Temperature-Biased miRNA Expression Patterns during European Sea Bass (Dicentrarchus labrax) Development
Abstract
:1. Introduction
2. Results
2.1. Morphological Data
2.1.1. Growth and Sex Ratios
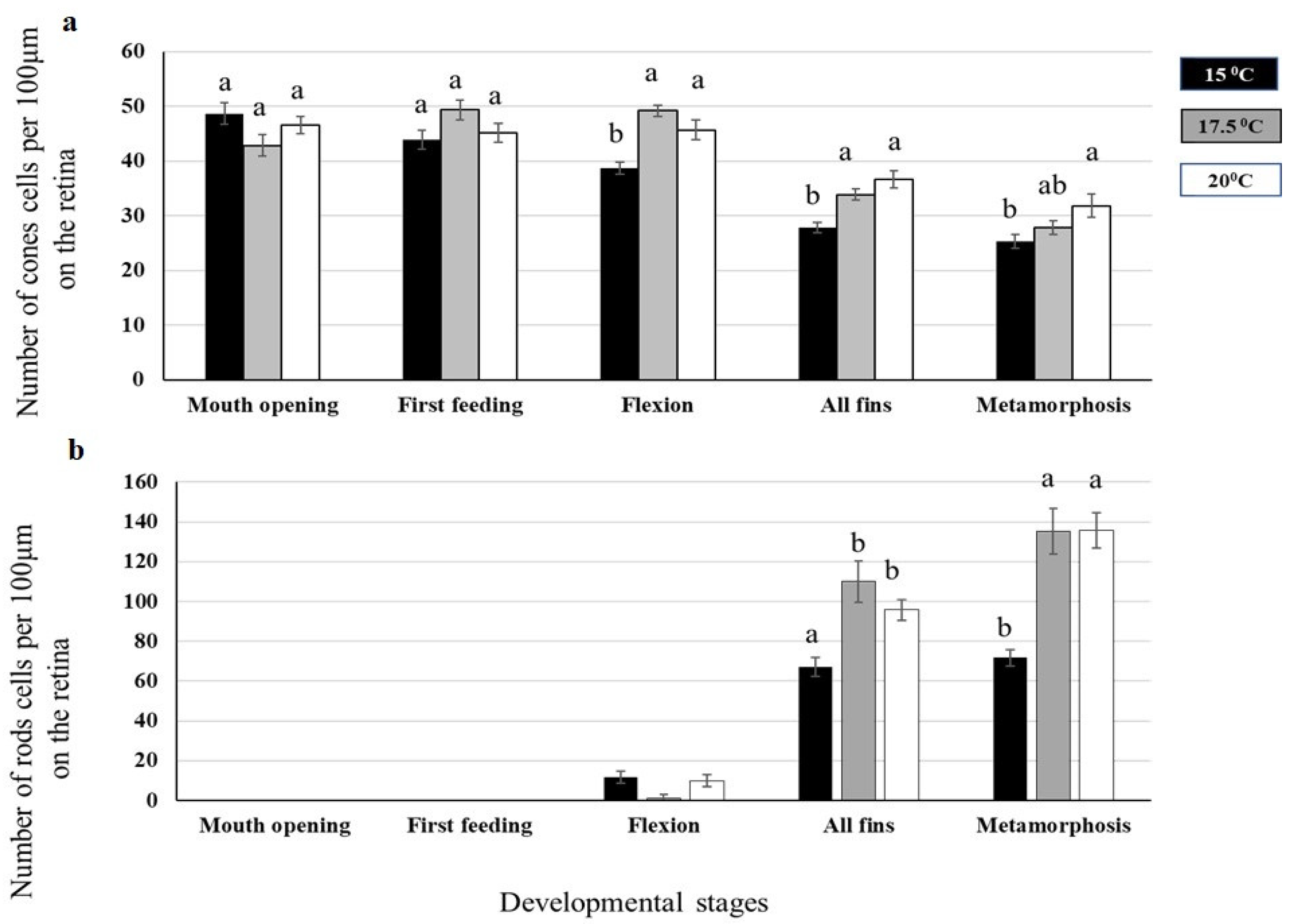
2.1.2. Eye Development
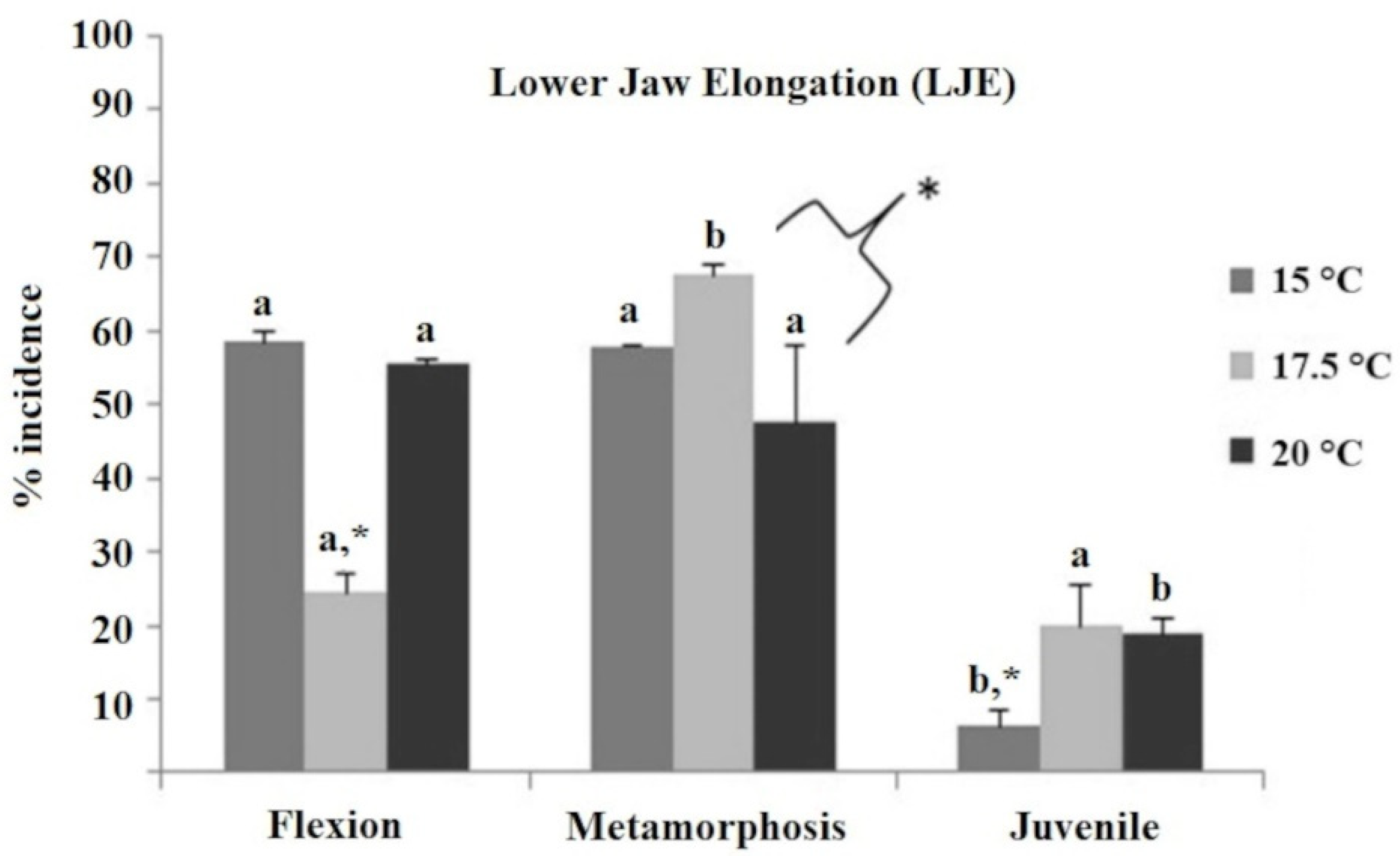
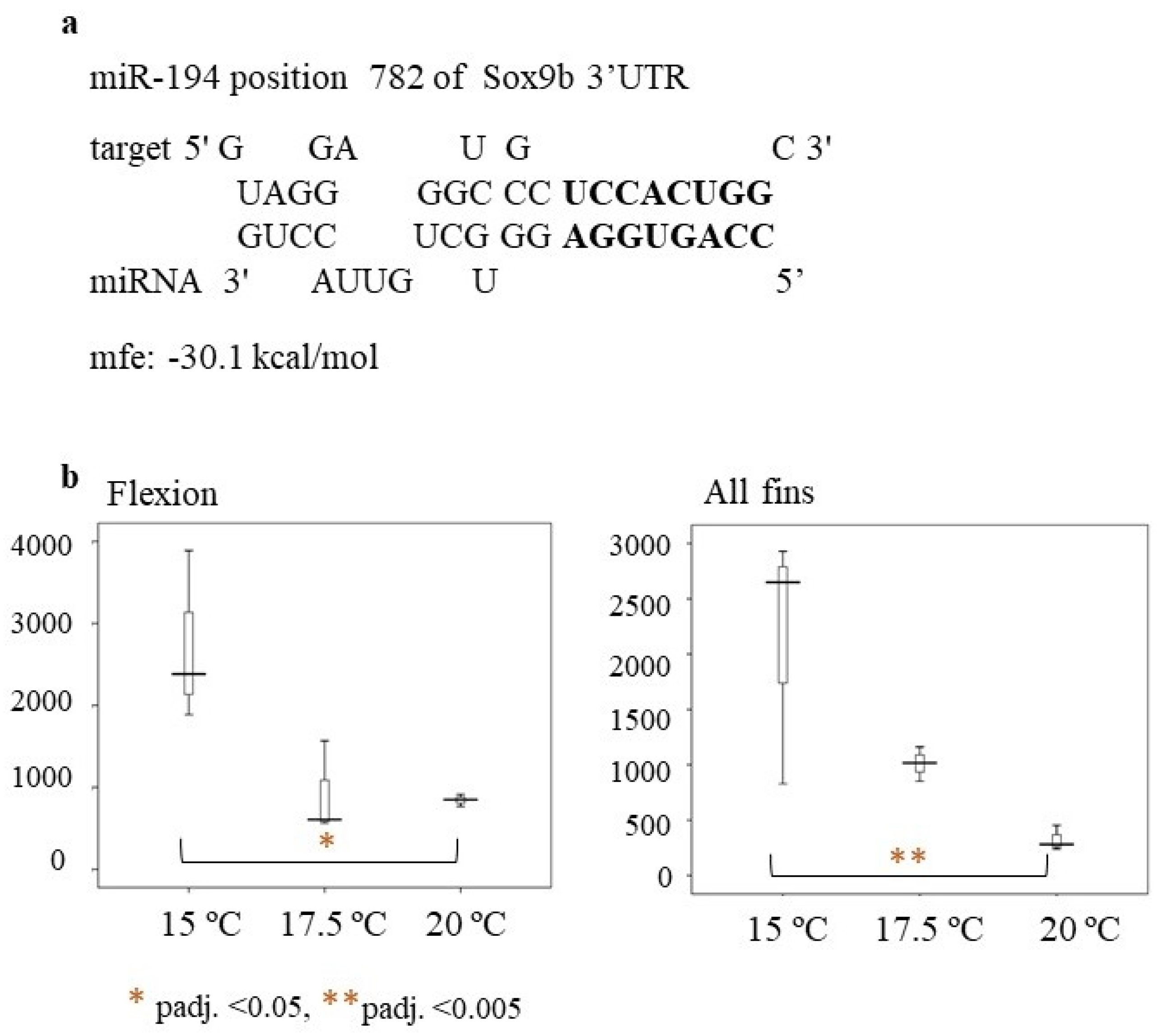
2.1.3. Skeletal Deformities
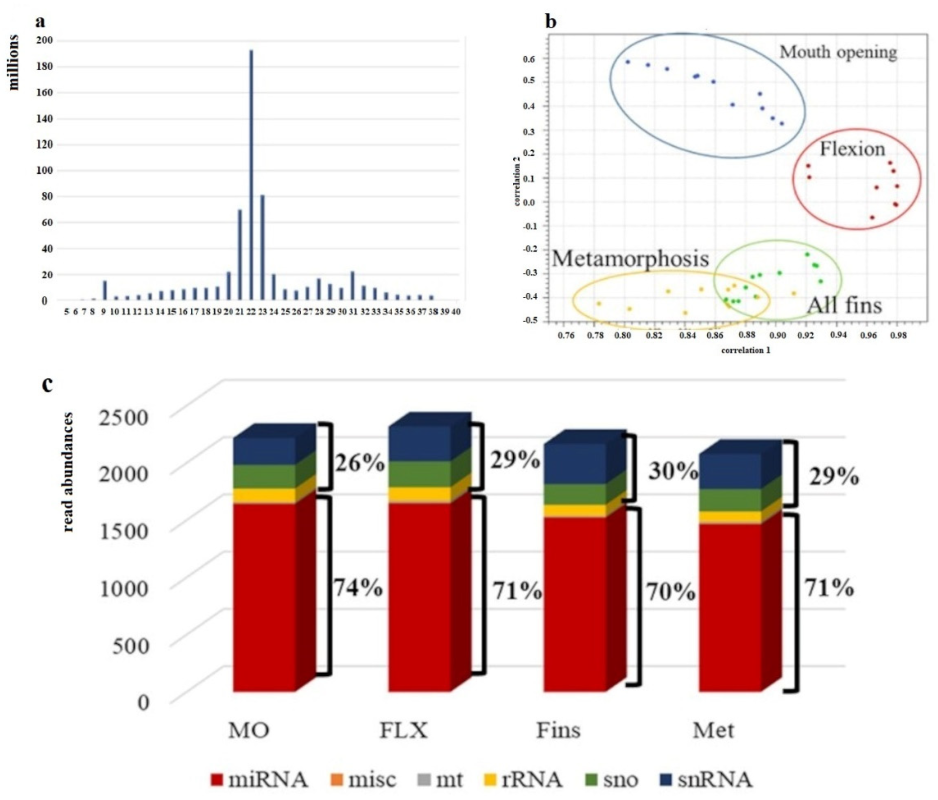
2.2. sncRNA Data Generation and Corroboration
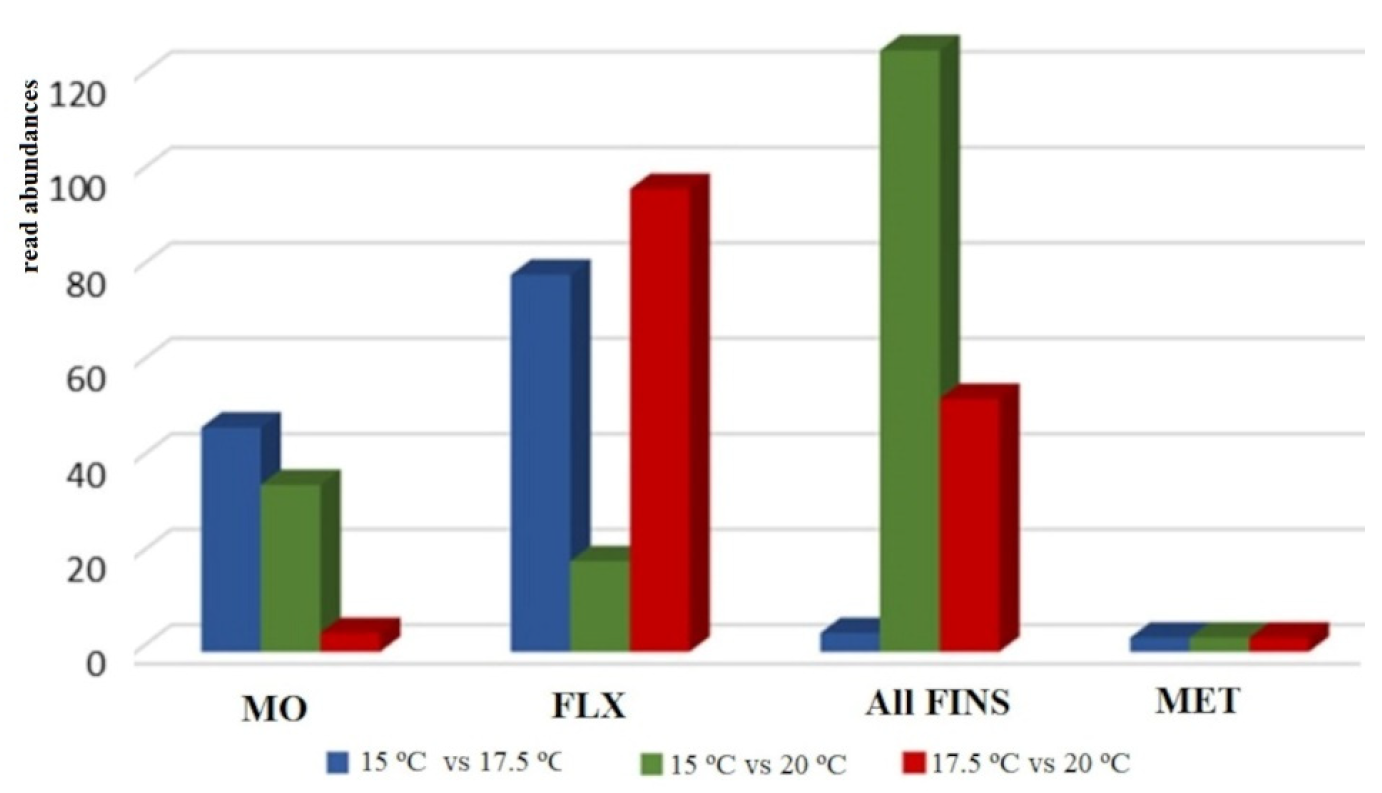
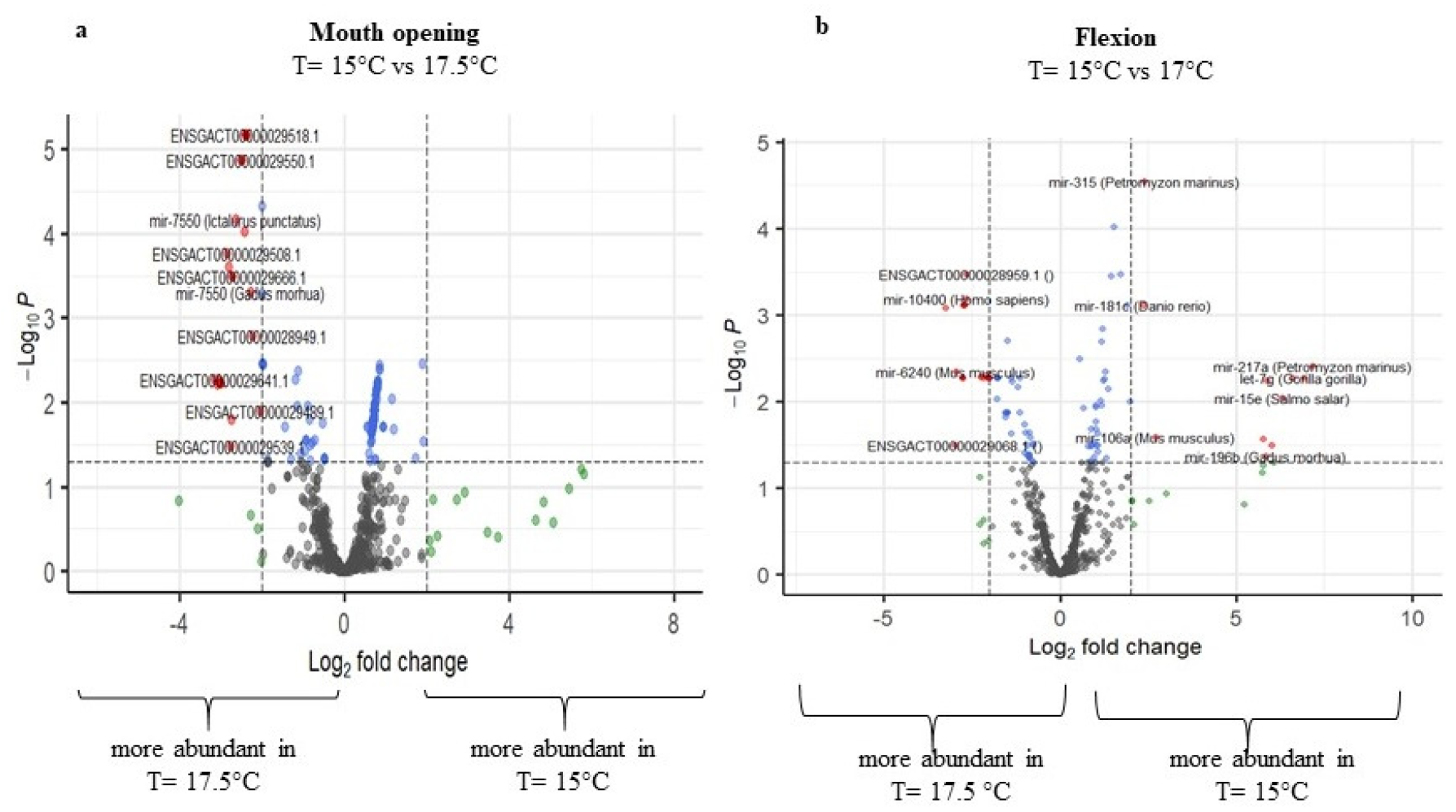
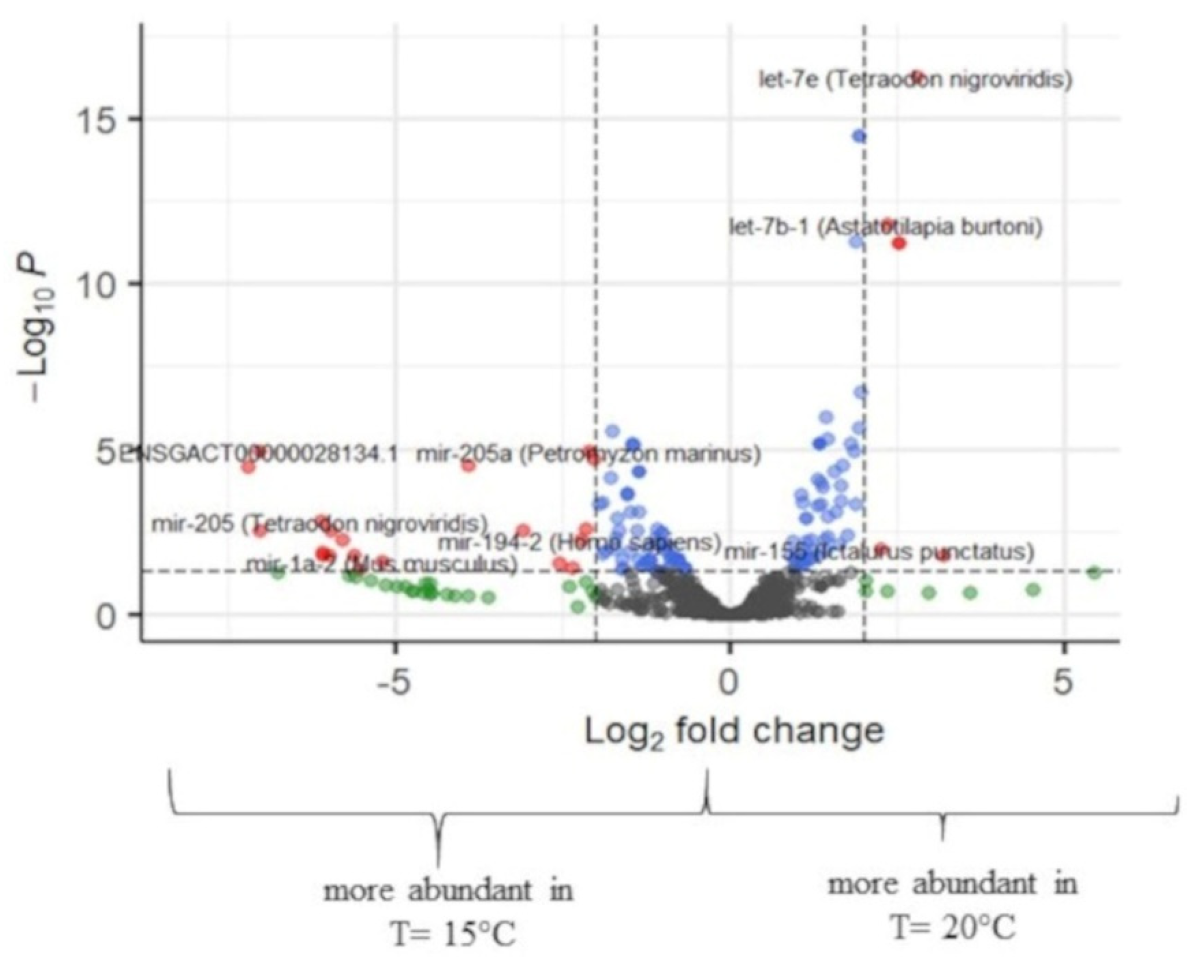
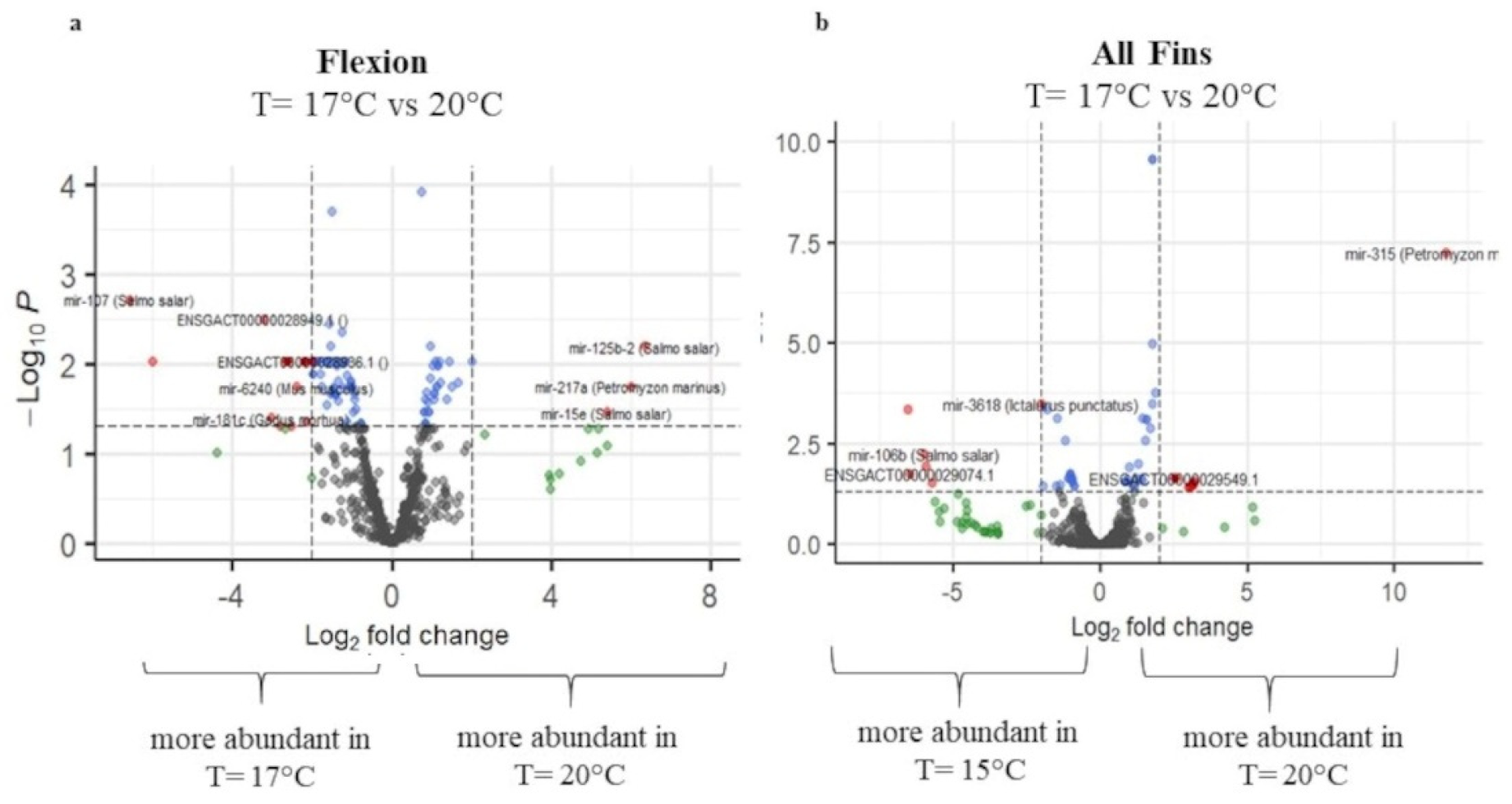
2.3. Differential Expression
2.3.1. Differential Expression of Temperature at 15 °C and 17.5 °C
2.3.2. Differential Expression of Temperature at 15 °C and 20 °C
2.3.3. Differential Expression of Temperature at 17.5 °C and 20 °C
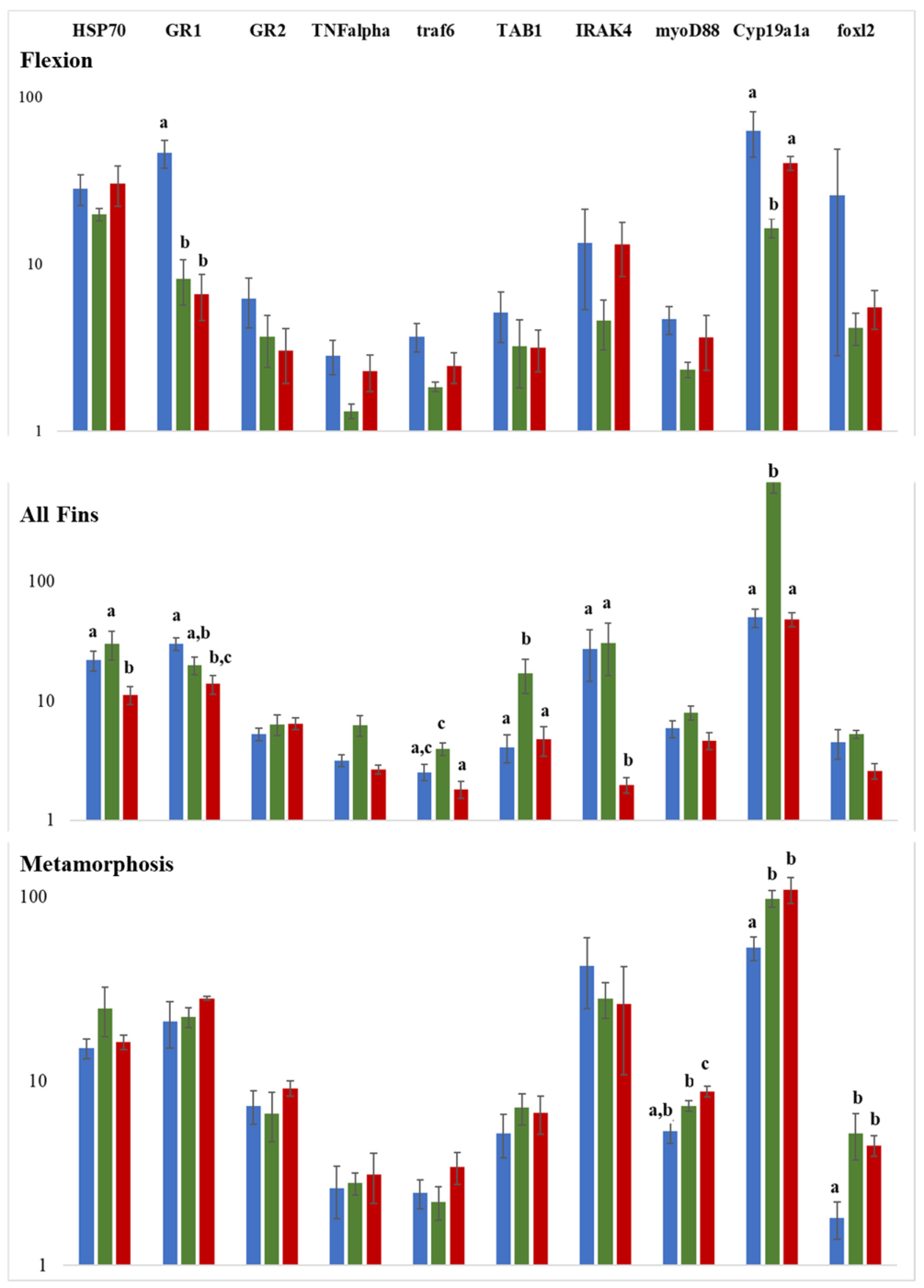
2.4. Expression Analysis of Selected Genes Involved in Reproduction and Stress Response
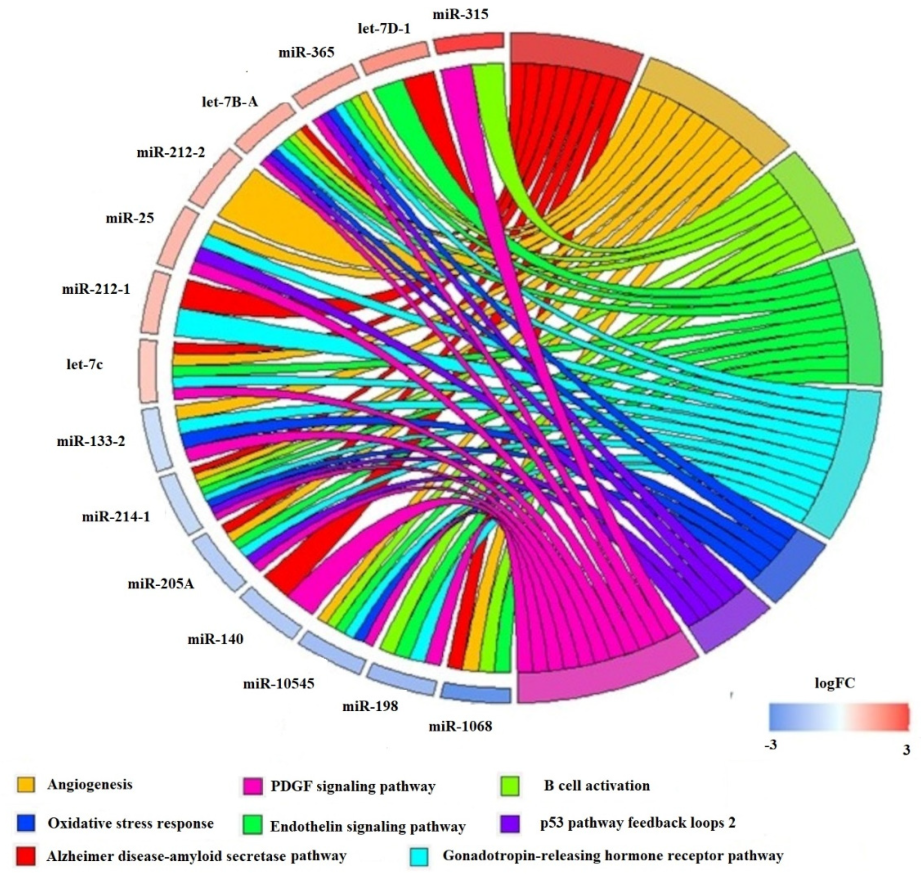
2.5. Target Search and Panther Pathway Analysis
3. Discussion
4. Materials and Methods
4.1. Ethics Approval
4.2. Sampling
4.3. Rod and Cone Cells Analysis
4.4. Statistics
4.5. RNA Extraction and Evaluation
4.6. sncRNA Libraries Construction and Sequencing
4.7. Sequencing Reads Analysis
4.8. Differential Expression Analysis
4.9. Quantitative Real-Time PCR (qPCR)
4.10. Target Search
4.11. Data Access
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, W.W.; Casterlin, M.E. The Role of Temperature in the Environmental Physiology of Fishes. In Environmental Physiology of Fishes; NATO Advanced Study Institutes Series; Springer: Boston, MA, USA, 1980; pp. 497–518. [Google Scholar]
- Bizuayehu, T.T.; Johansen, S.D.; Puvanendran, V.; Toften, H.; Babiak, I. Temperature during Early Development Has Long-Term Effects on MicroRNA Expression in Atlantic Cod. BMC Genom. 2015, 16, 305. [Google Scholar] [CrossRef] [PubMed]
