NMDA Receptor C-Terminal Domain Signalling in Development, Maturity, and Disease
Abstract
:1. Introduction
2. The Role of the CTD in Developmental and Activity-Dependent Changes in NMDAR Subunit Composition
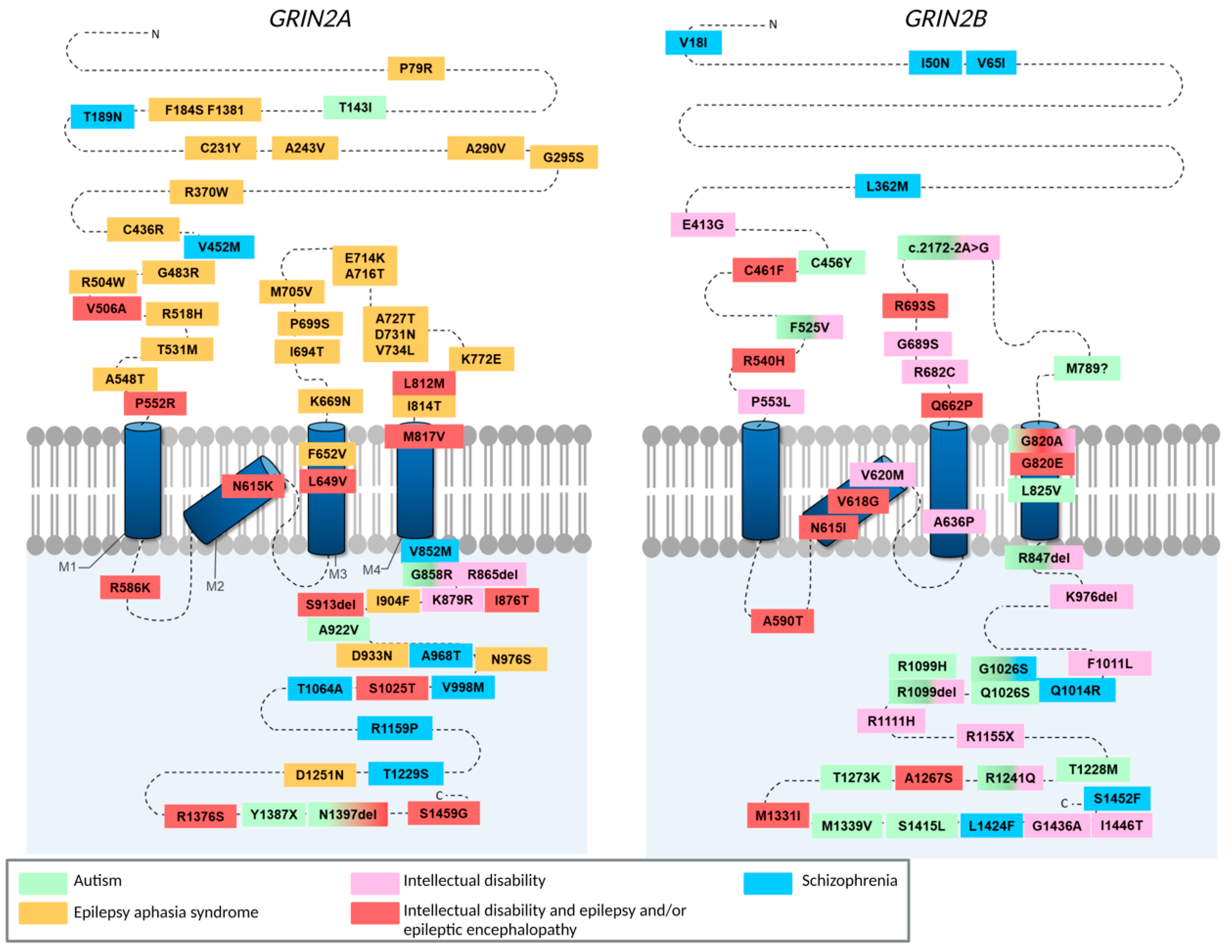
3. GluN2A/2B CTD Mutations Associated with Neurodevelopmental Disorders
4. A Critical Role for CTD Interactions in Acute Excitotoxicity
4.1. GluN2B Mediated Excitotoxicity through PSD95-nNOS Pathway
4.2. The Role of Extrasynaptic Specific Physical and Functional Coupling in Excitotoxicity
4.3. DAPK1 Interactions at CTD2B Do Not Mediate Excitotoxic Cell Death
4.4. Role of GluN2A and GluN2B in Tissue-Type Plasminogen Activator Mediated Neuroprotection
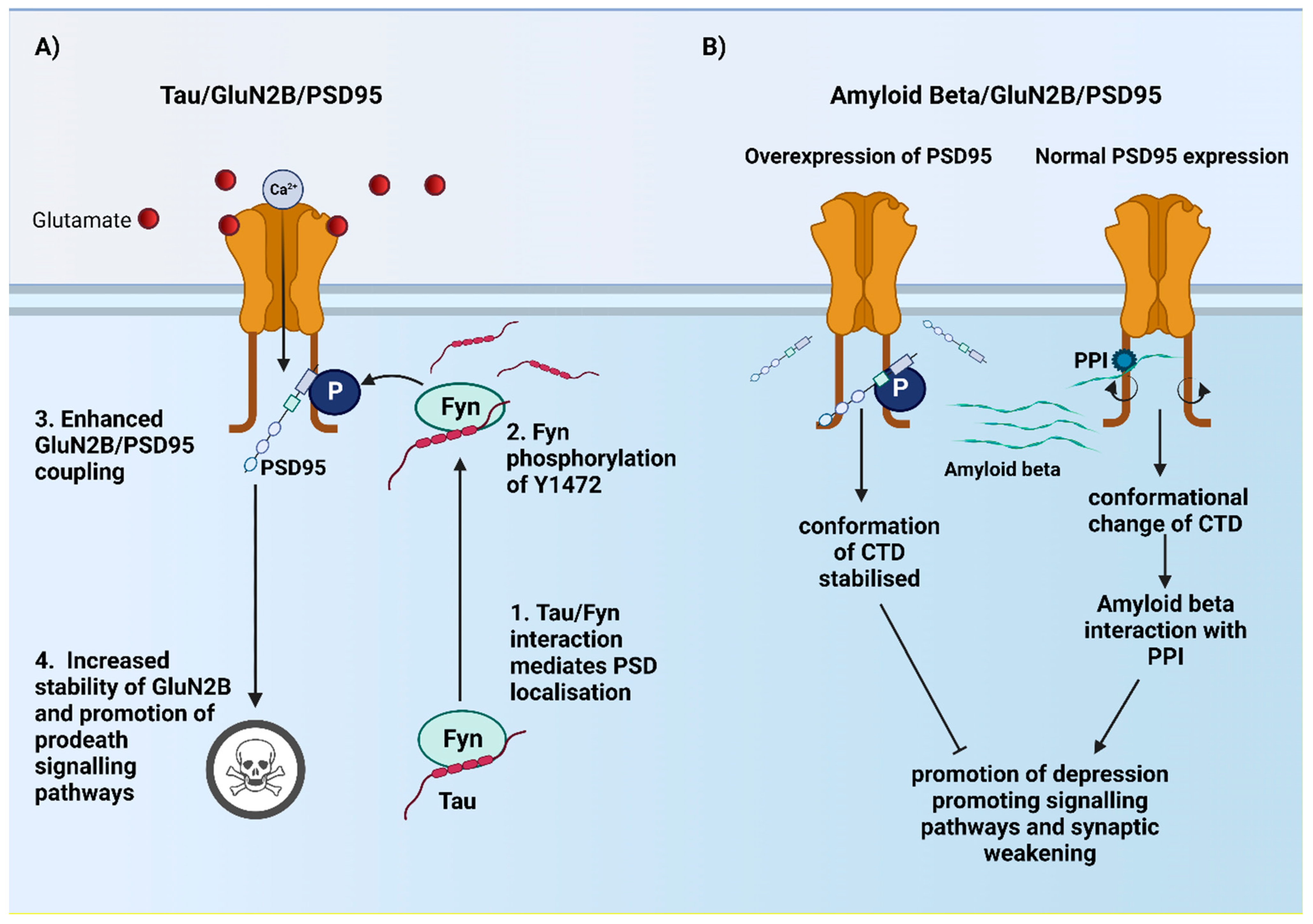
5. Role of CTD2B in Alzheimer’s Disease?
5.1. Role of CTD2B in Ionotropic and Metabotropic Dependent Pathways in AD
5.2. Implications for Astrocytic NMDA Subunits in AD?
6. Contribution of CTD2A and CTD2B in Other Disease Pathologies?
7. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef]
- Wyllie, D.J.; Livesey, M.R.; Hardingham, G.E. Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology 2013, 74, 4–17. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef]
- Parsons, M.P.; Raymond, L.A. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 2014, 82, 279–293. [Google Scholar] [CrossRef]
- Tymianski, M. Emerging mechanisms of disrupted cellular signaling in brain ischemia. Nat. Neurosci. 2011, 14, 1369–1373. [Google Scholar] [CrossRef]
- Vicini, S.; Wang, J.F.; Li, J.H.; Zhu, W.J.; Wang, Y.H.; Luo, J.H.; Wolfe, B.B.; Grayson, D.R. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J. Neurophysiol. 1998, 79, 555–566. [Google Scholar] [CrossRef]
- Monyer, H.; Burnashev, N.; Laurie, D.J.; Sakmann, B.; Seeburg, P.H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 1994, 12, 529–540. [Google Scholar] [CrossRef]
- Collins, M.O.; Grant, S.G. Supramolecular signalling complexes in the nervous system. Subcell. Biochem. 2007, 43, 185–207. [Google Scholar]
- Ryan, T.J.; Emes, R.D.; Grant, S.G.; Komiyama, N.H. Evolution of NMDA receptor cytoplasmic interaction domains: Implications for organisation of synaptic signalling complexes. BMC Neurosci. 2008, 9, 6. [Google Scholar] [CrossRef]
- Ryan, T.J.; Kopanitsa, M.V.; Indersmitten, T.; Nithianantharajah, J.; Afinowi, N.O.; Pettit, C.; Stanford, L.E.; Sprengel, R.; Saksida, L.M.; Bussey, T.J.; et al. Evolution of GluN2A/B cytoplasmic domains diversified vertebrate synaptic plasticity and behavior. Nat. Neurosci. 2013, 16, 25–32. [Google Scholar] [CrossRef]
- Chen, W.S.; Bear, M.F. Activity-dependent regulation of NR2B translation contributes to metaplasticity in mouse visual cortex. Neuropharmacology 2007, 52, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Philpot, B.D.; Espinosa, J.S.; Bear, M.F. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J. Neurosci. 2003, 23, 5583–5588. [Google Scholar] [CrossRef] [PubMed]
- Philpot, B.D.; Weisberg, M.P.; Ramos, M.S.; Sawtell, N.B.; Tang, Y.P.; Tsien, J.Z.; Bear, M.F. Effect of transgenic overexpression of NR2B on NMDA receptor function and synaptic plasticity in visual cortex. Neuropharmacology 2001, 41, 762–770. [Google Scholar] [CrossRef]
- Quinlan, E.M.; Olstein, D.H.; Bear, M.F. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc. Natl. Acad. Sci. USA 1999, 96, 12876–12880. [Google Scholar] [CrossRef]
- Quinlan, E.M.; Philpot, B.D.; Huganir, R.L.; Bear, M.F. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat. Neurosci. 1999, 2, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Clemente, A.; Gray, J.A.; Ogilvie, K.A.; Nicoll, R.A.; Roche, K.W. Activated CaMKII couples GluN2B and casein kinase 2 to control synaptic NMDA receptors. Cell Rep. 2013, 3, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Clemente, A.; Matta, J.A.; Isaac, J.T.; Roche, K.W. Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron 2010, 67, 984–996. [Google Scholar] [CrossRef]
- Chen, B.S.; Gray, J.A.; Sanz-Clemente, A.; Wei, Z.; Thomas, E.V.; Nicoll, R.A.; Roche, K.W. SAP102 mediates synaptic clearance of NMDA receptors. Cell Rep. 2012, 2, 1120–1128. [Google Scholar] [CrossRef]
- Halt, A.R.; Dallapiazza, R.F.; Zhou, Y.; Stein, I.S.; Qian, H.; Juntti, S.; Wojcik, S.; Brose, N.; Silva, A.J.; Hell, J.W. CaMKII binding to GluN2B is critical during memory consolidation. EMBO J. 2012, 31, 1203–1216. [Google Scholar] [CrossRef]
- McKay, S.; Ryan, T.J.; McQueen, J.; Indersmitten, T.; Marwick, K.F.M.; Hasel, P.; Kopanitsa, M.V.; Baxter, P.S.; Martel, M.A.; Kind, P.C.; et al. The Developmental Shift of NMDA Receptor Composition Proceeds Independently of GluN2 Subunit-Specific GluN2 C-Terminal Sequences. Cell Rep. 2018, 25, 841–851.e844. [Google Scholar] [CrossRef]
- Mota Vieira, M.; Nguyen, T.A.; Wu, K.; Badger, J.D., 2nd; Collins, B.M.; Anggono, V.; Lu, W.; Roche, K.W. An Epilepsy-Associated GRIN2A Rare Variant Disrupts CaMKIIalpha Phosphorylation of GluN2A and NMDA Receptor Trafficking. Cell Rep. 2020, 32, 108104. [Google Scholar] [CrossRef] [PubMed]
