopsis thaliana in an FLC-dependent manner. J. Pineal Res. 2019, 67, e12582. [Google Scholar] [CrossRef]
- Baz, L.; Mori, N.; Mi, J.; Jamil, M.; Kountche, B.A.; Guo, X.; Balakrishna, A.; Jia, K.-P.; Vermathen, M.; Akiyama, K.; et al. 3-Hydroxycarlactone, a Novel Product of the Strigolactone Biosynthesis Core Pathway. Mol. Plant 2018, 11, 1312–1314. [Google Scholar] [CrossRef]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The path from beta-carotene to carlactone, a strigolactone-like plant hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef]
- Rubio-Moraga, A.; Ahrazem, O.; Perez-Clemente, R.M.; Gomez-Cadenas, A.; Yoneyama, K.; Lopez-Raez, J.A.; Molina, R.V.; Go-mez-Gomez, L. Apical dominance in saffron and the involvement of the branching enzymes CCD7 and CCD8 in the control of bud sprouting. BMC Plant Biol. 2014, 14, 171. [Google Scholar] [CrossRef]
- Lin, H.; Wang, R.; Qian, Q.; Yan, M.; Meng, X.; Fu, Z.; Yan, C.; Jiang, B.; Su, Z.; Li, J.; et al. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 2009, 21, 1512–1525. [Google Scholar] [CrossRef]
- Waters, M.T.; Brewer, P.B.; Bussell, J.D.; Smith, S.M.; Beveridge, C.A. The Arabidopsis ortholog of rice DWARF27 acts up-stream of MAX1 in the control of plant development by strigolactones. Plant Physiol. 2012, 159, 1073–1085. [Google Scholar] [CrossRef] [Green Version]
- Klepikova, A.V.; Kasianov, A.S.; Gerasimov, E.S.; Logacheva, M.D.; Penin, A.A. A high resolution map of the Arabidopsis thaliana developmental transcriptome based on RNA-seq profiling. Plant J. 2016, 88, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.-P.; Mi, J.; Ali, S.; Ohyanagi, H.; Moreno, J.C.; Ablazov, A.; Balakrishna, A.; Berqdar, L.; Fiore, A.; Diretto, G.; et al. An alternative, zeaxanthin epoxidase-independent abscisic acid biosynthetic pathway in plants. Mol. Plant 2022, 15, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Abuauf, H.; Haider, I.; Jia, K.-P.; Ablazov, A.; Mi, J.; Blilou, I.; Al-Babili, S. The Arabidopsis DWARF27 gene encodes an all-trans-/9-cis-β-carotene isomerase and is induced by auxin, abscisic acid and phosphate deficiency. Plant Sci. 2018, 277, 33–42. [Google Scholar] [CrossRef] [PubMed]
- López-Ráez, J.A.; Kohlen, W.; Charnikhova, T.; Mulder, P.; Undas, A.K.; Sergeant, M.J.; Verstappen, F.; Bugg, T.D.H.; Thompson, A.J.; Ruyter-Spira, C.; et al. Does abscisic acid affect strigolactone biosynthesis? New Phytol. 2010, 187, 343–354. [Google Scholar] [CrossRef]
- Haider, I.; Andreo-Jimenez, B.; Bruno, M.; Bimbo, A.; Floková, K.; Abuauf, H.; Ntui, V.O.; Guo, X.; Charnikhova, T.; Al-Babili, S.; et al. The interaction of strigolactones with abscisic acid during the drought response in rice. J. Exp. Bot. 2018, 69, 2403–2414. [Google Scholar] [CrossRef]
- Liu, L.; Xie, T.; Peng, P.; Qiu, H.; Zhao, J.; Fang, J.; Patil, S.B.; Wang, Y.; Fang, S.; Chu, J.; et al. Mutations in the MIT3 gene en-coding a caroteniod isomerase lead to increased tiller number in rice. Plant Sci. 2018, 267, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Q.; Yan, J.; Sun, K.; Liang, Y.; Jia, M.; Meng, X.; Fang, S.; Wang, Y.; Jing, Y.; et al. ζ-Carotene Iso-merase Suppresses Tillering in Rice through the Coordinated Biosynthesis of Strigolactone and Abscisic Acid. Mol. Plant 2020, 13, 1784–1801. [Google Scholar] [CrossRef]
- Renau-Morata, B.; Nebauer, S.G.; García-Carpintero, V.; Cañizares, J.; Gómez Minguet, E.; de los Mozos, M.; Mo-lina, R.V. Flower induction and development in saffron: Timing and hormone signalling pathways. Ind. Crops Prod. 2021, 164, 113370. [Google Scholar] [CrossRef]
- Winterhalter, P.; Straubinger, M. Saffron-renewed interest in an ancient spice. Food Rev. Int. 2000, 16, 39–59. [Google Scholar] [CrossRef]