- Sfakianakis, D.G.; Papadakis, I.E.; Papadaki, M.; Sigelaki, I.; Mylonas, C.C. Influence of Rearing Temperature during Early Life on Sex Differentiation, Haemal Lordosis and Subsequent Growth during the Whole Production Cycle in European Sea Bass Dicentrarchus labrax. Aquaculture 2013, 412–413, 179–185. [Google Scholar] [CrossRef]
- Liu, T.; Li, R.; Liu, L.; Wu, S.; Zhang, L.; Li, Y.; Wei, H.; Shu, Y.; Yang, Y.; Wang, S.; et al. The Effect of Temperature on Gonadal Sex Differentiation of Yesso Scallop Patinopecten Yessoensis. Front. Cell Dev. Biol. 2022, 9, 803046. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Anezaki, L.; Divanach, P.; Zanuy, S.; Piferrer, F.; Ron, B.; Peduel, A.; Ben Atia, I.; Gorshkov, S.; Tandler, A. Influence of Rearing Temperature during the Larval and Nursery Periods on Growth and Sex Differentiation in Two Mediterranean Strains of Dicentrarchus labrax. J. Fish Biol. 2005, 67, 652–668. [Google Scholar] [CrossRef]
- Abram, Q.H.; Dixon, B. Impacts of Low Temperature on the Teleost Immune System. Biology 2017, 6, 39. [Google Scholar] [CrossRef]
- Zhang, Q.; Kopp, M.; Babiak, I.; Fernandes, J.M.O. Low Incubation Temperature during Early Development Negatively Affects Survival and Related Innate Immune Processes in Zebrafish Larvae Exposed to Lipopolysaccharide. Sci. Rep. 2018, 8, 4142. [Google Scholar] [CrossRef] [PubMed]
- Dey, M. The Influence of Low Temperature on the Immune System of Teleosts. Br. J. Biol. Stud. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Navarro-Martín, L.; Viñas, J.; Ribas, L.; Díaz, N.; Gutiérrez, A.; Di Croce, L.; Piferrer, F. DNA Methylation of the Gonadal Aromatase (Cyp19a) Promoter Is Involved in Temperature-Dependent Sex Ratio Shifts in the European Sea Bass. PLoS Genet. 2011, 7, e1002447. [Google Scholar] [CrossRef]
- Lutton, B.; Callard, I. Evolution of Reproductive-Immune Interactions. Integr. Comp. Biol. 2006, 46, 1060–1071. [Google Scholar] [CrossRef]
