Prenatal SAMe Treatment Induces Changes in Brain Monoamines and in the Expression of Genes Related to Monoamine Metabolism in a Mouse Model of Social Hierarchy and Depression, Probably via an Epigenetic Mechanism
Abstract
:1. Introduction
2. Results
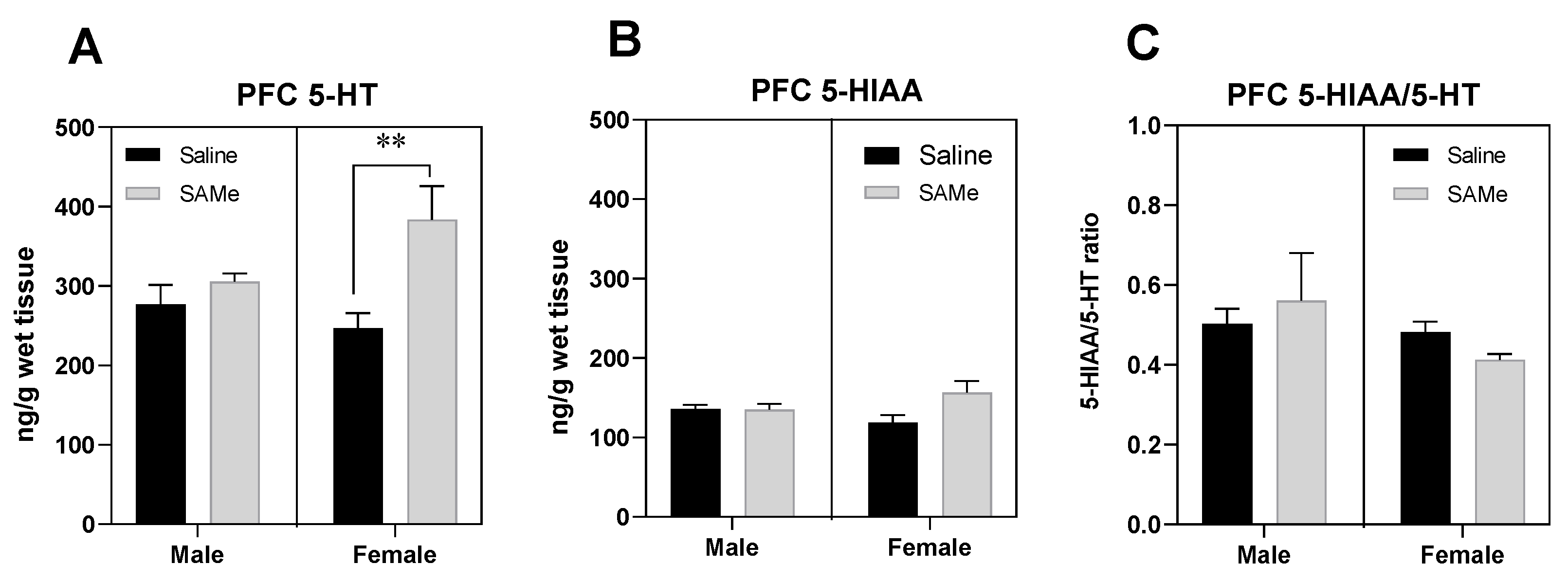
2.1. SAMe Effect on Serotonin Metabolism in the PFC
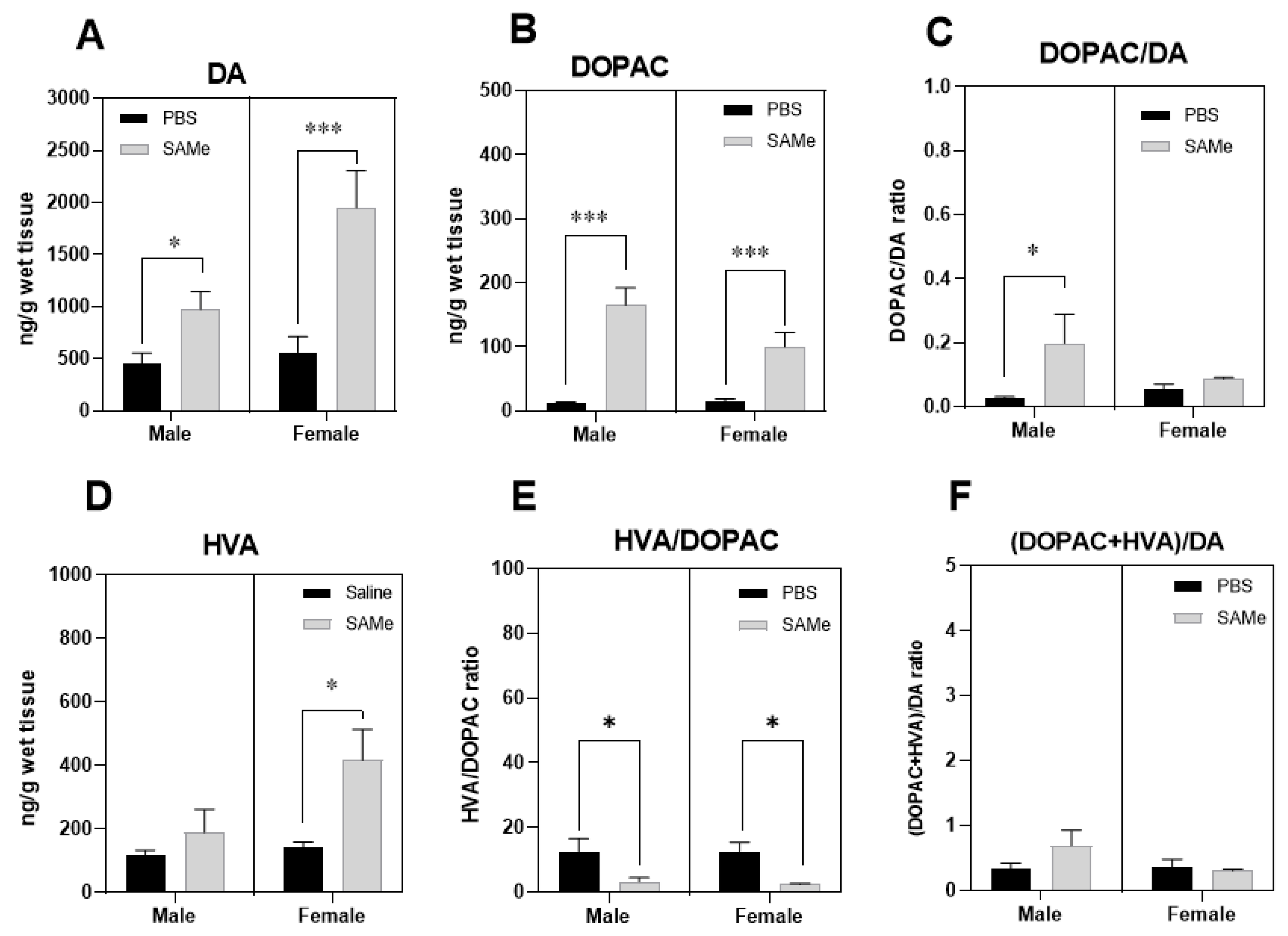
2.2. SAMe Effect on Dopamine, Dopamine Metabolites and of Metabolites/Dopamine Ratio in the PFC
2.3. SAMe Effect on Norepinephrine Metabolism in the PFC
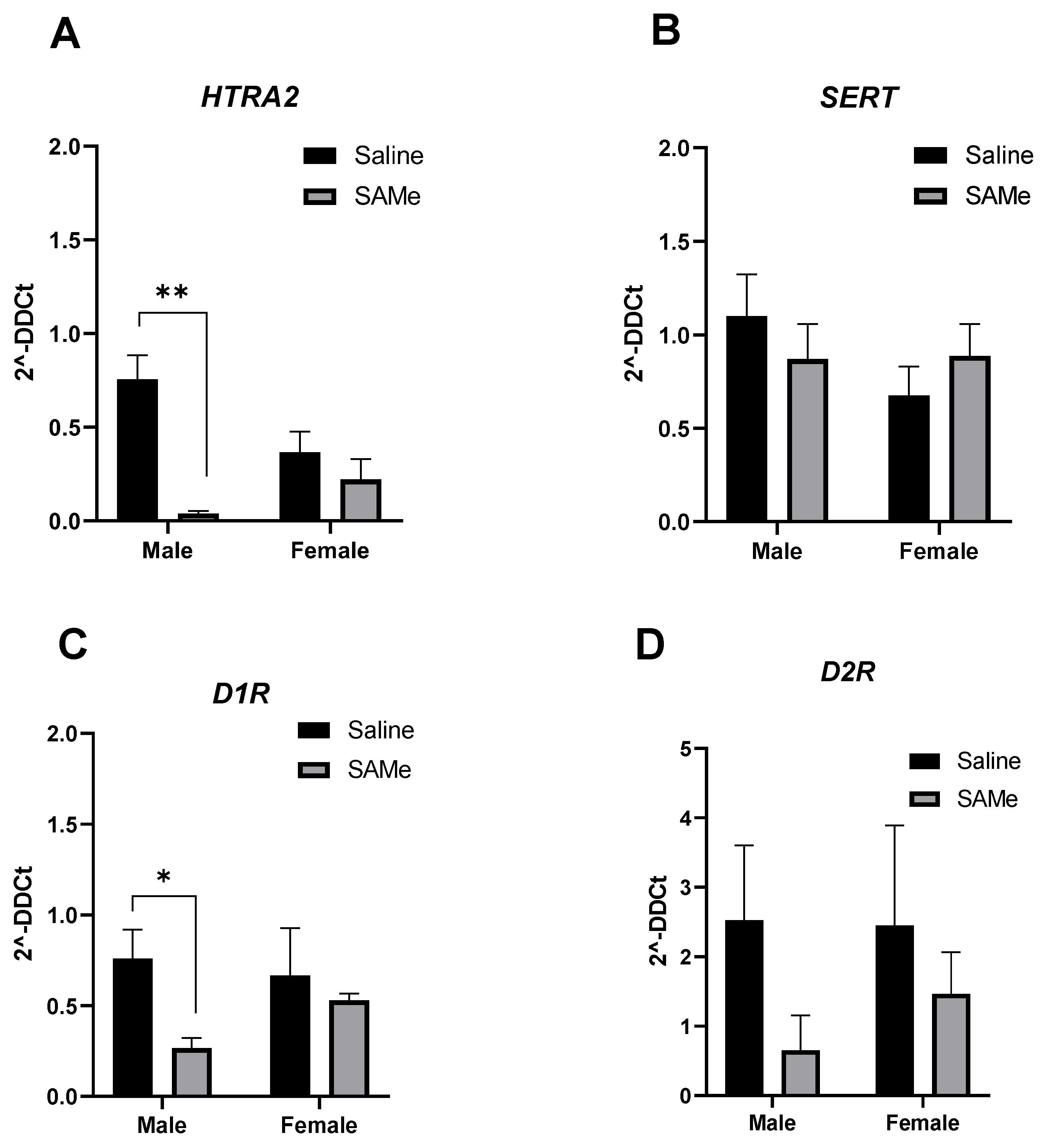
2.4. SAMe Effects on Tph2, Mao-a, Mao-b and Compt Gene Expression in the PFC
2.5. SAMe Treatment Effects on Htr2a, Sert, D1R and D2R Gene Expression in the PFC
3. Discussion
3.1. SAMe Effects on the Serotonergic Metabolism
3.2. SAMe Effects on Dopaminergic Metabolism
3.3. SAMe Effect on Gene Expression Are Possibly via Epigenetic Modulation
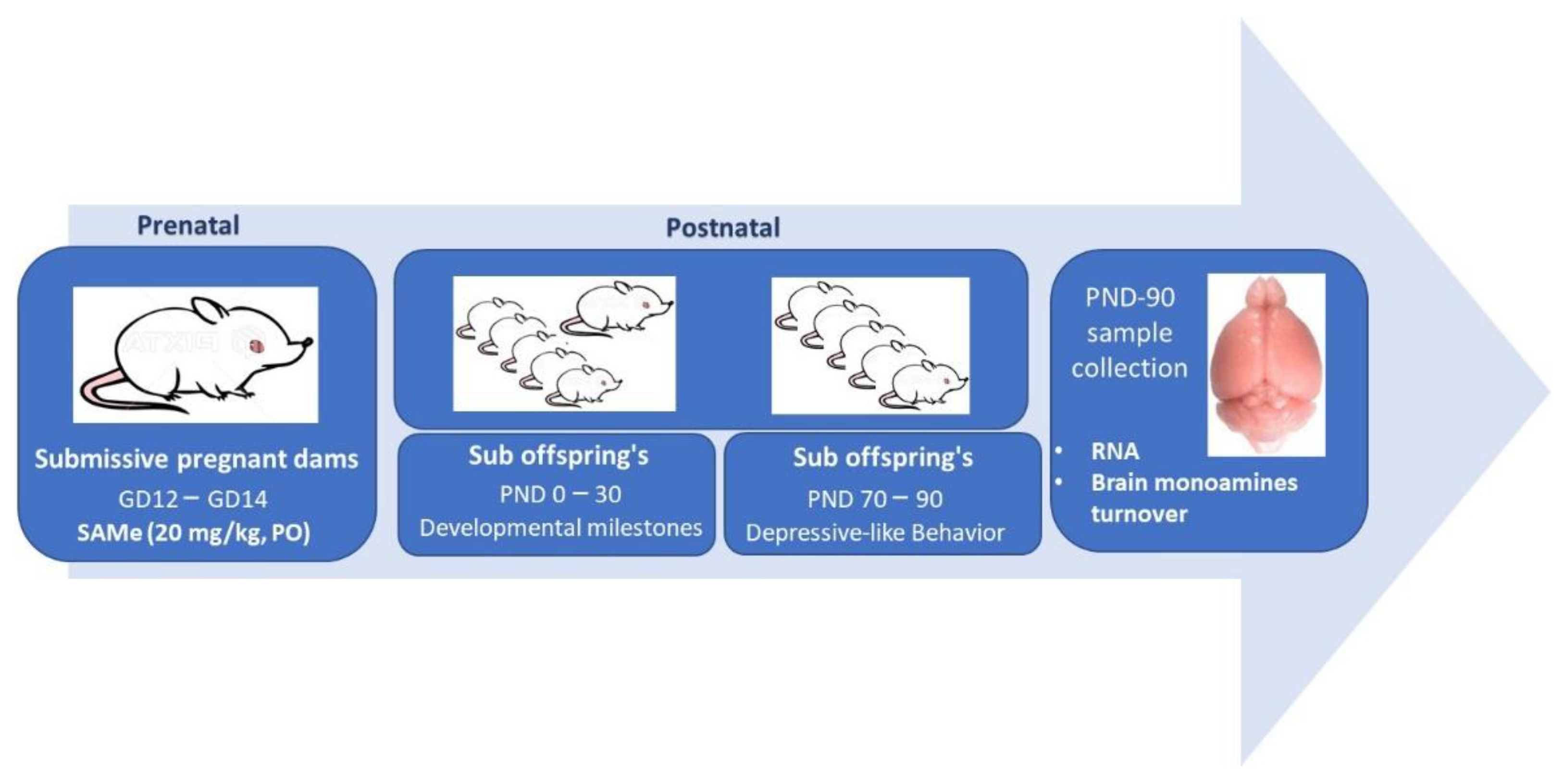
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HIAA | 5-hydroxyindoleacetic acid |
| 5-HT | 5-hydroxytryptamine or serotonin |
| HT1A | Gene 5-hydroxytryptamine receptor 1A gene |
| 5-HT1AR | 5-hydroxytryptamine receptor 1A |
| HT2A | Gene for 5-hydroxytryptamine receptor 2A |
| 5-HT2AR | 5-hydroxytryptamine receptor 2A |
| COMT | Catechol-O-methyl transferase |
| CSF | Cerebrospinal fluid |
| DA | Dopamine |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| D1R | Dopamine receptors D1 |
| D2R | Dopamine receptors D2 |
| EPM | Elevated Plus Maze |
| HPC | Hippocampus |
| HVA | Homovanillic acid |
| MAO-A | Monoamine oxidase A |
| MAO-B | Monoamine oxidase B |
| MDD | Major Depressive Disorder |
| MHPG | 4-hydroxy-3-Methoxyphenylglycol |
| NE | Norepinephrine |
| OF | Open field |
| PFC | Prefrontal cortex |
| SAMe | S-Adenosyl-methionine |
| SERT | Serotonin transporter |
| Sub mice | Submissive mice |
| Tph2 | Tryptophan hydroxylase enzyme 2 |
References
- Sghendo, L.; Mifsud, J. Understanding the molecular pharmacology of the serotonergic system: Using fluoxetine as a model. J. Pharm. Pharmacol. 2012, 64, 317–325. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, S.J.; Nestler, E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013, 14, 609–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, G.; Johansson, M.; Ågren, H.; Friberg, P. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: Evidence in support of the catecholamine hypothesis of mood disorders. Arch. Gen. Psychiatry 2000, 57, 787–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Yang, P.; Yang, C.; Yang, D.; Wu, X.; Cao, T.; Zeng, C.; Chen, Q.; Zhang, S.; Zhu, Z.; et al. Disturbance of neurotransmitter metabolism in drug-naïve, first-episode major depressive disorder: A comparative study on adult and adolescent cohorts. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 1283–1296. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Hattori, K.; Ogawa, S.; Sasayama, D.; Ota, M.; Teraishi, T.; Kunugi, H. Relationships of Cerebrospinal Fluid Monoamine Metabolite Levels With Clinical Variables in Major Depressive Disorder. J. Clin. Psychiatry 2017, 78, e947–e956. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Kopin, I.J.; Goldstein, D.S. Catecholamine Metabolism: A Contemporary View with Implications for Physiology and Medicine. Pharmacol. Rev. 2004, 56, 331. [Google Scholar] [CrossRef] [Green Version]
- Ornoy, A.; Becker, M.; Weinstein-Fudim, L.; Ergaz, Z. S-Adenosine Methionine (SAMe) and Valproic Acid (VPA) as Epigenetic Modulators: Special Emphasis on their Interactions Affecting Nervous Tissue during Pregnancy. Int. J. Mol. Sci. 2020, 21, 3721. [Google Scholar] [CrossRef]
- De Berardis, D.; Orsolini, L.; Serroni, N.; Girinelli, G.; Iasevoli, F.; Tomasetti, C.; de Bartolomeis, A.; Mazza, M.; Valchera, A.; Fornaro, M.; et al. A comprehensive review on the efficacy of S-Adenosyl-L-methionine in Major Depressive Disorder. CNS Neurol. Disord.—Drug Targets 2016, 15, 35–44. [Google Scholar] [CrossRef]
- Miller, J.W.; Shukitt-Hale, B.; Villalobos-Molina, R.; Nadeau, M.R.; Selhub, J.; Joseph, J.A. Effect of L-Dopa and the Catechol-O-Methyltransferase Inhibitor Ro 41–0960 on Sulfur Amino Acid Metabolites in Rats. Clin. Neuropharmacol. 1997, 20, 55–66. [Google Scholar] [CrossRef]
- Cheng, H.; Gomes-Trolin, C.; Aquilonius, S.M.; Steinberg, A.; Löfberg, C.; Ekblom, J.; Oreland, L. Levels ofl-MethionineS-Adenosyltranferase Activity in Erythrocytes and Concentrations ofS-Adenosylmethionine andS-Adenosylhomocysteine in Whole Blood of Patients with Parkinson’s Disease. Exp. Neurol. 1997, 145, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Mato, J.; Alvarez, L.; Ortiz, P.; Pajares, M.A. S-adenosylmethionine synthesis: Molecular mechanisms and clinical implications. Pharmacol. Ther. 1997, 73, 265–280. [Google Scholar] [CrossRef] [Green Version]