- Yong, X.L.H.; Zhang, L.; Yang, L.; Chen, X.; Tan, J.Z.A.; Yu, X.; Chandra, M.; Livingstone, E.; Widagdo, J.; Vieira, M.M.; et al. Regulation of NMDA receptor trafficking and gating by activity-dependent CaMKIIalpha phosphorylation of the GluN2A subunit. Cell Rep. 2021, 36, 109338. [Google Scholar] [CrossRef]
- Frank, R.A.; Komiyama, N.H.; Ryan, T.J.; Zhu, F.; O’Dell, T.J.; Grant, S.G. NMDA receptors are selectively partitioned into complexes and supercomplexes during synapse maturation. Nat. Commun. 2016, 7, 11264. [Google Scholar] [CrossRef] [PubMed]
- Edman, S.; McKay, S.; Macdonald, L.J.; Samadi, M.; Livesey, M.R.; Hardingham, G.E.; Wyllie, D.J. TCN 201 selectively blocks GluN2A-containing NMDARs in a GluN1 co-agonist dependent but non-competitive manner. Neuropharmacology 2012, 63, 441–449. [Google Scholar] [CrossRef] [PubMed]
- McKay, S.; Griffiths, N.H.; Butters, P.A.; Thubron, E.B.; Hardingham, G.E.; Wyllie, D.J. Direct pharmacological monitoring of the developmental switch in NMDA receptor subunit composition using TCN 213, a GluN2A-selective, glycine-dependent antagonist. Br. J. Pharm. 2012, 166, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Stroebel, D.; Casado, M.; Paoletti, P. Triheteromeric NMDA receptors: From structure to synaptic physiology. Curr. Opin. Physiol. 2018, 2, 1–12. [Google Scholar] [CrossRef]
- Hoffmann, H.; Gremme, T.; Hatt, H.; Gottmann, K. Synaptic activity-dependent developmental regulation of NMDA receptor subunit expression in cultured neocortical neurons. J. Neurochem. 2000, 75, 1590–1599. [Google Scholar] [CrossRef]
- Ishchenko, Y.; Carrizales, M.G.; Koleske, A.J. Regulation of the NMDA receptor by its cytoplasmic domains: (How) is the tail wagging the dog? Neuropharmacology 2021, 195, 108634. [Google Scholar] [CrossRef] [PubMed]
- Bramswig, N.C.; Ludecke, H.J.; Alanay, Y.; Albrecht, B.; Barthelmie, A.; Boduroglu, K.; Braunholz, D.; Caliebe, A.; Chrzanowska, K.H.; Czeschik, J.C.; et al. Exome sequencing unravels unexpected differential diagnoses in individuals with the tentative diagnosis of Coffin-Siris and Nicolaides-Baraitser syndromes. Hum. Genet. 2015, 134, 553–568. [Google Scholar] [CrossRef]
- Chen, W.; Shieh, C.; Swanger, S.A.; Tankovic, A.; Au, M.; McGuire, M.; Tagliati, M.; Graham, J.M.; Madan-Khetarpal, S.; Traynelis, S.F.; et al. GRIN1 mutation associated with intellectual disability alters NMDA receptor trafficking and function. J. Hum. Genet. 2017, 62, 589–597. [Google Scholar] [CrossRef]
- Dimassi, S.; Labalme, A.; Lesca, G.; Rudolf, G.; Bruneau, N.; Hirsch, E.; Arzimanoglou, A.; Motte, J.; de Saint Martin, A.; Boutry-Kryza, N.; et al. A subset of genomic alterations detected in rolandic epilepsies contains candidate or known epilepsy genes including GRIN2A and PRRT2. Epilepsia 2014, 55, 370–378. [Google Scholar] [CrossRef]
- Endele, S.; Rosenberger, G.; Geider, K.; Popp, B.; Tamer, C.; Stefanova, I.; Milh, M.; Kortum, F.; Fritsch, A.; Pientka, F.K.; et al. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat. Genet. 2010, 42, 1021–1026. [Google Scholar] [CrossRef]
- Firth, H.V.; Richards, S.M.; Bevan, A.P.; Clayton, S.; Corpas, M.; Rajan, D.; Van Vooren, S.; Moreau, Y.; Pettett, R.M.; Carter, N.P. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am. J. Hum. Genet. 2009, 84, 524–533. [Google Scholar] [CrossRef]
- Freunscht, I.; Popp, B.; Blank, R.; Endele, S.; Moog, U.; Petri, H.; Prott, E.C.; Reis, A.; Rubo, J.; Zabel, B.; et al. Behavioral phenotype in five individuals with de novo mutations within the GRIN2B gene. Behav. Brain Funct. 2013, 9, 20. [Google Scholar] [CrossRef]
- Grozeva, D.; Carss, K.; Spasic-Boskovic, O.; Tejada, M.I.; Gecz, J.; Shaw, M.; Corbett, M.; Haan, E.; Thompson, E.; Friend, K.; et al. Targeted Next-Generation Sequencing Analysis of 1,000 Individuals with Intellectual Disability. Hum. Mutat. 2015, 36, 1197–1204. [Google Scholar] [CrossRef]
- Lal, D.; Steinbrucker, S.; Schubert, J.; Sander, T.; Becker, F.; Weber, Y.; Lerche, H.; Thiele, H.; Krause, R.; Lehesjoki, A.E.; et al. Investigation of GRIN2A in common epilepsy phenotypes. Epilepsy Res. 2015, 115, 95–99. [Google Scholar] [CrossRef]
- Lemke, J.R.; Hendrickx, R.; Geider, K.; Laube, B.; Schwake, M.; Harvey, R.J.; James, V.M.; Pepler, A.; Steiner, I.; Hortnagel, K.; et al. GRIN2B mutations in West syndrome and intellectual disability with focal epilepsy. Ann. Neurol. 2014, 75, 147–154. [Google Scholar] [CrossRef]
- Lemke, J.R.; Lal, D.; Reinthaler, E.M.; Steiner, I.; Nothnagel, M.; Alber, M.; Geider, K.; Laube, B.; Schwake, M.; Finsterwalder, K.; et al. Mutations in GRIN2A cause idiopathic focal epilepsy with rolandic spikes. Nat. Genet. 2013, 45, 1067–1072. [Google Scholar] [CrossRef]
- Lesca, G.; Rudolf, G.; Bruneau, N.; Lozovaya, N.; Labalme, A.; Boutry-Kryza, N.; Salmi, M.; Tsintsadze, T.; Addis, L.; Motte, J.; et al. GRIN2A mutations in acquired epileptic aphasia and related childhood focal epilepsies and encephalopathies with speech and language dysfunction. Nat. Genet. 2013, 45, 1061–1066. [Google Scholar] [CrossRef]