- Anastasiadi, D.; Díaz, N.; Piferrer, F. Small Ocean Temperature Increases Elicit Stage-Dependent Changes in DNA Methylation and Gene Expression in a Fish, the European Sea Bass. Sci. Rep. 2017, 7, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Geffroy, B.; Besson, M.; Goikoetxea, A.; Sadoul, B.; Blanc, M.; Parrinello, H.; Hermet, S.; Blondeau-bidet, E.; Pratlong, M.; Piferrer, F.; et al. Unraveling the Genotype by Environment Interaction in a Thermosensitive Fish with a Polygenic Sex Determination System. Proc. Natl. Acad. Sci. USA 2021, 118, e2112660118. [Google Scholar] [CrossRef] [PubMed]
- Pittman, K.; Yúfera, M.; Pavlidis, M.; Geffen, A.J.; Koven, W.; Ribeiro, L.; Zambonino-Infante, J.L.; Tandler, A. Fantastically Plastic: Fish Larvae Equipped for a New World. Rev. Aquac. 2013, 5, S224–S267. [Google Scholar] [CrossRef]
- Navarro-Martín, L.; Blázquez, M.; Viñas, J.; Joly, S.; Piferrer, F. Balancing the Effects of Rearing at Low Temperature during Early Development on Sex Ratios, Growth and Maturation in the European Sea Bass (Dicentrarchus labrax). Limitations and Opportunities for the Production of Highly Female-Biased Stocks. Aquaculture 2009, 296, 347–358. [Google Scholar] [CrossRef]
- Koumoundouros, G.; Pavlidis, M.; Anezaki, L.; Kokkari, C.; Sterioti, A.; Divanach, P.; Kentouri, M. Temperature Sex Determination in the European Sea Bass, Dicentrarchus labrax (L., 1758) (Teleostei, Perciformes, Moronidae): Critical Sensitive Ontogenetic Phase. J. Exp. Zool. 2002, 292, 573–579. [Google Scholar] [CrossRef]
- Pavlidis, M.; Koumoundouros, G.; Sterioti, A.; Somarakis, S.; Divanach, P.; Kentouri, M. Evidence of Temperature-Dependent Sex Determination in the European Sea Bass (Dicentrarchus labrax L.). J. Exp. Zool. 2000, 287, 225–232. [Google Scholar] [CrossRef]
- Saillant, E.; Fostier, A.; Haffray, P.; Menu, B.; Thimonier, J.; Chatain, B. Temperature Effects and Genotype-Temperature Interactions on Sex Determination in the European Sea Bass (Dicentrarchus labrax L.). J. Exp. Zool. 2002, 292, 494–505. [Google Scholar] [CrossRef]
- Piferrer, F. Epigenetics of Sex Determination and Gonadogenesis. Dev. Dyn. 2013, 242, 360–370. [Google Scholar] [CrossRef]
- Somero, G.N. The Physiology of Climate Change: How Potentials for Acclimatization and Genetic Adaptation Will Determine “winners” and “Losers”. J. Exp. Biol. 2010, 213, 912–920. [Google Scholar] [CrossRef]