- Ornoy, A.; Weinstein-Fudim, L.; Becker, M. SAMe, Choline, and Valproic Acid as Possible Epigenetic Drugs: Their Effects in Pregnancy with a Special Emphasis on Animal Studies. Pharmaceuticals 2022, 15, 192. [Google Scholar] [CrossRef]
- Hanson, M.A.; Gluckman, P.D. Developmental origins of health and disease—Global public health implications. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H. Early life nutrition, epigenetics and programming of later life disease. Nutrients 2014, 6, 2165–2178. [Google Scholar] [CrossRef] [PubMed]
- Kuijper, B.; Hanson, M.A.; Vitikainen, E.I.K.; Marshall, H.H.; Ozanne, S.E.; Cant, M.A. Developing differences: Early-life effects and evolutionary medicine. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190039. [Google Scholar] [CrossRef] [PubMed]
- Bagot, R.C.; Labonté, B.; Peña, C.J.; Nestler, E.J. Epigenetic signaling in psychiatric disorders: Stress and depression. Dialogues. Clin. Neurosci. 2014, 16, 281–295. [Google Scholar] [CrossRef]
- Szyf, M. DNA Methylation, Behavior and Early Life Adversity. J. Genet. Genom. 2013, 40, 331–338. [Google Scholar] [CrossRef]
- Gross, M.; Romi, H.; Miller, A.; Pinhasov, A. Social dominance predicts hippocampal glucocorticoid receptor recruitment and resilience to prenatal adversity. Sci. Rep. 2018, 8, 9595. [Google Scholar] [CrossRef] [Green Version]
- Nesher, E.; Gross, M.; Lisson, S.; Tikhonov, T.; Yadid, G.; Pinhasov, A. Differential responses to distinct psychotropic agents of selectively bred dominant and submissive animals. Behav. Brain Res 2013, 236, 225–235. [Google Scholar] [CrossRef]
- Becker, M.; Abaev, K.; Pinhasov, A.; Ornoy, A. S-Adenosyl-Methionine alleviates sociability aversion and reduces changes in gene expression in a mouse model of social hierarchy. Behav. Brain Res. 2022, 427, 113866. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D.; Yohn, S.E.; López-Cruz, L.; San Miguel, N.; Correa, M. Activational and effort-related aspects of motivation: Neural mechanisms and implications for psychopathology. Brain 2016, 139, 1325–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmiter, R.D. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: Lessons from dopamine-deficient mice. Ann. N. Y. Acad. Sci. 2008, 1129, 35–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murlanova, K.; Michaelevski, I.; Kreinin, A.; Terrillion, C.; Pletnikov, M.; Pinhasov, A. Link between temperament traits, brain neurochemistry and response to SSRI: Insights from animal model of social behavior. J. Affect. Disord. 2020, 282, 1055–1066. [Google Scholar] [CrossRef]
- Asberg, M.; Träskman, L. Studies of CSF 5-HIAA in depression and suicidal behaviour. Adv. Exp. Med. Biol. 1981, 133, 739–752. [Google Scholar]
- Morrissette, D.A.; Stahl, S.M. Modulating the serotonin system in the treatment of major depressive disorder. CNS Spectr. 2014, 19 (Suppl. 1), 54–68. [Google Scholar] [CrossRef]
- Invernizzi, R.W. Role of TPH-2 in brain function: News from behavioral and pharmacologic studies. J. Neurosci. Res 2007, 85, 3030–3035. [Google Scholar] [CrossRef]
- Trillo, L.; Das, D.; Hsieh, W.; Medina, B.; Moghadam, S.; Lin, B.; Dang, V.; Sanchez, M.M.; De Miguel, Z.; Ashford, J.W.; et al. Ascending monoaminergic systems alterations in Alzheimer’s disease. translating basic science into clinical care. Neurosci. Biobehav. Rev. 2013, 37, 1363–1379. [Google Scholar] [CrossRef]
- Bach, H.; Huang, Y.-Y.; Underwood, M.D.; Dwork, A.J.; Mann, J.J.; Arango, V. Elevated serotonin and 5-HIAA in the brainstem and lower serotonin turnover in the prefrontal cortex of suicides. Synapse 2014, 68, 127–130. [Google Scholar] [CrossRef] [Green Version]
- Barton, D.A.; Esler, M.D.; Dawood, T.; Lambert, E.A.; Haikerwal, D.; Brenchley, C.; Socratous, F.; Hastings, J.; Guo, L.; Wiesner, G.; et al. Elevated Brain Serotonin Turnover in Patients With Depression: Effect of Genotype and Therapy. Arch. Gen. Psychiatry 2008, 65, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Esler, M.; Alvarenga, M.; Barton, D.; Jennings, G.; Kaye, D.; Guo, L.; Schwarz, R.; Lambert, G. Measurement of Noradrenaline and Serotonin Metabolites With Internal Jugular Vein Sampling: An Indicator of Brain Monoamine Turnover in Depressive Illness and Panic Disorder. Front. Psychiatry 2022, 13, 818012. [Google Scholar] [CrossRef]