- Li, Q.Q.; Chen, J.; Hu, P.; Jia, M.; Sun, J.H.; Feng, H.Y.; Qiao, F.C.; Zang, Y.Y.; Shi, Y.Y.; Chen, G.; et al. Enhancing GluN2A-type NMDA receptors impairs long-term synaptic plasticity and learning and memory. Mol. Psychiatry 2022. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, L.; Yuan, H.; Vieira, M.; Sanz-Clemente, A.; Badger, J.D., 2nd; Lu, W.; Traynelis, S.F.; Roche, K.W. A Rare Variant Identified Within the GluN2B C-Terminus in a Patient with Autism Affects NMDA Receptor Surface Expression and Spine Density. J. Neurosci. 2017, 37, 4093–4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, R.A.; Casals, F.; Gauthier, J.; Hamdan, F.F.; Keebler, J.; Boyko, A.R.; Bustamante, C.D.; Piton, A.M.; Spiegelman, D.; Henrion, E.; et al. A population genetic approach to mapping neurological disorder genes using deep resequencing. PLoS Genet. 2011, 7, e1001318. [Google Scholar] [CrossRef] [PubMed]
- O’Roak, B.J.; Vives, L.; Fu, W.; Egertson, J.D.; Stanaway, I.B.; Phelps, I.G.; Carvill, G.; Kumar, A.; Lee, C.; Ankenman, K.; et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 2012, 338, 1619–1622. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, J.; Guo, H.; Ou, J.; Peng, Y.; Liu, Q.; Shen, Y.; Shi, L.; Liu, Y.; Xiong, Z.; et al. Association of genetic variants of GRIN2B with autism. Sci. Rep. 2015, 5, 8296. [Google Scholar] [CrossRef] [PubMed]
- Platzer, K.; Yuan, H.; Schutz, H.; Winschel, A.; Chen, W.; Hu, C.; Kusumoto, H.; Heyne, H.O.; Helbig, K.L.; Tang, S.; et al. GRIN2B encephalopathy: Novel findings on phenotype, variant clustering, functional consequences and treatment aspects. J. Med. Genet. 2017, 54, 460–470. [Google Scholar] [CrossRef]
- Rauch, A.; Wieczorek, D.; Graf, E.; Wieland, T.; Endele, S.; Schwarzmayr, T.; Albrecht, B.; Bartholdi, D.; Beygo, J.; Di Donato, N.; et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: An exome sequencing study. Lancet 2012, 380, 1674–1682. [Google Scholar] [CrossRef]
- Stessman, H.A.; Xiong, B.; Coe, B.P.; Wang, T.; Hoekzema, K.; Fenckova, M.; Kvarnung, M.; Gerdts, J.; Trinh, S.; Cosemans, N.; et al. Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat. Genet. 2017, 49, 515–526. [Google Scholar] [CrossRef]
- Takasaki, Y.; Koide, T.; Wang, C.; Kimura, H.; Xing, J.; Kushima, I.; Ishizuka, K.; Mori, D.; Sekiguchi, M.; Ikeda, M.; et al. Mutation screening of GRIN2B in schizophrenia and autism spectrum disorder in a Japanese population. Sci. Rep. 2016, 6, 33311. [Google Scholar] [CrossRef]
- Tarabeux, J.; Kebir, O.; Gauthier, J.; Hamdan, F.F.; Xiong, L.; Piton, A.; Spiegelman, D.; Henrion, E.; Millet, B.; S2D team; et al. Rare mutations in N-methyl-D-aspartate glutamate receptors in autism spectrum disorders and schizophrenia. Transl. Psychiatry 2011, 1, e55. [Google Scholar] [CrossRef]
- Venkateswaran, S.; Myers, K.A.; Smith, A.C.; Beaulieu, C.L.; Schwartzentruber, J.A.; Consortium, F.C.; Majewski, J.; Bulman, D.; Boycott, K.M.; Dyment, D.A. Whole-exome sequencing in an individual with severe global developmental delay and intractable epilepsy identifies a novel, de novo GRIN2A mutation. Epilepsia 2014, 55, e75–e79. [Google Scholar] [CrossRef]
- Von Stulpnagel, C.; Ensslen, M.; Moller, R.S.; Pal, D.K.; Masnada, S.; Veggiotti, P.; Piazza, E.; Dreesmann, M.; Hartlieb, T.; Herberhold, T.; et al. Epilepsy in patients with GRIN2A alterations: Genetics, neurodevelopment, epileptic phenotype and response to anticonvulsive drugs. Eur. J. Paediatr. Neurol. 2017, 21, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.J.; Georgieva, L.; Dwyer, S.; Kirov, G.; Owen, M.J.; O’Donovan, M.C. Absence of de novo point mutations in exons of GRIN2B in a large schizophrenia trio sample. Schizophr. Res. 2012, 141, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Keith, R.E.; Azcarate, J.M.; Keith, M.J.; Hung, C.W.; Badakhsh, M.F.; Dumas, T.C. Direct Intracellular Signaling by the Carboxy terminus of NMDA Receptor GluN2 Subunits Regulates Dendritic Morphology in Hippocampal CA1 Pyramidal Neurons. Neuroscience 2019, 396, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Addis, L.; Virdee, J.K.; Vidler, L.R.; Collier, D.A.; Pal, D.K.; Ursu, D. Epilepsy-associated GRIN2A mutations reduce NMDA receptor trafficking and agonist potency—Molecular profiling and functional rescue. Sci. Rep. 2017, 7, 66. [Google Scholar] [CrossRef]
- Cooper, L.N.; Bear, M.F. The BCM theory of synapse modification at 30: Interaction of theory with experiment. Nat. Rev. Neurosci. 2012, 13, 798–810. [Google Scholar] [CrossRef]
- Smith, G.B.; Heynen, A.J.; Bear, M.F. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philos. Trans. R Soc. Lond. B Biol. Sci. 2009, 364, 357–367. [Google Scholar] [CrossRef]
