Functional Heterogeneity of Bone Marrow Mesenchymal Stem Cell Subpopulations in Physiology and Pathology
Abstract
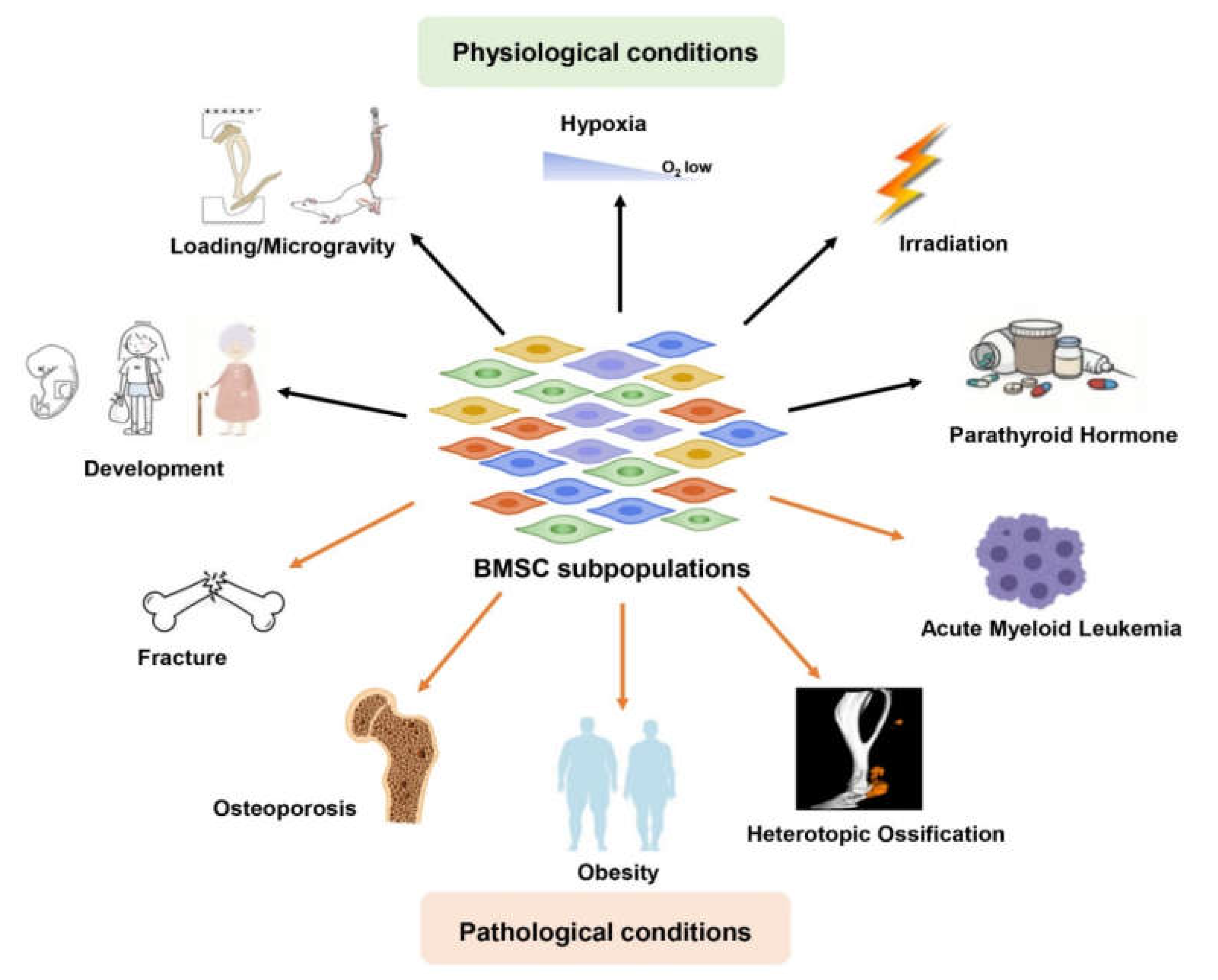
:1. Introduction
2. Functional Heterogeneity of BMSC Subpopulations in Physiology (Table 1)
2.1. Development
2.1.1. Embryonic Development
| Physiological Conditions | BMSC Subpopulations | Functions | References |
|---|---|---|---|
| Development | |||
| Embryonic development | Mice: CD105+, Grem1+, 6C3+, LepR+, Axin2+, Gli1+, Osx+, Sox9+, Prx1+ Human: CADM1+ PDPN+ | Supports hematopoiesis: CD105+, 6C3+, and LepR+ | [5,22,29] |
| Promotes growth and regeneration: Axin2+ and Gli1+ (label cranial SSCs) | [16,30,31,32] | ||
| Multi-lineage differentiation: Osx+ (bone marrow), Grem1+ and Sox9+ (cartilage templates), Prx1+ (limb bud) | [25,33,34,35,36] | ||
| Promotes self-renewal and generates the osteochongenesis but no adipocytes and no hematopoietic supportive function: CADM1+ PDPN+ | [37] | ||
| Adulthood | Mice: PDGFRα+ (PDGFRa+ Sca-1− CD45− Ter119−), Nestin+ (Nestin-GFPlow cells), Osx+, LepR+, Acan+, Mx1+, Prx1+, PTHrP+, Grem1+, Gli1+, mpMSCs | Promote osteogenesis and support the hematopoiesis: PDGFRα+, Nestin+, Osx+, LepR+, Acan+ Mx1+, Sca1+, CD105+ | [11,38,39,40,41,42,43,44,45] |
| Label specific bone regions: bone marrow of limb bones (Prx1+); chondrocytes of the resting zone in the growth plate of long bones (PTHrP+); metaphyseal areas (Grem1+, Gli1+, mpMSCs) | [46,47,48,49,50,51,52] | ||
| Human: CD105+, CD140a+, CD73+, CD90+, STRO1+, CD271+, CD44+, CD146+ CD271+PDGFRαlow, STRO-1+, CD45−Ter119− Tie2− Thy1− 6C3− CD51+, PDPN+ CD73+ CD164+ CD235− CD45− CD146− Tie2− CD31−, FGFR2+, FGF5+, PLAT+ VCAM1+ | Exhibits high CFU-F ability and multi-lineage differentiation potential: CD105+, CD140a+, CD73+, CD90+, STRO-1+, CD271+, CD44+, PDPN+ CD73+ CD164+ CD235− CD45− CD146− Tie2− CD31−, FGFR2+, FGF5+, PLAT+ VCAM1+ | [7,8,9,10,15,22,29,53,54,55,56,57,58] | |
| Supports hematopoiesis: CD146+, CD146+ CD271+ PDGFRαlow, STRO-1+ | [29,53,54,55] | ||
| Label specific bone regions: bone cartilage stromal (CD45− Ter119− Tie2− Thy1− 6C3− CD51+); hypertrophic zones of the growth plate (PDPN+ CD73+ CD164+ CD235− CD45− CD146− Tie2− CD31−) | [15,22,56,57] | ||