- Sheline, Y.; Bardgett, M.E.; Csernansky, J.G. Correlated reductions in cerebrospinal fluid 5-HIAA and MHPG concentrations after treatment with selective serotonin reuptake inhibitors. J. Clin. Psychopharmacol. 1997, 17, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Pech, J.; Forman, J.; Kessing, L.V.; Knorr, U. Poor evidence for putative abnormalities in cerebrospinal fluid neurotransmitters in patients with depression versus healthy non-psychiatric individuals: A systematic review and meta-analyses of 23 studies. J. Affect Disord 2018, 240, 6–16. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J.J.; Wei, J.; Dilley, G.; Pittman, S.D.; Meltzer, H.Y.; Overholser, J.C.; Roth, B.L.; Stockmeier, C.A. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry 1999, 45, 1085–1098. [Google Scholar] [CrossRef]
- Cotter, D.; Mackay, D.; Chana, G.; Beasley, C.; Landau, S.; Everall, I.P. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb. Cortex 2002, 12, 386–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, F.A.; McGahuey, C.A.; Freeman, M.P.; Delgado, P.L. Sex differences in depressive response during monoamine depletions in remitted depressive subjects. J. Clin. Psychiatry 2006, 67, 1618–1623. [Google Scholar] [CrossRef]
- Moreno, F.A.; Erickson, R.P.; Garriock, H.A.; Gelernter, J.; Mintz, J.; Oas-Terpstra, J.; Davies, M.A.; Delgado, P.L. Association Study of Genotype by Depressive Response during Tryptophan Depletion in Subjects Recovered from Major Depression. Mol. Neuropsychiatry 2015, 1, 165–174. [Google Scholar] [CrossRef]
- Smith, K.A.; Fairburn, C.G.; Cowen, P.J. Relapse of depression after rapid depletion of tryptophan. Lancet 1997, 349, 915–919. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022. [Google Scholar] [CrossRef]
- Mosienko, V.; Matthes, S.; Hirth, N.; Beis, D.; Flinders, M.; Bader, M.; Hansson, A.C.; Alenina, N. Adaptive changes in serotonin metabolism preserve normal behavior in mice with reduced TPH2 activity. Neuropharmacology 2014, 85, 73–80. [Google Scholar] [CrossRef]
- Delva, N.C.; Stanwood, G.D. Dysregulation of brain dopamine systems in major depressive disorder. Exp. Biol. Med. 2021, 246, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Shumay, E.; Logan, J.; Volkow, N.D.; Fowler, J.S. Evidence that the methylation state of the monoamine oxidase A (MAOA) gene predicts brain activity of MAO A enzyme in healthy men. Epigenetics 2012, 7, 1151–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melas, P.A.; Forsell, Y. Hypomethylation of MAOA׳s first exon region in depression: A replication study. Psychiatry Res. 2015, 226, 389–391. [Google Scholar] [CrossRef]