- Hanson, J.E.; Ma, K.; Elstrott, J.; Weber, M.; Saillet, S.; Khan, A.S.; Simms, J.; Liu, B.; Kim, T.A.; Yu, G.Q.; et al. GluN2A NMDA Receptor Enhancement Improves Brain Oscillations, Synchrony, and Cognitive Functions in Dravet Syndrome and Alzheimer’s Disease Models. Cell Rep. 2020, 30, 381–396.e384. [Google Scholar] [CrossRef]
- Acutain, M.F.; Griebler Luft, J.; Vazquez, C.A.; Popik, B.; Cercato, M.C.; Epstein, A.; Salvetti, A.; Jerusalinsky, D.A.; de Oliveira Alvares, L.; Baez, M.V. Reduced Expression of Hippocampal GluN2A-NMDAR Increases Seizure Susceptibility and Causes Deficits in Contextual Memory. Front. Neurosci. 2021, 15, 644100. [Google Scholar] [CrossRef]
- Gorlewicz, A.; Pijet, B.; Orlova, K.; Kaczmarek, L.; Knapska, E. Epileptiform GluN2B-driven excitation in hippocampus as a therapeutic target against temporal lobe epilepsy. Exp. Neurol. 2022, 354, 114087. [Google Scholar] [CrossRef]
- Chen, B.; Feng, B.; Tang, Y.; You, Y.; Wang, Y.; Hou, W.; Hu, W.; Chen, Z. Blocking GluN2B subunits reverses the enhanced seizure susceptibility after prolonged febrile seizures with a wide therapeutic time-window. Exp. Neurol. 2016, 283 Pt A, 29–38. [Google Scholar] [CrossRef]
- Mares, P.; Kozlova, L.; Mikulecka, A.; Kubova, H. The GluN2B-Selective Antagonist Ro 25-6981 Is Effective against PTZ-Induced Seizures and Safe for Further Development in Infantile Rats. Pharmaceutics 2021, 13, 1482. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.Q.; Chung, Y.H.; Shin, E.J.; Tran, T.V.; Jeong, J.H.; Jang, C.G.; Nah, S.Y.; Yamada, K.; Nabeshima, T.; Kim, H.C. MK-801, but not naloxone, attenuates high-dose dextromethorphan-induced convulsive behavior: Possible involvement of the GluN2B receptor. Toxicol. Appl. Pharm. 2017, 334, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Amador, A.; Bostick, C.D.; Olson, H.; Peters, J.; Camp, C.R.; Krizay, D.; Chen, W.; Han, W.; Tang, W.; Kanber, A.; et al. Modelling and treating GRIN2A developmental and epileptic encephalopathy in mice. Brain 2020, 143, 2039–2057. [Google Scholar] [CrossRef]
- Benke, T.A.; Park, K.; Krey, I.; Camp, C.R.; Song, R.; Ramsey, A.J.; Yuan, H.; Traynelis, S.F.; Lemke, J. Clinical and therapeutic significance of genetic variation in the GRIN gene family encoding NMDARs. Neuropharmacology 2021, 199, 108805. [Google Scholar] [CrossRef] [PubMed]
- Epi, P.M.C. A roadmap for precision medicine in the epilepsies. Lancet Neurol. 2015, 14, 1219–1228. [Google Scholar]
- Han, W.; Yuan, H.; Allen, J.P.; Kim, S.; Shaulsky, G.H.; Perszyk, R.E.; Traynelis, S.F.; Myers, S.J. Opportunities for Precision Treatment of GRIN2A and GRIN2B Gain-of-Function Variants in Triheteromeric N-Methyl-D-Aspartate Receptors. J. Pharm. Exp. Ther. 2022, 381, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Strehlow, V.; Rieubland, C.; Gallati, S.; Kim, S.; Myers, S.J.; Peterson, V.; Ramsey, A.J.; Teuscher, D.D.; Traynelis, S.F.; Lemke, J.R. Compound-heterozygous GRIN2A null variants associated with severe developmental and epileptic encephalopathy. Epilepsia 2022, 1–6. [Google Scholar] [CrossRef]
- Sapkota, K.; Dore, K.; Tang, K.; Irvine, M.; Fang, G.; Burnell, E.S.; Malinow, R.; Jane, D.E.; Monaghan, D.T. The NMDA receptor intracellular C-terminal domains reciprocally interact with allosteric modulators. Biochem. Pharm. 2019, 159, 140–153. [Google Scholar] [CrossRef]
- Olney, J.W. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 1969, 164, 719–721. [Google Scholar] [CrossRef]
- Choi, D.W. Ionic dependence of glutamate neurotoxicity. J. Neurosci. 1987, 7, 369–379. [Google Scholar] [CrossRef]
- Tymianski, M.; Charlton, M.P.; Carlen, P.L.; Tator, C.H. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J. Neurosci. 1993, 13, 2085–2104. [Google Scholar] [CrossRef] [PubMed]
- Sattler, R.; Charlton, M.P.; Hafner, M.; Tymianski, M. Distinct influx pathways, not calcium load, determine neuronal vulnerability to calcium neurotoxicity. J. Neurochem. 1998, 71, 2349–2364. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Bading, H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003, 26, 81–89. [Google Scholar] [CrossRef]
- Lipton, S.A.; Kater, S.B. Neurotransmitter regulation of neuronal outgrowth, plasticity and survival. Trends Neurosci. 1989, 12, 265–270. [Google Scholar] [CrossRef]
- Weilinger, N.L.; Lohman, A.W.; Rakai, B.D.; Ma, E.M.; Bialecki, J.; Maslieieva, V.; Rilea, T.; Bandet, M.V.; Ikuta, N.T.; Scott, L.; et al. Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat. Neurosci. 2016, 19, 432–442. [Google Scholar]
- Chen, M.; Lu, T.J.; Chen, X.J.; Zhou, Y.; Chen, Q.; Feng, X.Y.; Xu, L.; Duan, W.H.; Xiong, Z.Q. Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke 2008, 39, 3042–3048. [Google Scholar] [CrossRef]
- Zheng, M.; Liao, M.; Cui, T.; Tian, H.; Fan, D.S.; Wan, Q. Regulation of nuclear TDP-43 by NR2A-containing NMDA receptors and PTEN. J. Cell Sci. 2012, 125 Pt 6, 1556–1567. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Fukunaga, Y.; Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002, 5, 405–414. [Google Scholar] [CrossRef]