- Abdullaev, F.I.; Espinosa-Aguirre, J.J. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect. Prev. 2004, 28, 426–432. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Sadeghnia, H.R.; Ghaeni, F.A.; Motamedshariaty, V.S.; Mohajeri, S.A. Effects of saffron (Crocus sativus L.) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytother. Res. 2012, 26, 381–386. [Google Scholar] [CrossRef]
- Poma, A.; Fontecchio, G.; Carlucci, G.; Chichiricco, G. Anti-inflammatory properties of drugs from saffron crocus. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2012, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, C.Z.; Wen, X.D.; Shoyama, Y.; Yuan, C.S. Role of saffron and its constituents on cancer chemoprevention. Pharm. Biol. 2013, 51, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Sarwat, M.; Khan, T.H. Mechanism behind the anti-tumour potential of saffron (Crocus sativus L.): The molecular perspective. Crit. Rev. Oncol. Hematol. 2017, 115, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Mzabri, I.; Addi, M.; Berrichi, A. Traditional and Modern Uses of Saffron (Crocus Sativus). Cosmetics 2019, 6, 63. [Google Scholar] [CrossRef]
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Evaluation of corm origin and climatic conditions on saffron (Crocus sativus L.) yield and quality. J. Sci. Food Agric. 2019, 99, 5858–5869. [Google Scholar] [CrossRef]
- Chi, C.; Xu, X.; Wang, M.; Zhang, H.; Fang, P.; Zhou, J.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Strigolactones positively regulate abscisic acid-dependent heat and cold tolerance in tomato. Hortic. Res. 2021, 8, 237. [Google Scholar] [CrossRef]
- Ahrazem, O.; Diretto, G.; Argandona Picazo, J.; Fiore, A.; Rubio-Moraga, A.; Rial, C.; Varela, R.M.; Macias, F.A.; Castillo, R.; Romano, E.; et al. The Specialized Roles in Carotenogenesis and Apocarotenogenesis of the Phytoene Synthase Gene Family in Saffron. Front. Plant Sci. 2019, 10, 249. [Google Scholar] [CrossRef]
- Ahrazem, O.; Rubio-Moraga, A.; Argandona-Picazo, J.; Castillo, R.; Gomez-Gomez, L. Intron retention and rhythmic diel pattern regulation of carotenoid cleavage dioxygenase 2 during crocetin biosynthesis in saffron. Plant Mol. Biol. 2016, 91, 355–374. [Google Scholar] [CrossRef]
- Wu, H.; Li, H.; Chen, H.; Qi, Q.; Ding, Q.; Xue, J.; Ding, J.; Jiang, X.; Hou, X.; Li, Y. Identification and expression analysis of strigolactone biosynthetic and signaling genes reveal strigolactones are involved in fruit development of the woodland strawberry (Fragaria vesca). BMC Plant Biol. 2019, 19, 73. [Google Scholar] [CrossRef] [Green Version]
- Bruno, M.; Al-Babili, S. On the substrate specificity of the rice strigolactone biosynthesis enzyme DWARF27. Planta 2016, 243, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Booker, J.; Auldridge, M.; Wills, S.; McCarty, D.; Klee, H.; Leyser, C. MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr. Biol. 2004, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Hofmann, M.; Vermathen, M.; Alder, A.; Beyer, P.; Al-Babili, S. On the substrate- and stereo-specificity of the plant carotenoid cleavage dioxygenase 7. FEBS Lett. 2014, 588, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Katayama, N.; Hashimoto, H.; Koyama, Y.; Shimamura, T. High-performance liquid chromatography of cis-trans isomers of neurosporene: Discrimination of cis and trans configurations at the end of an open con-jugated chain. J. Chromatogr. 1990, 519, 6. [Google Scholar] [CrossRef]
- Liu, W.; Kohlen, W.; Lillo, A.; Op den Camp, R.; Ivanov, S.; Hartog, M.; Limpens, E.; Jamil, M.; Smaczniak, C.; Kaufmann, K.; et al. Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell 2011, 23, 3853–3865. [Google Scholar] [CrossRef] [PubMed]
- van Zeijl, A.; Liu, W.; Xiao, T.T.; Kohlen, W.; Yang, W.C.; Bisseling, T.; Geurts, R. The strigolactone biosynthesis gene DWARF27 is co-opted in rhizobium symbiosis. BMC Plant Biol. 2015, 15, 260. [Google Scholar] [CrossRef]
