A New Chemotactic Mechanism Governs Long-Range Angiogenesis Induced by Patching an Arterial Graft into a Vein
Abstract
:1. Introduction
2. Results
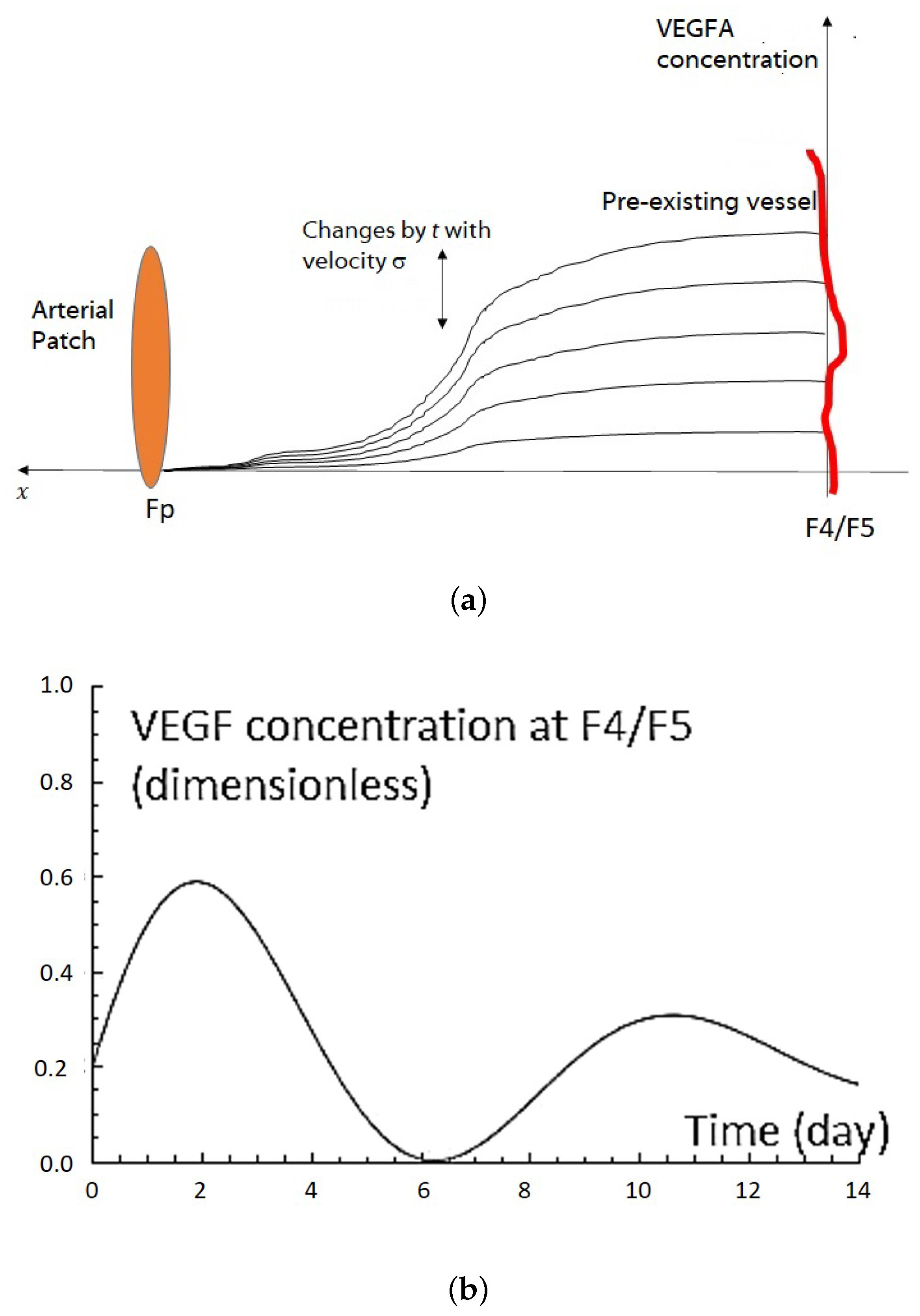
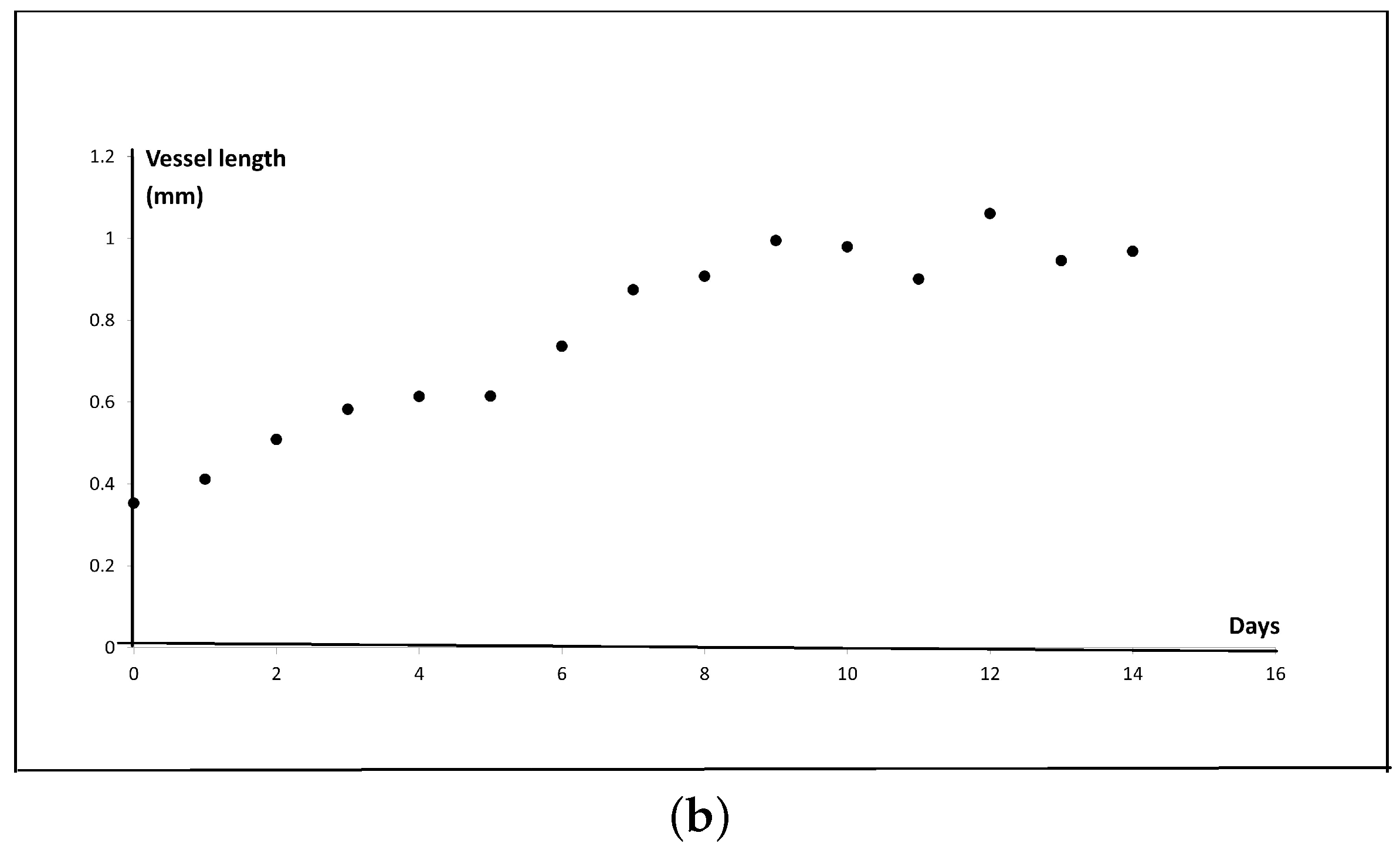
2.1. VEGF Distribution Model
2.2. VEGFA Quantification in In Vivo Angiogenesis Model
2.3. VEGFA Distribution Equation Based on In Vivo Quantification Data
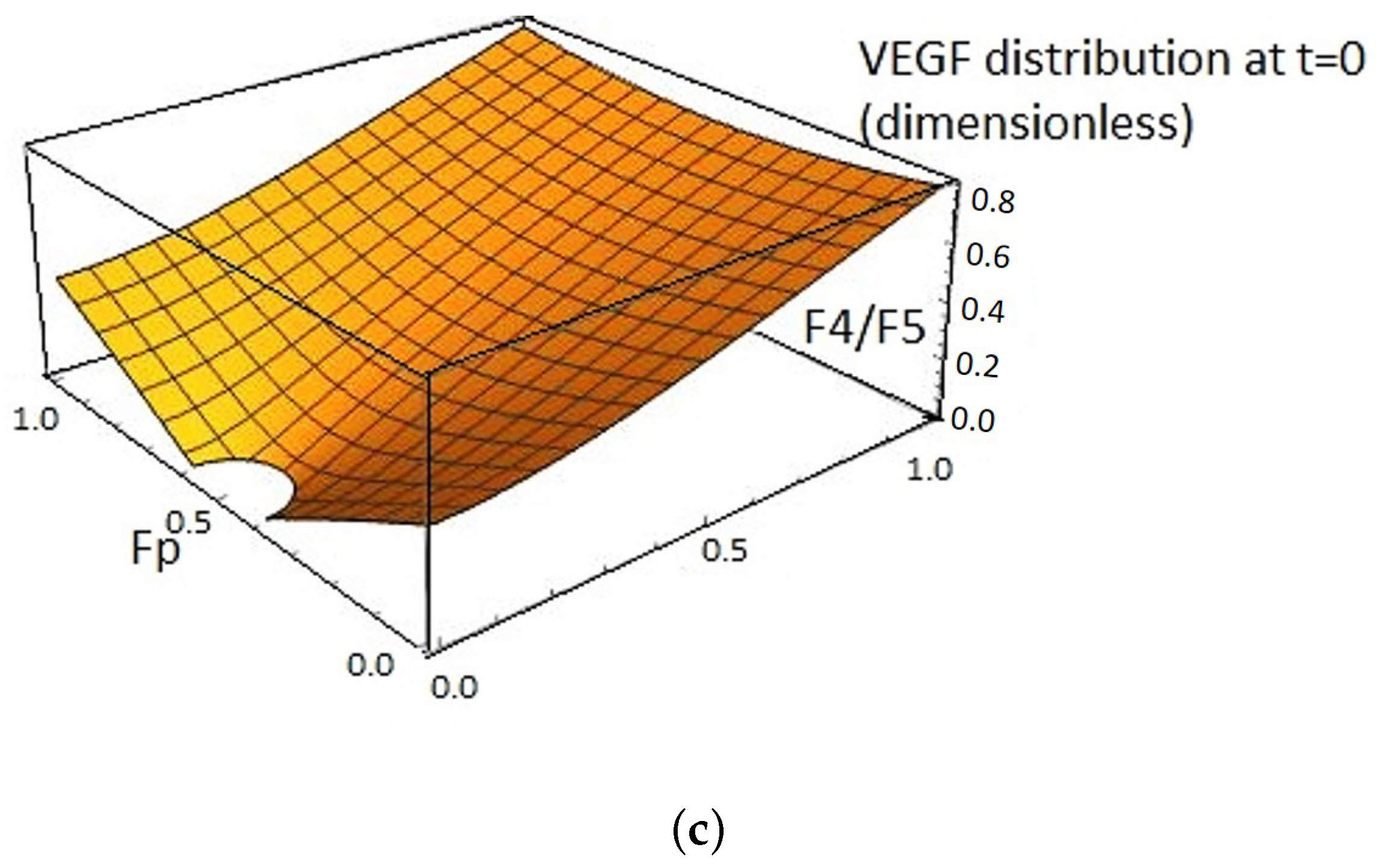
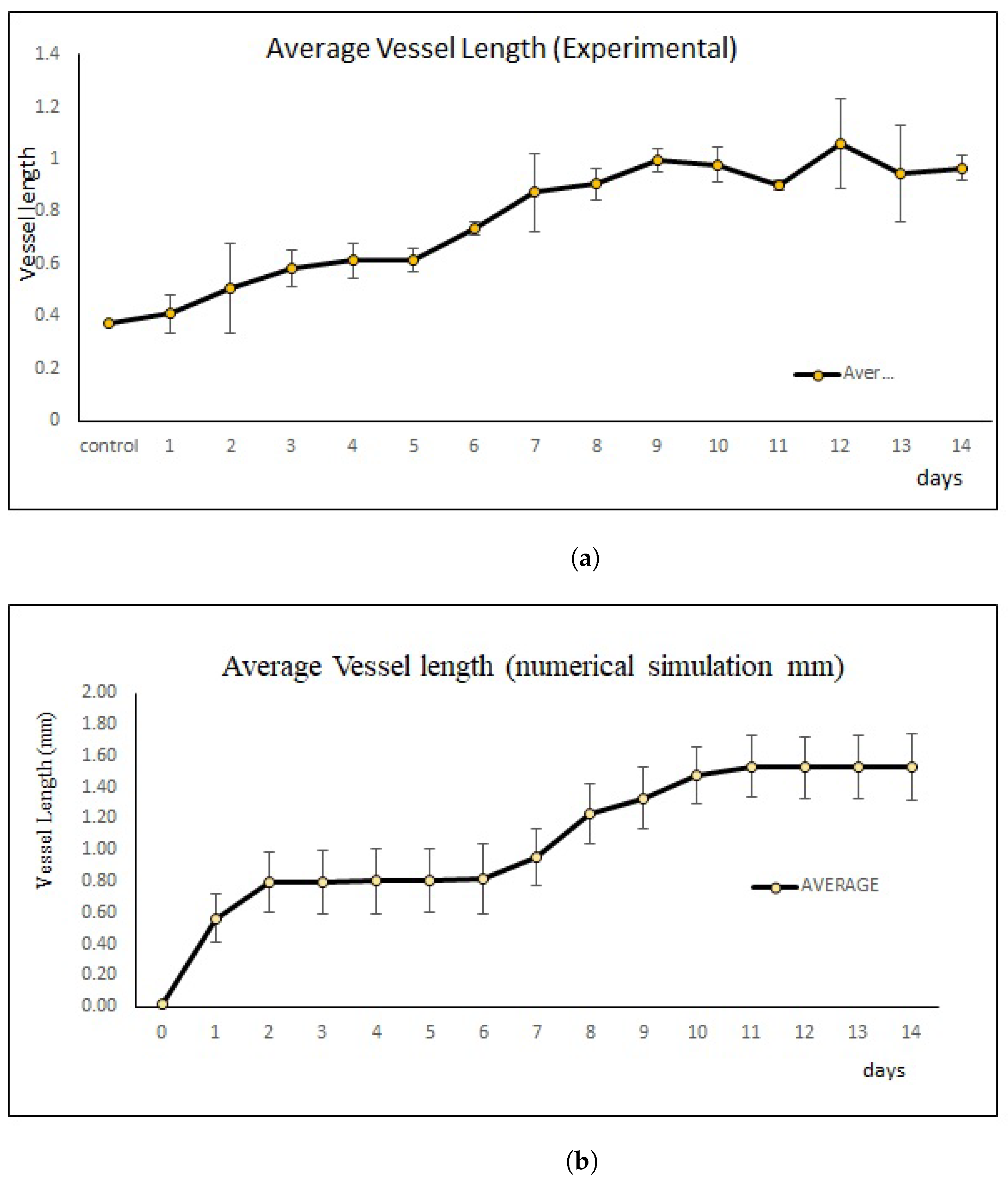
2.4. New Blood Vessel Length Measurement in In Vivo Angiogenesis Model
2.5. Sprout Tip Distribution Equation with New Chemotactic Velocity
3. Discussion
4. Material and Methods
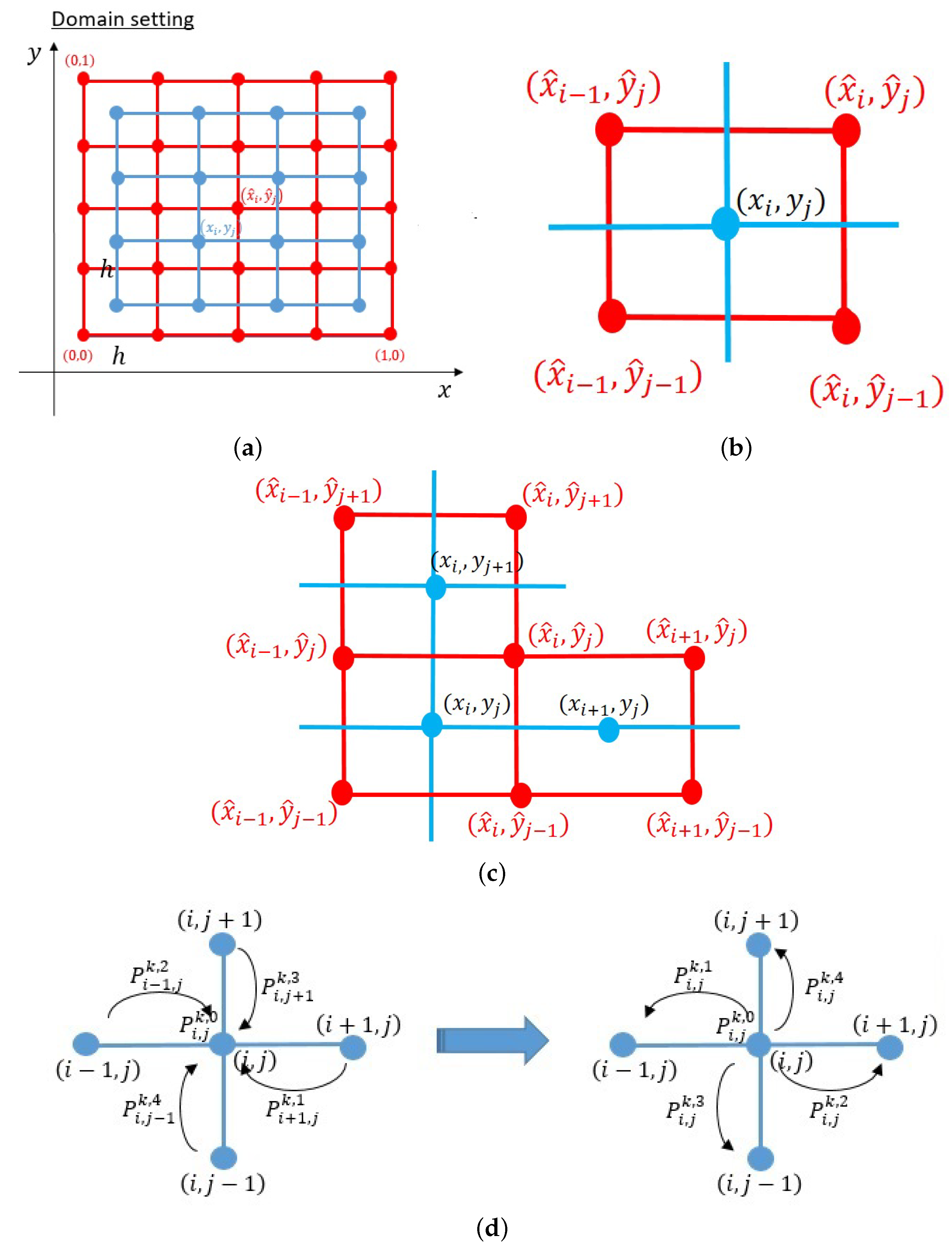
4.1. Numerical Simulation Method and Results
4.1.1. Derivation of Numerical Scheme
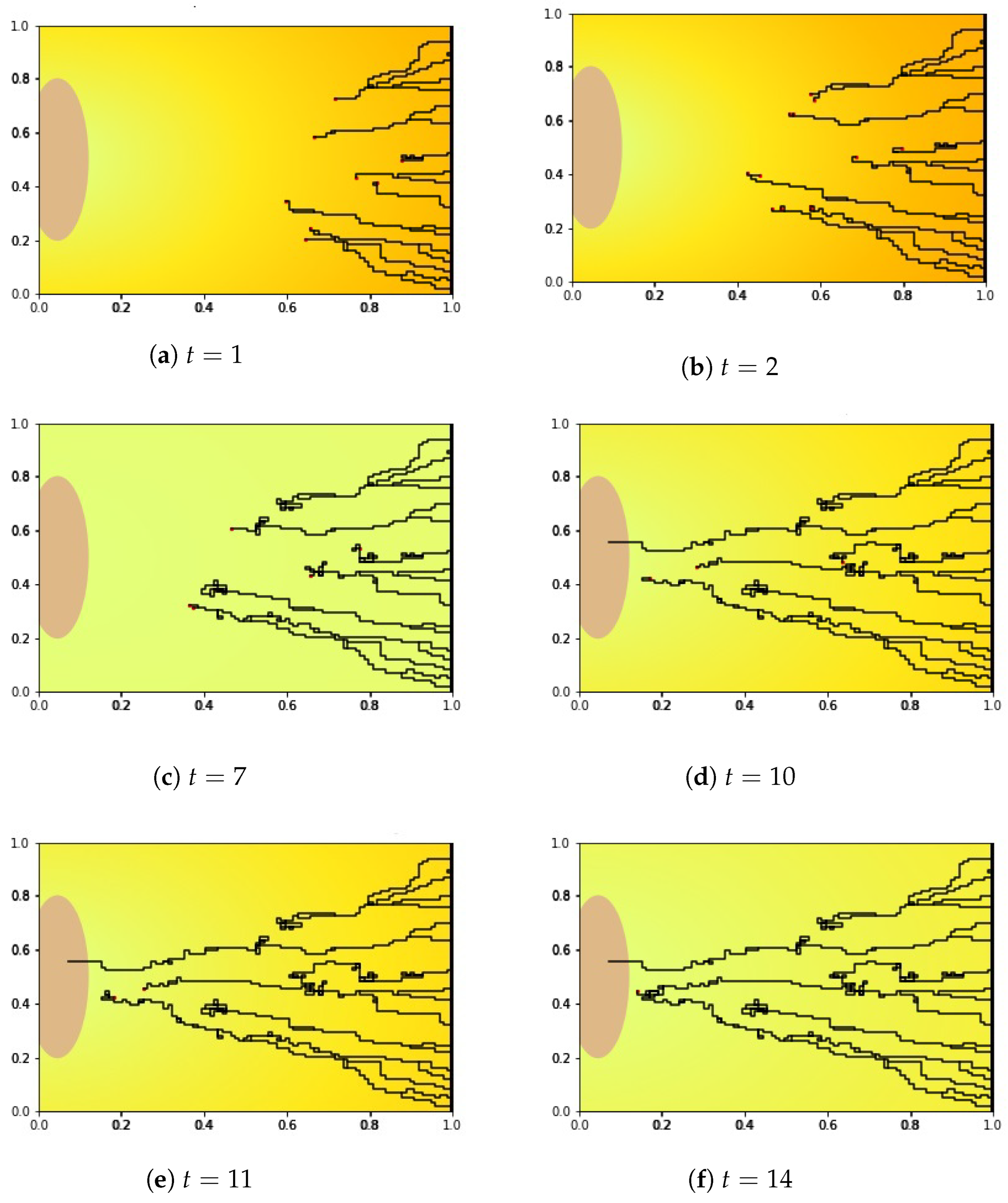
4.1.2. Numerical Simulation Results
4.2. Material and Methods: In Vivo
4.2.1. Quantification of Vegfa in In Vivo Angiogenesis Model
4.2.2. Measurement of New Blood Vessel Length
4.2.3. Numerical Simulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VEGF | Vascular endothelial growth factor |