- Chang, N.; Li, L.; Hu, R.; Shan, Y.; Liu, B.; Li, L.; Wang, H.; Feng, H.; Wang, D.; Cheung, C.; et al. Differential regulation of NMDA receptor function by DJ-1 and PINK1. Aging Cell 2010, 9, 837–850. [Google Scholar] [CrossRef]
- Bell, K.F.; Hardingham, G.E. The influence of synaptic activity on neuronal health. Curr. Opin. Neurobiol. 2011, 21, 299–305. [Google Scholar] [CrossRef]
- Hagenston, A.M.; Bading, H. Calcium signaling in synapse-to-nucleus communication. Cold Spring Harb. Perspect. Biol. 2011, 3, a004564. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Bading, H. Nuclear calcium: A key regulator of gene expression. Biometals 1998, 11, 345–358. [Google Scholar] [CrossRef]
- McKenzie, G.J.; Stevenson, P.; Ward, G.; Papadia, S.; Bading, H.; Chawla, S.; Privalsky, M.; Hardingham, G.E. Nuclear Ca2+ and CaM kinase IV specify hormonal- and Notch-responsiveness. J. Neurochem. 2005, 93, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Soriano, F.X.; Hardingham, G.E. Compartmentalized NMDA receptor signalling to survival and death. J. Physiol. 2007, 584, 381–387. [Google Scholar] [CrossRef]
- Wahl, A.S.; Buchthal, B.; Rode, F.; Bomholt, S.F.; Freitag, H.E.; Hardingham, G.E.; Ronn, L.C.; Bading, H. Hypoxic/ischemic conditions induce expression of the putative pro-death gene Clca1 via activation of extrasynaptic N-methyl-D-aspartate receptors. Neuroscience 2009, 158, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Martel, M.A.; Wyllie, D.J.; Hardingham, G.E. In developing hippocampal neurons, NR2B-containing N-methyl-D-aspartate receptors (NMDARs) can mediate signaling to neuronal survival and synaptic potentiation, as well as neuronal death. Neuroscience 2009, 158, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Papadia, S.; Soriano, F.X.; Leveille, F.; Martel, M.A.; Dakin, K.A.; Hansen, H.H.; Kaindl, A.; Sifringer, M.; Fowler, J.; Stefovska, V.; et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat. Neurosci. 2008, 11, 476–487. [Google Scholar] [CrossRef]
- Gupta, K.; Hardingham, G.E.; Chandran, S. NMDA receptor-dependent glutamate excitotoxicity in human embryonic stem cell-derived neurons. Neurosci. Lett. 2013, 543, 95–100. [Google Scholar] [CrossRef]
- Puddifoot, C.; Martel, M.A.; Soriano, F.X.; Camacho, A.; Vidal-Puig, A.; Wyllie, D.J.; Hardingham, G.E. PGC-1alpha negatively regulates extrasynaptic NMDAR activity and excitotoxicity. J. Neurosci. 2012, 32, 6995–7000. [Google Scholar] [CrossRef]
- Martel, M.A.; Ryan, T.J.; Bell, K.F.; Fowler, J.H.; McMahon, A.; Al-Mubarak, B.; Komiyama, N.H.; Horsburgh, K.; Kind, P.C.; Grant, S.G.; et al. The subtype of GluN2 C-terminal domain determines the response to excitotoxic insults. Neuron 2012, 74, 543–556. [Google Scholar] [CrossRef]
- Aarts, M.; Liu, Y.; Liu, L.; Besshoh, S.; Arundine, M.; Gurd, J.W.; Wang, Y.T.; Salter, M.W.; Tymianski, M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science 2002, 298, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Shin, K.S.; Ryu, J.H.; Kang, K.; Kim, J.; Ahn, H.; Huh, Y. The inhibition of nitric oxide synthase enhances PSA-NCAM expression and CREB phosphorylation in the rat hippocampus. Neuroreport 2004, 15, 231–234. [Google Scholar] [CrossRef]
- Zhu, X.J.; Hua, Y.; Jiang, J.; Zhou, Q.G.; Luo, C.X.; Han, X.; Lu, Y.M.; Zhu, D.Y. Neuronal nitric oxide synthase-derived nitric oxide inhibits neurogenesis in the adult dentate gyrus by down-regulating cyclic AMP response element binding protein phosphorylation. Neuroscience 2006, 141, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.W. Glutamate-based therapeutic approaches: Clinical trials with NMDA antagonists. Curr. Opin. Pharm. 2006, 6, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Teves, L.; Tymianski, M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature 2012, 483, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.D.; Goyal, M.; Menon, B.K.; Nogueira, R.G.; McTaggart, R.A.; Demchuk, A.M.; Poppe, A.Y.; Buck, B.H.; Field, T.S.; Dowlatshahi, D.; et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): A multicentre, double-blind, randomised controlled trial. Lancet 2020, 395, 878–887. [Google Scholar] [CrossRef]
- Marko, M.; Cimflova, P.; Poppe, A.Y.; Kashani, N.; Singh, N.; Ospel, J.; Mayank, A.; van Adel, B.; McTaggart, R.A.; Nogueira, R.G.; et al. Management and outcome of patients with acute ischemic stroke and tandem carotid occlusion in the ESCAPE-NA1 trial. J. Neurointerv. Surg. 2022, 14, 429–433. [Google Scholar] [CrossRef]
- Yan, J.; Bengtson, C.P.; Buchthal, B.; Hagenston, A.M.; Bading, H. Coupling of NMDA receptors and TRPM4 guides discovery of unconventional neuroprotectants. Science 2020, 370, eaay3302. [Google Scholar] [CrossRef]
- Zong, P.; Feng, J.; Yue, Z.; Li, Y.; Wu, G.; Sun, B.; He, Y.; Miller, B.; Yu, A.S.; Su, Z.; et al. Functional coupling of TRPM2 and extrasynaptic NMDARs exacerbates excitotoxicity in ischemic brain injury. Neuron 2022, 110, 1944–1958.e1948. [Google Scholar] [CrossRef]