- Anastasiadi, D.; Vandeputte, M.; Sánchez-Baizán, N.; Allal, F.; Piferrer, F. Dynamic Epimarks in Sex-Related Genes Predict Gonad Phenotype in the European Sea Bass, a Fish with Mixed Genetic and Environmental Sex Determination. Epigenetics 2018, 13, 988–1011. [Google Scholar] [CrossRef]
- Campos, C.; Sundaram, A.Y.M.; Valente, L.M.P.; Conceição, L.E.C.; Engrola, S.; Fernandes, J.M.O. Thermal Plasticity of the MiRNA Transcriptome during Senegalese Sole Development. BMC Genom. 2014, 15, 525. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Xu, W.; Lucas, A.S.; Wang, Z.; Liu, Y. Identifying MicroRNA Targets in Different Gene Regions. BMC Bioinform. 2014, 15, S4. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.J.; Legesse-Miller, A.; Coller, H.A. A Search for Conserved Sequences in Coding Regions Reveals That the Let-7 MicroRNA Targets Dicer within Its Coding Sequence. Proc. Natl. Acad. Sci. USA 2008, 105, 14879–14884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, W.; Liu, Y.; Liu, T.; Li, C.; Wang, L. Oncogenic Role of MicroRNA-532-5p in Human Colorectal Cancer via Targeting of the 5′UTR of RUNX3. Oncol. Lett. 2018, 15, 7215–7220. [Google Scholar] [CrossRef]
- Dharap, A.; Pokrzywa, C.; Murali, S.; Pandi, G.; Vemuganti, R. MicroRNA MiR-324-3p Induces Promoter-Mediated Expression of RelA Gene. PLoS ONE 2013, 8, e79467. [Google Scholar] [CrossRef]
- Chang, T.C.; Mendell, J.T. MicroRNAs in Vertebrate Physiology and Human Disease. Annu. Rev. Genom. Hum. Genet. 2007, 8, 215–239. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Sun, Z.; Evans, J.; Bhagwate, A.; Middha, S.; Bockol, M.; Yan, H.; Kocher, J.P. CAP-MiRSeq: A Comprehensive Analysis Pipeline for MicroRNA Sequencing Data. BMC Genom. 2014, 15, 423. [Google Scholar] [CrossRef]
- Sarropoulou, E.; Kaitetzidou, E.; Papandroulaki, N.; Tsalafouta, A.; Pavlidis, M. Inventory of European Sea Bass (Dicentrarchus labrax) SncRNAs Vital during Early Teleost Development. Front. Genet. 2019, 10, 657. [Google Scholar] [CrossRef]