| AVF | Arteriovenous fistula |
| AVM | Arteriovenous malformation |
| VEGFR | Vascular endothelial growth factor receptor |
| mm | Millimeter |
| SD | Standard deviation |
| MN | Minnesota |
Appendix A
References
- Bautch, V.L. VEGF-directed blood vessel patterning: From cells to organism. Cold Spring Harb. Perspect. Med. 2012, 2, a006452. [Google Scholar] [CrossRef] [PubMed]
- Baeriswyl, V.; Christofori, G. The angiogenic switch in carcinogenesis. In Seminars in Cancer Biology; Elsevier: Berlin/Heidelberg, Germany, 2009; Volume 19, pp. 329–337. [Google Scholar]
- Patel, R.; Nicholson, A. Arteriovenous fistulas: Etiology and treatment. Endovasc. Today 2012, 11, 45–51. [Google Scholar]
- Shatzkes, D.R. Vascular anomalies: Description, classification and nomenclature. Appl. Radiol. 2018, 18, 7–13. [Google Scholar] [CrossRef]
- Faughnan, M.E.; Lui, Y.W.; Wirth, J.A.; Pugash, R.A.; Redelmeier, D.A.; Hyland, R.H.; White Jr, R.I. Diffuse pulmonary arteriovenous malformations: Characteristics and prognosis. Chest 2000, 117, 31–38. [Google Scholar] [CrossRef]