- Yavich, L.; Forsberg, M.M.; Karayiorgou, M.; Gogos, J.A.; Männistö, P.T. Site-Specific Role of Catechol-O-Methyltransferase in Dopamine Overflow within Prefrontal Cortex and Dorsal Striatum. J. Neurosci. 2007, 27, 10196. [Google Scholar] [PubMed] [Green Version]
- Zhang, Y.; Chang, Z.; Chen, J.; Ling, Y.; Liu, X.; Feng, Z.; Chen, C.; Xia, M.; Zhao, X.; Ying, W.; et al. Methylation of the tryptophan hydroxylase-2 gene is associated with mRNA expression in patients with major depression with suicide attempts. Mol. Med. Rep. 2015, 12, 3184–3190. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Zhang, Z.; Li, W.; He, L.; Xu, J.; Shi, Y. Association study of the TPH2 Gene with Major Depressive Disorder in the Han Chinese Population. Eur. J. Psychiatry 2016, 30, 131–140. [Google Scholar]
- Shen, X.; Wu, Y.; Qian, M.; Wang, X.; Hou, Z.; Liu, Y.; Sun, J.; Zhong, H.; Yang, J.; Lin, M.; et al. Tryptophan hydroxylase 2 gene is associated with major depressive disorder in a female Chinese population. J. Affect. Disord. 2011, 133, 619–624. [Google Scholar]
- Shishkina, G.; Kalinina, T.; Dygalo, N. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience 2007, 150, 404–412. [Google Scholar] [CrossRef]
- Muguruza, C.; Miranda-Azpiazu, P.; Díez-Alarcia, R.; Morentin, B.; González-Maeso, J.; Callado, L.F.; Meana, J.J. Evaluation of 5-HT2A and mGlu2/3 receptors in postmortem prefrontal cortex of subjects with major depressive disorder: Effect of antidepressant treatment. Neuropharmacology 2014, 86, 311–318. [Google Scholar] [CrossRef]
- Rosel, P.; Arranz, B.; Urretavizcaya, M.; Oros, M.; San, L.; Navarro, M.A. Altered 5-HT2A and 5-HT4 postsynaptic receptors and their intracellular signalling systems IP3 and cAMP in brains from depressed violent suicide victims. Neuropsychobiology 2004, 49, 189–195. [Google Scholar] [CrossRef]
- Feder, Y.; Nesher, E.; Ogran, A.; Kreinin, A.; Malatynska, E.; Yadid, G.; Pinhasov, A. Selective breeding for dominant and submissive behavior in Sabra mice. J. Affect. Disord. 2010, 126, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Malatynska, E.; Pinhasov, A.; Crooke, J.J.; Smith-Swintosky, V.L.; Brenneman, D.E. Reduction of dominant or submissive behaviors as models for antimanic or antidepressant drug testing: Technical considerations. J. Neurosci. Methods 2007, 165, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wolf, W.A.; Youdim, M.B.H.; Kuhn, D.M. Does brain 5-HIAA indicate serotonin release or monoamine oxidase activity? Eur. J. Pharmacol. 1985, 109, 381–387. [Google Scholar] [CrossRef]