- Rasmussen, K.D.; Simmini, S.; Abreu-Goodger, C.; Bartonicek, N.; Di Giacomo, M.; Bilbao-Cortes, D.; Horos, R.; Von Lindern, M.; Enright, A.J.; O’Carroll, D. The MiR-144/451 Locus is required for erythroid homeostasis. J. Exp. Med. 2010, 207, 1351–1358. [Google Scholar] [CrossRef]
- Kretov, D.A.; Walawalkar, I.A.; Mora-Martin, A.; Shafik, A.M.; Moxon, S.; Cifuentes, D. Ago2-Dependent Processing Allows MiR-451 to Evade the Global MicroRNA Turnover Elicited during Erythropoiesis. Mol. Cell 2020, 78, 317–328.e6. [Google Scholar] [CrossRef]
- Pase, L.; Layton, J.E.; Kloosterman, W.P.; Carradice, D.; Waterhouse, P.M.; Lieschke, G.J. MiR-451 Regulates Zebrafish Erythroid Maturation in Vivo via Its Target Gata2. Blood 2009, 113, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- Shahjahan, M.; Khatun, M.S.; Mun, M.M.; Islam, S.M.M.; Uddin, M.H.; Badruzzaman, M.; Khan, S. Nuclear and Cellular Abnormalities of Erythrocytes in Response to Thermal Stress in Common Carp Cyprinus Carpio. Front. Physiol. 2020, 11, 543. [Google Scholar] [CrossRef]
- Cohen, W.D.; Sorokina, Y.; Sanchez, I. Elliptical versus Circular Erythrocyte Marginal Bands: Isolation, Shape Conversion, and Mechanical Properties. Cell Motil. Cytoskelet. 1998, 40, 238–248. [Google Scholar] [CrossRef]
- Cohen, W.D.; Bartelt, D.; Jaeger, R.; Langford, G.; Nemhauser, I. The Cytoskeletal System of Nucleated Erythrocytes. I. Composition and Function of Major Elements. J. Cell Biol. 1982, 93, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Jaree, P.; Wongdontri, C.; Somboonwiwat, K. White Spot Syndrome Virus-Induced Shrimp MiR-315 Attenuates Prophenoloxidase Activation via PPAE3 Gene Suppression. Front. Immunol. 2018, 9, 2184. [Google Scholar] [CrossRef]
- Nusse, R. Wnt Signaling in Development and Disease. Cell Res. 2005, 15, 28–32. [Google Scholar] [CrossRef]
- Tsalafouta, A.; Sarropoulou, E.; Papandroulakis, N.; Pavlidis, M. Characterization and Expression Dynamics of Key Genes Involved in the Gilthead Sea Bream (Sparus aurata) Cortisol Stress Response during Early Ontogeny. Mar. Biotechnol. 2018, 20, 611–622. [Google Scholar] [CrossRef]