- Nakabayashi, T.; Kudo, M.; Hirasawa, T.; Kuwano, H. Arteriovenous malformation of the jejunum detected by arterial-phase enhanced helical CT, a case report. Hepato-Gastroenterology 2004, 51, 1066–1068. [Google Scholar] [PubMed]
- Steinmetz, M.P.; Chow, M.M.; Krishnaney, A.A.; Andrews-Hinders, D.; Benzel, E.C.; Masaryk, T.J.; Mayberg, M.R.; Rasmussen, P.A. Outcome after the treatment of spinal dural arteriovenous fistulae: A contemporary single-institution series and meta-analysis. Neurosurgery 2004, 55, 77–88. [Google Scholar] [CrossRef]
- Lalitha, N.; Seetha, P.; Shanmugasundaram, R.; Rajendiran, G. Uterine arteriovenous malformation: Case series and literature review. J. Obstet. Gynecol. India 2016, 66, 282–286. [Google Scholar] [CrossRef]
- Milton, I.; Ouyang, D.; Allen, C.J.; Yanasak, N.E.; Gossage, J.R.; Alleyne, C.H., Jr.; Seki, T. Age-dependent lethality in novel transgenic mouse models of central nervous system arteriovenous malformations. Stroke 2012, 43, 1432–1435. [Google Scholar] [CrossRef]