- Wen, C.; Xi, L.; Gao, B.; Wang, K.; Lv, S.; Kou, Y.; Ma, N.; Zhao, L. Roles of DgD14 in regulation of shoot branching in chrysanthemum (Dendranthema grandiflorum ‘Jinba’). Plant Physiol. Biochem. 2015, 96, 241–253. [Google Scholar] [CrossRef]
- Wen, C.; Zhao, Q.; Nie, J.; Liu, G.; Shen, L.; Cheng, C.; Xi, L.; Ma, N.; Zhao, L. Physiological controls of chrysanthemum DgD27 gene expression in regulation of shoot branching. Plant Cell Rep. 2016, 35, 1053–1070. [Google Scholar] [CrossRef]
- Delaux, P.-M.; Xie, X.; Timme, R.E.; Puech-Pages, V.; Dunand, C.; Lecompte, E.; Delwiche, C.F.; Yoneyama, K.; Bé-card, G.; Sé-jalon-Delmas, N. Origin of strigolactones in the green lineage. New Phytol. 2012, 195, 857–871. [Google Scholar] [CrossRef]
- Kobae, Y.; Kameoka, H.; Sugimura, Y.; Saito, K.; Ohtomo, R.; Fujiwara, T.; Kyozuka, J. Strigolactone Bio-synthesis Genes of Rice are Required for the Punctual Entry of Arbuscular Mycorrhizal Fungi into the Roots. Plant Cell Physiol. 2018, 59, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Takeda, N.; Kawaguchi, M. Common symbiosis genes CERBERUS and NSP1 provide additional insight into the establishment of arbuscular mycorrhizal and root nodule symbioses in Lotus japonicus. Plant Signal. Behav. 2014, 9, e28544. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Long, Y.; Zhao, Z.; Huang, G.; Huang, K.; Zhang, T.; Jiang, Y.; Yuan, Q.; Pei, X. Isolation and Characterization of a Green-Tissue Promoter from Common Wild Rice (Oryza rufipogon Griff.). Int. J. Mol. Sci. 2018, 19, 2009. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, X.; Chory, J. The Arabidopsis Transcriptome Responds Specifically and Dynamically to High Light Stress. Cell Rep. 2019, 29, 4186–4199.e3. [Google Scholar] [CrossRef]
- Salvi, D.; Bournais, S.; Moyet, L.; Bouchnak, I.; Kuntz, M.; Bruley, C.; Rolland, N. AT_CHLORO: The First Step When Looking for Information About Subplastidial Localization of Proteins. Methods Mol. Biol. 2018, 1829, 395–406. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Hou, X. Similarities and Differences of Photosynthesis Establishment Related mRNAs and Novel lncRNAs in Early Seedlings (Coleoptile/Cotyledon vs. True Leaf) of Rice and Arabidopsis. Front. Genet. 2020, 11, 565006. [Google Scholar] [CrossRef]
- Berger, N.; Vignols, F.; Touraine, B.; Taupin-Broggini, M.; Rofidal, V.; Demolombe, V.; Santoni, V.; Rouhier, N.; Gaymard, F.; Dubos, C. A Global Proteomic Approach Sheds New Light on Potential Iron-Sulfur Client Proteins of the Chloroplastic Mat-uration Factor NFU3. Int. J. Mol. Sci. 2020, 21, 8121. [Google Scholar] [CrossRef]
- Balk, J.; Lobréaux, S. Biogenesis of iron-sulfur proteins in plants. Trends Plant Sci. 2005, 10, 324–331. [Google Scholar] [CrossRef]
- Satyanarayan, M.B.; Zhao, J.; Zhang, J.; Yu, F.; Lu, Y. Functional relationships of three NFU proteins in the biogenesis of chloroplastic iron-sulfur clusters. Plant Direct 2021, 5, e00303. [Google Scholar] [CrossRef]
- Zhang, Y.; van Dijk, A.D.; Scaffidi, A.; Flematti, G.R.; Hofmann, M.; Charnikhova, T.; Verstappen, F.; Hepworth, J.; van der Krol, S.; Leyser, O.; et al. Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat. Chem. Biol. 2014, 10, 1028–1033. [Google Scholar] [CrossRef]
- Harrison, P.J.; Newgas, S.A.; Descombes, F.; Shepherd, S.A.; Thompson, A.J.; Bugg, T.D.H. Biochemical characterization and selective inhibition of β-carotene cis–trans isomerase D27 and carotenoid cleavage dioxygenase CCD8 on the strigolactone biosynthetic pathway. FEBS J. 2015, 282, 3986–4000. [Google Scholar] [CrossRef] [Green Version]