- Alim, I.; Teves, L.; Li, R.; Mori, Y.; Tymianski, M. Modulation of NMDAR subunit expression by TRPM2 channels regulates neuronal vulnerability to ischemic cell death. J. Neurosci. 2013, 33, 17264–17277. [Google Scholar] [CrossRef]
- Lan, J.Y.; Skeberdis, V.A.; Jover, T.; Grooms, S.Y.; Lin, Y.; Araneda, R.C.; Zheng, X.; Bennett, M.V.; Zukin, R.S. Protein kinase C modulates NMDA receptor trafficking and gating. Nat. Neurosci. 2001, 4, 382–390. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, L.; Wang, A.P.; Bennett, M.V.; Zukin, R.S. Protein kinase C potentiation of N-methyl-D-aspartate receptor activity is not mediated by phosphorylation of N-methyl-D-aspartate receptor subunits. Proc. Natl. Acad. Sci. USA 1999, 96, 15262–15267. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Xu, X.; Peng, L.; Zhong, X.; Zhang, W.; Soundarapandian, M.M.; Balel, C.; Wang, M.; Jia, N.; Zhang, W.; et al. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell 2010, 140, 222–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQueen, J.; Ryan, T.J.; McKay, S.; Marwick, K.; Baxter, P.; Carpanini, S.M.; Wishart, T.M.; Gillingwater, T.H.; Manson, J.C.; Wyllie, D.J.A.; et al. Pro-death NMDA receptor signaling is promoted by the GluN2B C-terminus independently of Dapk1. Elife 2017, 6, e17161. [Google Scholar] [CrossRef]
- Tullis, J.E.; Buonarati, O.R.; Coultrap, S.J.; Bourke, A.M.; Tiemeier, E.L.; Kennedy, M.J.; Herson, P.S.; Bayer, K.U. GluN2B S1303 phosphorylation by CaMKII or DAPK1: No indication for involvement in ischemia or LTP. iScience 2021, 24, 103214. [Google Scholar] [CrossRef] [PubMed]
- Buonarati, O.R.; Cook, S.G.; Goodell, D.J.; Chalmers, N.E.; Rumian, N.L.; Tullis, J.E.; Restrepo, S.; Coultrap, S.J.; Quillinan, N.; Herson, P.S.; et al. CaMKII versus DAPK1 Binding to GluN2B in Ischemic Neuronal Cell Death after Resuscitation from Cardiac Arrest. Cell Rep. 2020, 30, 1–8.e4. [Google Scholar] [CrossRef]
- Marquez-Jurado, S.; Diaz-Colunga, J.; das Neves, R.P.; Martinez-Lorente, A.; Almazan, F.; Guantes, R.; Iborra, F.J. Mitochondrial levels determine variability in cell death by modulating apoptotic gene expression. Nat. Commun. 2018, 9, 389. [Google Scholar] [CrossRef]
- Spencer, S.L.; Sorger, P.K. Measuring and modeling apoptosis in single cells. Cell 2011, 144, 926–939. [Google Scholar] [CrossRef]
- Khan, Z.A.; Sumsuzzman, D.M.; Choi, J.; Hong, Y. Neurodegenerative effect of DAPK1 after cerebral hypoxia-ischemia is associated with its post-transcriptional and signal transduction regulations: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 76, 101593. [Google Scholar] [CrossRef]
- Lesept, F.; Chevilley, A.; Jezequel, J.; Ladepeche, L.; Macrez, R.; Aimable, M.; Lenoir, S.; Bertrand, T.; Rubrecht, L.; Galea, P.; et al. Tissue-type plasminogen activator controls neuronal death by raising surface dynamics of extrasynaptic NMDA receptors. Cell Death Dis. 2016, 7, e2466. [Google Scholar] [CrossRef]
- Wu, F.; Echeverry, R.; Wu, J.; An, J.; Haile, W.B.; Cooper, D.S.; Catano, M.; Yepes, M. Tissue-type plasminogen activator protects neurons from excitotoxin-induced cell death via activation of the ERK1/2-CREB-ATF3 signaling pathway. Mol. Cell Neurosci. 2013, 52, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wu, J.; Nicholson, A.D.; Echeverry, R.; Haile, W.B.; Catano, M.; An, J.; Lee, A.K.; Duong, D.; Dammer, E.B.; et al. Tissue-type plasminogen activator regulates the neuronal uptake of glucose in the ischemic brain. J. Neurosci. 2012, 32, 9848–9858. [Google Scholar] [CrossRef]
- Harris, A.Z.; Pettit, D.L. Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. J. Physiol. 2007, 584 Pt 2, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Petralia, R.S.; Wang, Y.X.; Hua, F.; Yi, Z.; Zhou, A.; Ge, L.; Stephenson, F.A.; Wenthold, R.J. Organization of NMDA receptors at extrasynaptic locations. Neuroscience 2010, 167, 68–87. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.G.; Miller, A.J.; Westbrook, G.L. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J. Neurophysiol. 2006, 95, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Hedou, E.; Douceau, S.; Chevilley, A.; Varangot, A.; Thiebaut, A.M.; Triniac, H.; Bardou, I.; Ali, C.; Maillasson, M.; Crepaldi, T.; et al. Two-Chains Tissue Plasminogen Activator Unifies Met and NMDA Receptor Signalling to Control Neuronal Survival. Int. J. Mol. Sci. 2021, 22, 13483. [Google Scholar] [CrossRef]
- Fuller, S.; Steele, M.; Munch, G. Activated astroglia during chronic inflammation in Alzheimer’s disease—Do they neglect their neurosupportive roles? Mutat. Res. 2010, 690, 40–49. [Google Scholar] [CrossRef]
- Ong, W.Y.; Tanaka, K.; Dawe, G.S.; Ittner, L.M.; Farooqui, A.A. Slow excitotoxicity in Alzheimer’s disease. J. Alzheimers Dis. 2013, 35, 643–668. [Google Scholar] [CrossRef]
- Talantova, M.; Sanz-Blasco, S.; Zhang, X.; Xia, P.; Akhtar, M.W.; Okamoto, S.; Dziewczapolski, G.; Nakamura, T.; Cao, G.; Pratt, A.E.; et al. Abeta induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc. Natl. Acad. Sci. USA 2013, 110, E2518–E2527. [Google Scholar] [CrossRef]