- Ito, Y.; Yoshida, M.; Maeda, D.; Takahashi, M.; Nanjo, H.; Masuda, H.; Goto, A. Neovasculature can be induced by patching an arterial graft into a vein: A novel in vivo model of spontaneous arteriovenous fistula formation. Sci. Rep. 2018, 8, 3156. [Google Scholar] [CrossRef]
- Bauer, A.L.; Jackson, T.L.; Jiang, Y. A cell-based model exhibiting branching and anastomosis during tumor-induced angiogenesis. Biophys. J. 2007, 92, 3105–3121. [Google Scholar] [CrossRef]
- Scianna, M. A multiscale hybrid model for pro-angiogenic calcium signals in a vascular endothelial cell. Bull. Math. Biol. 2012, 74, 1253–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boas, S.E.; Jiang, Y.; Merks, R.M.; Prokopiou, S.A.; Rens, E.G. Cellular potts model: Applications to vasculogenesis and angiogenesis. In Probabilistic Cellular Automata; Springer: Berlin/Heidelberg, Germany, 2018; pp. 279–310. [Google Scholar]
- Stokes, C.L.; Lauffenburger, D.A. Analysis of the roles of microvessel endothelial cell random motility and chemotaxis in angiogenesis. J. Theor. Biol. 1991, 152, 377–403. [Google Scholar] [CrossRef]