- Ogawa, N.; Tanaka, K.; Asanuma, M. Bromocriptine markedly suppresses levodopa-induced abnormal increase of dopamine turnover in the parkinsonian striatum. Neurochem. Res. 2000, 25, 755–758. [Google Scholar] [CrossRef]
- Megyeri, K.; Marko, B.; Sziray, N.; Gacsalyi, I.; Juranyi, Z.; Levay, G.; Harsing, L.G., Jr. Effects of 2,3-benzodiazepine AMPA receptor antagonists on dopamine turnover in the striatum of rats with experimental parkinsonism. Brain Res. Bull. 2007, 71, 501–507. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Incerti, M.; Vink, J.; Roberson, R.; Abebe, D.; Spong, C.Y. Treatment with neuropeptides attenuates c-fos expression in a mouse model of fetal alcohol syndrome. Am. J. Perinatol. 2010, 27, 743–748. [Google Scholar] [CrossRef]
- Weinstein-Fudim, L.; Ergaz, Z.; Szyf, M.; Ornoy, A. Prenatal S-Adenosine Methionine (SAMe) Induces Changes in Gene Expression in the Brain of Newborn Mice That Are Prevented by Co-Administration of Valproic Acid (VPA). Int. J. Mol. Sci. 2020, 21, 2834. [Google Scholar] [CrossRef]

| MONOAMINES and METABOLITES | Male | Female | GENES | Male | Female |
|---|---|---|---|---|---|
| 5-HT | no change | increase ↑ | Tph2 | no change | no change |
| 5-HIAA | no change | no change | Mao-a | significantly downregulated ↓ | trend to decrease |
| DA | increases ↑ | increases ↑ | Htr2a | significantly downregulated ↓ | no change |
| DOPAC | increase ↑ | increase ↑ | Sert | no change | no change |
| HVA | no change | increase ↑ | Mao-b | significantly downregulated ↓ | trend to decrease |
| NE | no changes | no change | Comt | significantly downregulated ↓ | significantly downregulated ↓ |
| MHPG | no changes | no change | D1R | significantly downregulated ↓ | no change |
| D2R | trend to decrease | no change |
| Oligo Name | Sequence 5′ to 3′ | |
|---|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase–GAPDH | F | GGGGCTCTCTGCTCCTCCCTGT |
| R | TGACCCTTTTGGCCCCACCCT | |
| Serotonin receptor–Htr2a | F | CCTGATGTCACTTGCCATAGCTG |