- Bordji, K.; Becerril-Ortega, J.; Nicole, O.; Buisson, A. Activation of extrasynaptic, but not synaptic, NMDA receptors modifies amyloid precursor protein expression pattern and increases amyloid-ss production. J. Neurosci. 2010, 30, 15927–15942. [Google Scholar] [CrossRef]
- Hoey, S.E.; Williams, R.J.; Perkinton, M.S. Synaptic NMDA receptor activation stimulates alpha-secretase amyloid precursor protein processing and inhibits amyloid-beta production. J. Neurosci. 2009, 29, 4442–4460. [Google Scholar] [CrossRef] [PubMed]
- Dore, K.; Aow, J.; Malinow, R. The Emergence of NMDA Receptor Metabotropic Function: Insights from Imaging. Front. Synaptic Neurosci. 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Aow, J.; Dore, K.; Malinow, R. Conformational signaling required for synaptic plasticity by the NMDA receptor complex. Proc. Natl. Acad. Sci. USA 2015, 112, 14711–14716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dore, K.; Carrico, Z.; Alfonso, S.; Marino, M.; Koymans, K.; Kessels, H.W.; Malinow, R. PSD-95 protects synapses from beta-amyloid. Cell Rep. 2021, 35, 109194. [Google Scholar] [CrossRef]
- Birnbaum, J.H.; Bali, J.; Rajendran, L.; Nitsch, R.M.; Tackenberg, C. Calcium flux-independent NMDA receptor activity is required for Abeta oligomer-induced synaptic loss. Cell Death Dis. 2015, 6, e1791. [Google Scholar] [CrossRef]
- Kessels, H.W.; Nabavi, S.; Malinow, R. Metabotropic NMDA receptor function is required for beta-amyloid-induced synaptic depression. Proc. Natl. Acad. Sci. USA 2013, 110, 4033–4038. [Google Scholar] [CrossRef]
- Ittner, L.M.; Ke, Y.D.; Delerue, F.; Bi, M.; Gladbach, A.; van Eersel, J.; Wolfing, H.; Chieng, B.C.; Christie, M.J.; Napier, I.A.; et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 2010, 142, 387–397. [Google Scholar] [CrossRef]
- Lalo, U.; Pankratov, Y.; Kirchhoff, F.; North, R.A.; Verkhratsky, A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J. Neurosci. 2006, 26, 2673–2683. [Google Scholar] [CrossRef]
- Schipke, C.G.; Ohlemeyer, C.; Matyash, M.; Nolte, C.; Kettenmann, H.; Kirchhoff, F. Astrocytes of the mouse neocortex express functional N-methyl-D-aspartate receptors. FASEB J. 2001, 15, 1270–1272. [Google Scholar] [CrossRef]
- Alsaad, H.A.; DeKorver, N.W.; Mao, Z.; Dravid, S.M.; Arikkath, J.; Monaghan, D.T. In the Telencephalon, GluN2C NMDA Receptor Subunit mRNA is Predominately Expressed in Glial Cells and GluN2D mRNA in Interneurons. Neurochem. Res 2019, 44, 61–77. [Google Scholar] [CrossRef]
- Ravikrishnan, A.; Gandhi, P.J.; Shelkar, G.P.; Liu, J.; Pavuluri, R.; Dravid, S.M. Region-specific Expression of NMDA Receptor GluN2C Subunit in Parvalbumin-Positive Neurons and Astrocytes: Analysis of GluN2C Expression using a Novel Reporter Model. Neuroscience 2018, 380, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.H.Y.; Walby, J.L.; Palpagama, T.H.; Turner, C.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Glutamatergic receptor expression changes in the Alzheimer’s disease hippocampus and entorhinal cortex. Brain Pathol. 2021, 31, e13005. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Blasco, D.; Santofimia-Castano, P.; Gonzalez, A.; Almeida, A.; Bolanos, J.P. Astrocyte NMDA receptors’ activity sustains neuronal survival through a Cdk5-Nrf2 pathway. Cell Death Differ. 2015, 22, 1877–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obara-Michlewska, M.; Ruszkiewicz, J.; Zielinska, M.; Verkhratsky, A.; Albrecht, J. Astroglial NMDA receptors inhibit expression of Kir4.1 channels in glutamate-overexposed astrocytes in vitro and in the brain of rats with acute liver failure. Neurochem. Int. 2015, 88, 20–25. [Google Scholar] [CrossRef]
- Skowronska, K.; Obara-Michlewska, M.; Czarnecka, A.; Dabrowska, K.; Zielinska, M.; Albrecht, J. Persistent Overexposure to N-Methyl-D-Aspartate (NMDA) Calcium-Dependently Downregulates Glutamine Synthetase, Aquaporin 4, and Kir4.1 Channel in Mouse Cortical Astrocytes. Neurotox. Res. 2019, 35, 271–280. [Google Scholar] [CrossRef]
- Paille, V.; Picconi, B.; Bagetta, V.; Ghiglieri, V.; Sgobio, C.; Di Filippo, M.; Viscomi, M.T.; Giampa, C.; Fusco, F.R.; Gardoni, F.; et al. Distinct levels of dopamine denervation differentially alter striatal synaptic plasticity and NMDA receptor subunit composition. J. Neurosci. 2010, 30, 14182–14193. [Google Scholar] [CrossRef]
- Gardoni, F.; Picconi, B.; Ghiglieri, V.; Polli, F.; Bagetta, V.; Bernardi, G.; Cattabeni, F.; Di Luca, M.; Calabresi, P. A critical interaction between NR2B and MAGUK in L-DOPA induced dyskinesia. J. Neurosci. 2006, 26, 2914–2922. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.B.; Sharma, S.; Surmeier, D.J.; Eberwine, J.H.; Chesselet, M.F. Multiplicity of glutamate receptor subunits in single striatal neurons: An RNA amplification study. Mol. Pharm. 1996, 49, 852–859. [Google Scholar]