- Geffroy, B.; Wedekind, C. Effects of Global Warming on Sex Ratios in Fishes. Fish Biol. 2020, 97, 596–606. [Google Scholar] [CrossRef]
- Wong, T.T.; Gothilf, Y.; Zmora, N.; Kight, K.E.; Meiri, I.; Elizur, A.; Zohar, Y. Developmental Expression of Three Forms of Gonadotropin-Releasing Hormone and Ontogeny of the Hypothalamic-Pituitary-Gonadal Axis in Gilthead Seabream (Sparus aurata). Biol. Reprod. 2004, 71, 1026–1035. [Google Scholar] [CrossRef] [Green Version]
- González-Martínez, D.; Zmora, N.; Mañanos, E.; Saligaut, D.; Zanuy, S.; Zohar, Y.; Elizur, A.; Kah, O.; Muñoz-Cueto, J.A. Immunohistochemical Localization of Three Different Prepro-GnRHs in the Brain and Pituitary of the European Sea Bass (Dicentrarchus labrax) Using Antibodies to the Corresponding GnRH-Associated Peptides. J. Comp. Neurol. 2002, 446, 95–113. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, D.; Zmora, N.; Saligaut, D.; Zanuy, S.; Elizur, A.; Kah, O.; Muñoz-Cueto, J.A. New Insights in Developmental Origins of Different GnRH (Gonadotrophin-Releasing Hormone) Systems in Perciform Fish: An Immunohistochemical Study in the European Sea Bass (Dicentrarchus labrax). J. Chem. Neuroanat. 2004, 28, 1–15. [Google Scholar] [CrossRef]
- Abraham, E.; Palevitch, O.; Gothilf, Y.; Zohar, Y. Targeted Gonadotropin-Releasing Hormone-3 Neuron Ablation in Zebrafish: Effects on Neurogenesis, Neuronal Migration, and Reproduction. Endocrinology 2010, 151, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lin, M.-C.A.; Farajzadeh, M.; Wayne, N.L. Early Development of the Gonadotropin-Releasing Hormone Neuronal Network in Transgenic Zebrafish. Front. Endocrinol. 2013, 4, 107. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.S.; Benninghoff, A.D.; Holt, G.J.; Khan, I.A. Developmental Expression of the G Protein-Couple Receptor 54 and Three GnRH MRNAs in the Teleost Fish Cobia. J. Mol. Endocrinol. 2007, 38, 235–244. [Google Scholar] [CrossRef]
- Sherwood, N.M.; Wu, S. Developmental Role of GnRH and PACAP in a Zebrafish Model. Gen. Comp. Endocrinol. 2005, 142, 74–80. [Google Scholar] [CrossRef]