- Przybyla-Toscano, J.; Boussardon, C.; Law, S.R.; Rouhier, N.; Keech, O. Gene atlas of iron-containing proteins in Arabidopsis thaliana. Plant J. 2021, 106, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Avendano-Vazquez, A.O.; Cordoba, E.; Llamas, E.; San Roman, C.; Nisar, N.; De la Torre, S.; Ramos-Vega, M.; Gutierrez-Nava, M.D.; Cazzonelli, C.I.; Pogson, B.J.; et al. An Uncharacterized Apocarotenoid-Derived Signal Generated in zeta-Carotene Desaturase Mutants Regulates Leaf Development and the Expression of Chloroplast and Nuclear Genes in Arabidopsis. Plant Cell 2014, 26, 2524–2537. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sreelakshmi, Y.; Sharma, R. A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods 2015, 11, 5. [Google Scholar] [CrossRef]
- Schaub, P.; Rodriguez-Franco, M.; Cazzonelli, C.I.; Álvarez, D.; Wüst, F.; Welsch, R. Establishment of an Arabidopsis callus system to study the interrelations of biosynthesis, degradation and accumulation of carotenoids. PLoS ONE 2018, 13, e0192158. [Google Scholar] [CrossRef]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid Metabolism in Plants: The Role of Plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.; Pogson, B.J.; Tissue, D.T.; Cazzonelli, C.I. A foliar pigment-based bioassay for interrogating chloroplast signalling revealed that carotenoid isomerisation regulates chlorophyll abundance. Plant Methods 2022, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Alagoz, Y.; Nayak, P.; Dhami, N.; Cazzonelli, C.I. cis-carotene biosynthesis, evolution and regulation in plants: The emergence of novel signaling metabolites. Arch. Biochem. Biophys. 2018, 654, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Cazzonelli, C.I.; Hou, X.; Alagoz, Y.; Rivers, J.; Dhami, N.; Lee, J.; Marri, S.; Pogson, B.J. A cis-carotene de-rived apocarotenoid regulates etioplast and chloroplast development. Elife 2020, 9, e45310. [Google Scholar] [CrossRef] [PubMed]
- Rubio, A.; Rambla, J.L.; Santaella, M.; Gomez, M.D.; Orzaez, D.; Granell, A.; Gomez-Gomez, L. Cytosolic and plastoglo-bule-targeted carotenoid dioxygenases from Crocus sativus are both involved in beta-ionone release. J. Biol. Chem. 2008, 283, 24816–24825. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martí, M.; Diretto, G.; Aragonés, V.; Frusciante, S.; Ahrazem, O.; Gómez-Gómez, L.; Daròs, J.-A. Efficient production of saffron crocins and picrocrocin in Nicotiana benthamiana using a virus-driven system. Metab. Eng. 2020, 61, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.; Fernandez, J.A.; Gomez-Gomez, L. Implications of carotenoid biosynthetic genes in apo-carotenoid for-mation during the stigma development of Crocus sativus and its closer relatives. Plant Physiol. 2005, 139, 674–689. [Google Scholar] [CrossRef] [PubMed]
- Sarrion-Perdigones, A.; Palaci, J.; Granell, A.; Orzaez, D. Design and construction of multigenic con-structs for plant biotechnology using the GoldenBraid cloning strategy. Methods Mol. Biol. 2014, 1116, 133–151. [Google Scholar] [CrossRef]
- Diretto, G.; Ahrazem, O.; Rubio-Moraga, A.; Fiore, A.; Sevi, F.; Argandona, J.; Gomez-Gomez, L. UGT709G1: A novel uridine diphosphate glycosyltransferase involved in the biosynthesis of picrocrocin, the precursor of safranal in saffron (Crocus sativus). New Phytol. 2019, 224, 725–740. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Jiménez, A.J.; Morote, L.; Niza, E.; Mondéjar, M.; Rubio-Moraga, Á.; Diretto, G.; Ahrazem, O.; Gómez-Gómez, L. Subfunctionalization of D27 Isomerase Genes in Saffron. Int. J. Mol. Sci. 2022, 23, 10543. https://doi.org/10.3390/ijms231810543
López-Jiménez AJ, Morote L, Niza E, Mondéjar M, Rubio-Moraga Á, Diretto G, Ahrazem O, Gómez-Gómez L. Subfunctionalization of D27 Isomerase Genes in Saffron. International Journal of Molecular Sciences. 2022; 23(18):10543. https://doi.org/10.3390/ijms231810543
Chicago/Turabian StyleLópez-Jiménez, Alberto José, Lucía Morote, Enrique Niza, María Mondéjar, Ángela Rubio-Moraga, Gianfranco Diretto, Oussama Ahrazem, and Lourdes Gómez-Gómez. 2022. "Subfunctionalization of D27 Isomerase Genes in Saffron" International Journal of Molecular Sciences 23, no. 18: 10543. https://doi.org/10.3390/ijms231810543