| R | CAGGTAAATCCAGACGGCACAG | |
| Tryptophan hydroxylase 2–Tph2 | F | GTTGATGCTGCGGCTCAGATCT |
| R | GAAGCTCGTCATGCAGTTCACC | |
| Serotonin Transporter–Sert | F | TCGCCTCCTACTACAACACC |
| R | ATGTTGTCCTGGGCGAAGTA | |
| Catechol-O-Methyltransferase–Comt | F | ACGAGGGGATGAGAGAGTCCT |
| R | AGCAGCCAACAGCATTTATGGG | |
| Monoamine oxidase A–Mao-a | F | CGTGATCGGAGGTGGCATTTC |
| R | AAAGGCGCCCCGAAAGG-3 | |
| Monoamine oxidase B–Mao-b | F | GGGGGCGGCATCTCAGGTAT |
| R | TGCTTCCAGAACCACCACACT | |
| Dopamine receptor D1–D1R | F | AGATGACTCCGAAGGCAGCCTT |
| R | GCCATGTAGGTTTTGCCTTGTGC | |
| Dopamine receptor D2–D2R | F | TTCCCAGTGAACAGGCGGAGA |
| R | TTTGGCAGGACTGTCAGGGTT | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, M.; Abaev, K.; Shmerkin, E.; Weinstein-Fudim, L.; Pinhasov, A.; Ornoy, A. Prenatal SAMe Treatment Induces Changes in Brain Monoamines and in the Expression of Genes Related to Monoamine Metabolism in a Mouse Model of Social Hierarchy and Depression, Probably via an Epigenetic Mechanism. Int. J. Mol. Sci. 2022, 23, 11898. https://doi.org/10.3390/ijms231911898
Becker M, Abaev K, Shmerkin E, Weinstein-Fudim L, Pinhasov A, Ornoy A. Prenatal SAMe Treatment Induces Changes in Brain Monoamines and in the Expression of Genes Related to Monoamine Metabolism in a Mouse Model of Social Hierarchy and Depression, Probably via an Epigenetic Mechanism. International Journal of Molecular Sciences. 2022; 23(19):11898. https://doi.org/10.3390/ijms231911898
Chicago/Turabian StyleBecker, Maria, Karin Abaev, Elena Shmerkin, Liza Weinstein-Fudim, Albert Pinhasov, and Asher Ornoy. 2022. "Prenatal SAMe Treatment Induces Changes in Brain Monoamines and in the Expression of Genes Related to Monoamine Metabolism in a Mouse Model of Social Hierarchy and Depression, Probably via an Epigenetic Mechanism" International Journal of Molecular Sciences 23, no. 19: 11898. https://doi.org/10.3390/ijms231911898
APA StyleBecker, M., Abaev, K., Shmerkin, E., Weinstein-Fudim, L., Pinhasov, A., & Ornoy, A. (2022). Prenatal SAMe Treatment Induces Changes in Brain Monoamines and in the Expression of Genes Related to Monoamine Metabolism in a Mouse Model of Social Hierarchy and Depression, Probably via an Epigenetic Mechanism. International Journal of Molecular Sciences, 23(19), 11898. https://doi.org/10.3390/ijms231911898