- Feng, K.; Cui, X.; Song, Y.; Tao, B.; Chen, J.; Wang, J.; Liu, S.; Sun, Y.; Zhu, Z.; Trudeau, V.L.; et al. GnRH3 Regulates PGC Proliferation and Sex Differentiation in Developing Zebrafish. Endocrinology 2020, 161, bqz024. [Google Scholar] [CrossRef]
- Anonymous. Guidelines for the Treatment of Animals in Behavioural Research and Teaching. Anim. Behav. 1998, 55, 251–257. [Google Scholar] [CrossRef]
- Metcalfe, J.D.; Craig, J.F. Ethical Justification for the Use and Treatment of Fishes in Research: An Update. J. Fish Biol. 2011, 78, 393–394. [Google Scholar] [CrossRef]
- McDowell, E.M.; Trump, B.F. Histologic Fixatives Suitable for Diagnostic Light and Electron Microscopy. Arch. Pathol. Lab. Med. 1976, 100, 405–414. [Google Scholar]
- Markle, D.F. Phosphate Buffered Formalin for Long Term Preservation of Formalin Fixed Ichthyoplankton. Copeia 1984, 2, 525–528. [Google Scholar] [CrossRef]
- Park, E.H.; Kim, D.S. A Procedure for Staining Cartilage and Bone of Whole Vertebrate Larvae While Rendering All Other Tissues Transparent. Biotech. Histochem. 1984, 59, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S.; Saini, H.K.; Van Dongen, S.; Enright, A.J. MiRBase: Tools for MicroRNA Genomics. Nucleic Acids Res. 2008, 36, D154–D158. [Google Scholar] [CrossRef]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.Y.; Dillies, M.A. SARTools: A DESeq2- and EdgeR-Based R Pipeline for Comprehensive Differential Analysis of RNA-Seq Data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Zhao, S.; Fernald, R.D. Comprehensive Algorithm for Quantitative Real-Time Polymerase Chain Reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef]
- Krüger, J.; Rehmsmeier, M. RNAhybrid: MicroRNA Target Prediction Easy, Fast and Flexible. Nucleic Acids Res. 2006, 34, W451–W454. [Google Scholar] [CrossRef]
| Male Percentage (%) | Female Percentage (%) | |
|---|---|---|
| 15 °C | 64.1 ± 6.3% | 35.9 ± 6.3% |
| 17.5 °C | 53.6 ± 4.2% | 46.4 ± 4.2% |
| 20 °C | 72.7 ± 0.9% | 27.3 ± 0.9% |