- Bonilla, L.; Capasso, V.; Alvaro, M.; Carretero, M. Hybrid modeling of tumor-induced angiogenesis. Phys. Rev. E 2014, 90, 062716. [Google Scholar] [CrossRef] [PubMed]
- Perfahl, H.; Hughes, B.D.; Alarcón, T.; Maini, P.K.; Lloyd, M.C.; Reuss, M.; Byrne, H.M. 3D hybrid modelling of vascular network formation. J. Theor. Biol. 2017, 414, 254–268. [Google Scholar] [CrossRef]
- Spill, F.; Guerrero, P.; Alarcon, T.; Maini, P.K.; Byrne, H.M. Mesoscopic and continuum modelling of angiogenesis. J. Math. Biol. 2015, 70, 485–532. [Google Scholar] [CrossRef]
- Byrne, H.M.; Chaplain, M.A. Mathematical models for tumour angiogenesis: Numerical simulations and nonlinear wave solutions. Bull. Math. Biol. 1995, 57, 461–486. [Google Scholar] [CrossRef]
- Connor, A.J.; Nowak, R.P.; Lorenzon, E.; Thomas, M.; Herting, F.; Hoert, S.; Quaiser, T.; Shochat, E.; Pitt-Francis, J.; Cooper, J.; et al. An integrated approach to quantitative modelling in angiogenesis research. J. R. Soc. Interface 2015, 12, 20150546. [Google Scholar] [CrossRef]