- Landwehrmeyer, G.B.; Standaert, D.G.; Testa, C.M.; Penney, J.B., Jr.; Young, A.B. NMDA receptor subunit mRNA expression by projection neurons and interneurons in rat striatum. J. Neurosci. 1995, 15 Pt 2, 5297–5307. [Google Scholar] [CrossRef]
- Rigby, M.; Le Bourdelles, B.; Heavens, R.P.; Kelly, S.; Smith, D.; Butler, A.; Hammans, R.; Hills, R.; Xuereb, J.H.; Hill, R.G.; et al. The messenger RNAs for the N-methyl-D-aspartate receptor subunits show region-specific expression of different subunit composition in the human brain. Neuroscience 1996, 73, 429–447. [Google Scholar] [CrossRef]
- Standaert, D.G.; Friberg, I.K.; Landwehrmeyer, G.B.; Young, A.B.; Penney, J.B., Jr. Expression of NMDA glutamate receptor subunit mRNAs in neurochemically identified projection and interneurons in the striatum of the rat. Brain Res. Mol. Brain Res. 1999, 64, 11–23. [Google Scholar] [CrossRef]
- Fan, M.M.; Fernandes, H.B.; Zhang, L.Y.; Hayden, M.R.; Raymond, L.A. Altered NMDA receptor trafficking in a yeast artificial chromosome transgenic mouse model of Huntington’s disease. J. Neurosci. 2007, 27, 3768–3779. [Google Scholar] [CrossRef] [PubMed]
- Milnerwood, A.J.; Gladding, C.M.; Pouladi, M.A.; Kaufman, A.M.; Hines, R.M.; Boyd, J.D.; Ko, R.W.; Vasuta, O.C.; Graham, R.K.; Hayden, M.R.; et al. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington’s disease mice. Neuron 2010, 65, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Pouladi, M.A.; Talantova, M.; Yao, D.; Xia, P.; Ehrnhoefer, D.E.; Zaidi, R.; Clemente, A.; Kaul, M.; Graham, R.K.; et al. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat. Med. 2009, 15, 1407–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javitt, D.C. Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia. Hillside J. Clin. Psychiatry 1987, 9, 12–35. [Google Scholar]
- Javitt, D.C.; Zukin, S.R. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry 1991, 148, 1301–1308. [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, W.; Axerio-Cilies, P.; Wang, Y.T. NMDARs in Cell Survival and Death: Implications in Stroke Pathogenesis and Treatment. Trends Mol. Med. 2020, 26, 533–551. [Google Scholar] [CrossRef]
- Bai, N.; Aida, T.; Yanagisawa, M.; Katou, S.; Sakimura, K.; Mishina, M.; Tanaka, K. NMDA receptor subunits have different roles in NMDA-induced neurotoxicity in the retina. Mol. Brain 2013, 6, 34. [Google Scholar] [CrossRef]
- Bai, N.; Hayashi, H.; Aida, T.; Namekata, K.; Harada, T.; Mishina, M.; Tanaka, K. Dock3 interaction with a glutamate-receptor NR2D subunit protects neurons from excitotoxicity. Mol. Brain 2013, 6, 22. [Google Scholar] [CrossRef]
- Jullienne, A.; Montagne, A.; Orset, C.; Lesept, F.; Jane, D.E.; Monaghan, D.T.; Maubert, E.; Vivien, D.; Ali, C. Selective inhibition of GluN2D-containing N-methyl-D-aspartate receptors prevents tissue plasminogen activator-promoted neurotoxicity both in vitro and in vivo. Mol. Neurodegener. 2011, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Hubalkova, P.; Ladislav, M.; Vyklicky, V.; Smejkalova, T.; Hrcka Krausova, B.; Kysilov, B.; Krusek, J.; Naimova, Z.; Korinek, M.; Chodounska, H.; et al. Palmitoylation Controls NMDA Receptor Function and Steroid Sensitivity. J. Neurosci. 2021, 41, 2119–2134. [Google Scholar] [CrossRef] [PubMed]

| Model | Treatment | Findings | Study |
|---|---|---|---|
| HEK293 | Coexpression of GluN1/GluN2B and constitutively active DAPK1 | ↑ GluN1/GluN2B peak amplitude of receptor currents ↑ S1303 phosphorylation | [103] |
| DAPK1+/+ cortical neurons | OGD | ↑ S1303 phosphorylation ↑ amplitude of extrasynaptic NMDA mediated currents and Ca2+ transients | [103] |
| DAPK1+/+ cortical neurons | Bath application of NMDA (20 µM and 50 µM) and OGD | No change in s1303 phosphorylation | [104] |
| DAPK1−/− cortical neurons | OGD | No change in s1301 phosphorylation | [103] |
| DAPK1−/− in vivo | MCAO | ↓ infarct volume as measured by TTC staining | [103] |
| DAPK1−/− in vivo | MCAO | No change to infarct volume as measured by H-E staining | [104] |
| DAPK1+/+ in vivo | CA/CPR | No change in s1303 phosphorylation | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haddow, K.; Kind, P.C.; Hardingham, G.E. NMDA Receptor C-Terminal Domain Signalling in Development, Maturity, and Disease. Int. J. Mol. Sci. 2022, 23, 11392. https://doi.org/10.3390/ijms231911392
Haddow K, Kind PC, Hardingham GE. NMDA Receptor C-Terminal Domain Signalling in Development, Maturity, and Disease. International Journal of Molecular Sciences. 2022; 23(19):11392. https://doi.org/10.3390/ijms231911392
Chicago/Turabian StyleHaddow, Kirsty, Peter C. Kind, and Giles E. Hardingham. 2022. "NMDA Receptor C-Terminal Domain Signalling in Development, Maturity, and Disease" International Journal of Molecular Sciences 23, no. 19: 11392. https://doi.org/10.3390/ijms231911392
APA StyleHaddow, K., Kind, P. C., & Hardingham, G. E. (2022). NMDA Receptor C-Terminal Domain Signalling in Development, Maturity, and Disease. International Journal of Molecular Sciences, 23(19), 11392. https://doi.org/10.3390/ijms231911392