| Mouth Opening | Flexion | AllFins | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Statistical Overrepresentation of Pathways | 15 °C vs. 17.5 °C | 17.5 °C vs. 20 °C | 15 °C vs. 20 °C | 15 °C vs. 17.5 °C | 17.5 °C vs. 20 °C | 15 °C vs. 20 °C | 15 °C vs. 17.5 °C | 17.5 °C vs. 20 °C | 15 °C vs. 20 °C |
| Alzheimer disease-amyloid secretase pathway (P00003) | No statistically significant results | No statistically significant results | No statistically significant results | ||||||
| Angiogenesis (P00005) | |||||||||
| B cell activation (P00010) | |||||||||
| Cadherin signaling pathway (P00012) | |||||||||
| EGF receptor signaling pathway (P00018) | |||||||||
| Endothelin signaling pathway (P00019) | |||||||||
| FGF signaling pathway (P00021) | |||||||||
| Gonadotropin-releasing hormone receptor pathway (P06664) | |||||||||
| Oxidative stress response (P00046) | |||||||||
| p53 pathway feedback loops 2 (P04398) | |||||||||
| PDGF signaling pathway (P00047) | |||||||||
| Wnt signaling pathway (P00057) | |||||||||
| Hypoxia response via HIF activation | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadaki, M.; Kaitetzidou, E.; Papadakis, I.E.; Sfakianakis, D.G.; Papandroulakis, N.; Mylonas, C.C.; Sarropoulou, E. Temperature-Biased miRNA Expression Patterns during European Sea Bass (Dicentrarchus labrax) Development. Int. J. Mol. Sci. 2022, 23, 11164. https://doi.org/10.3390/ijms231911164
Papadaki M, Kaitetzidou E, Papadakis IE, Sfakianakis DG, Papandroulakis N, Mylonas CC, Sarropoulou E. Temperature-Biased miRNA Expression Patterns during European Sea Bass (Dicentrarchus labrax) Development. International Journal of Molecular Sciences. 2022; 23(19):11164. https://doi.org/10.3390/ijms231911164
Chicago/Turabian StylePapadaki, Maria, Elisavet Kaitetzidou, Ioannis E. Papadakis, Dimitris G. Sfakianakis, Nikos Papandroulakis, Constantinos C. Mylonas, and Elena Sarropoulou. 2022. "Temperature-Biased miRNA Expression Patterns during European Sea Bass (Dicentrarchus labrax) Development" International Journal of Molecular Sciences 23, no. 19: 11164. https://doi.org/10.3390/ijms231911164