- Giverso, C.; Ciarletta, P. Tumour angiogenesis as a chemo-mechanical surface instability. Sci. Rep. 2016, 6, 22610. [Google Scholar] [CrossRef]
- Phillips, C.M.; Lima, E.A.; Woodall, R.T.; Brock, A.; Yankeelov, T.E. A hybrid model of tumor growth and angiogenesis: In silico experiments. PLoS ONE 2020, 15, e0231137. [Google Scholar] [CrossRef]
- Scianna, M.; Bell, C.; Preziosi, L. A review of mathematical models for the formation of vascular networks. J. Theor. Biol. 2013, 333, 174–209. [Google Scholar] [CrossRef]
- Anderson, A.R.; Chaplain, M.A. Continuous and discrete mathematical models of tumor-induced angiogenesis. Bull. Math. Biol. 1998, 60, 857–899. [Google Scholar] [CrossRef] [PubMed]
- Braile, M.; Marcella, S.; Cristinziano, L.; Galdiero, M.R.; Modestino, L.; Ferrara, A.L.; Varricchi, G.; Marone, G.; Loffredo, S. VEGF-A in cardiomyocytes and heart diseases. Int. J. Mol. Sci. 2020, 21, 5294. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti-and pro-angiogenic therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Saito, N. Conservative upwind finite-element method for a simplified Keller–Segel system modelling chemotaxis. IMA J. Numer. Anal. 2007, 27, 332–365. [Google Scholar] [CrossRef]
- Paweletz, N.; Knierim, M. Tumor-related angiogenesis. Crit. Rev. Oncol. 1989, 9, 197–242. [Google Scholar] [CrossRef]
- Diaz, R.J.; Ali, S.; Qadir, M.G.; De La Fuente, M.I.; Ivan, M.E.; Komotar, R.J. The role of bevacizumab in the treatment of glioblastoma. J. Neuro-Oncol. 2017, 133, 455–467. [Google Scholar] [CrossRef]
- Rosen, L.S.; Jacobs, I.A.; Burkes, R.L. Bevacizumab in colorectal cancer: Current role in treatment and the potential of biosimilars. Target. Oncol. 2017, 12, 599–610. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Levchenko, A.; Iglesias, P.A. Models of eukaryotic gradient sensing: Application to chemotaxis of amoebae and neutrophils. Biophys. J. 2002, 82, 50–63. [Google Scholar] [CrossRef]
- Nakajima, A.; Ishihara, S.; Imoto, D.; Sawai, S. Rectified directional sensing in long-range cell migration. Nat. Commun. 2014, 5, 5367. [Google Scholar] [CrossRef]
- Devreotes, P.N.; Bhattacharya, S.; Edwards, M.; Iglesias, P.A.; Lampert, T.; Miao, Y. Excitable signal transduction networks in directed cell migration. Annu. Rev. Cell Dev. Biol. 2017, 33, 103. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minerva, D.; Othman, N.L.; Nakazawa, T.; Ito, Y.; Yoshida, M.; Goto, A.; Suzuki, T. A New Chemotactic Mechanism Governs Long-Range Angiogenesis Induced by Patching an Arterial Graft into a Vein. Int. J. Mol. Sci. 2022, 23, 11208. https://doi.org/10.3390/ijms231911208
Minerva D, Othman NL, Nakazawa T, Ito Y, Yoshida M, Goto A, Suzuki T. A New Chemotactic Mechanism Governs Long-Range Angiogenesis Induced by Patching an Arterial Graft into a Vein. International Journal of Molecular Sciences. 2022; 23(19):11208. https://doi.org/10.3390/ijms231911208
Chicago/Turabian StyleMinerva, Dhisa, Nuha Loling Othman, Takashi Nakazawa, Yukinobu Ito, Makoto Yoshida, Akiteru Goto, and Takashi Suzuki. 2022. "A New Chemotactic Mechanism Governs Long-Range Angiogenesis Induced by Patching an Arterial Graft into a Vein" International Journal of Molecular Sciences 23, no. 19: 11208. https://doi.org/10.3390/ijms231911208