| Specific functional subpopulation of UC-MSCs: high immune response/regulatory activities (group 1 of UC-MSCs); bone and cartilage growth related group 2 of UC-MSCs | [59] | ||
| Aging | Mice: Sca1+, Prx1+, LepR+, LepR+ Notch3+, LepR+ MALPs | Decreases number and impairs paracrine support for hematopoiesis: Sca1+, Prx1+, LepR+, LepR+ Notch3+ | [60,61,62,63] |
| Increases the number and promotes adipogenesis: LepR+ MALPs | [19] | ||
| Human:CD29+ CD44+ CD90+ CD105+ CD34− CD45− HLA-DR− | Self-renewal related subpopulation: high expression, CDCA5, MYBL2, FAM64A, CENP-A, PAQR4, Asf1b, CAF-1, HMGB2 | [64,65,66,67,68,69,70,71,72] | |
| Multidirectional differentiation-related subpopulation: high express TGM2, COL11A1, NEAT1, Type V collagen | [64,73,74,75,76] | ||
| Immune regulation and damage repair related subpopulation: high express Cyba, TIMP-1, ANXA1, LUM, DPT, ERp44, and HSPA5 | [64,77,78,79,80,81,82,83] | ||
| Environmental Stresses | |||
| Loading | Sca-1+ Prx1+, Osx+, CXCL12+, LepR+ osteolectin+ | Responds to loading and participating in bone formation | [11,38,84,85,86,87,88,89] |
| Microgravity | Sca+ CD90.2+, Lin− LepR+, LepR+ osteolectin+ | Declines number and exhibits more quiescence and lower bone anabolism | [89,90,91] |
| Hypoxia | CD13+ CD29+ CD44+ CD73+ CD90+ CD105+ CD151+ CD34−, PDGFRα+, LepR+, SP7+, 7AAD− CD45− Ter119− Tie2− CD51+ CD105− CD90.2− CD249− CD200− | Exhibits high proliferative activity: CD13+ CD29+ CD44+ CD73+ CD90+ CD105+ CD151+ CD34-, PDGFRα+ and LepR+ | [92,93] |
| Osteogenic and chondrogenic differentiation: SP7+ (also know as Osx+), 7AAD− CD45− Ter119− Tie2− CD51+ CD105− CD90.2− CD249− CD200+ | [94,95] | ||
| Irradiation | Mice: LepR+, Nestin+, CD73+ NGFRhigh, LepR+ MALPs | Declines number after irradiation: LepR +, Nestin + | [96] |
| Expansion, supports hematopoietic and bone marrow repair: CD73+ NGFRhigh, LepR+ MALPs, LepR+ BMSCs with high expression of Npdc1/ Hoxb2 | [19,63,96] | ||
| Human: CD73+ CD90+ CD105+ CD14− CD34− CD45− HLA-DR− | Exhibits senescence and impairs immunomodulation capacity: CD73+ CD90+ CD105+ CD14− CD34− CD45− HLA-DR− | [97] | |
| PTH | LepR+, LepR+ Runx2-GFPlow | Promotes osteogenic differentiation by promoting osteolectin expression or increasing numbers of type H endothelial cells | [98,99,100,101,102] |
2.1.2. Adulthood
Mouse BMSCs
Human BMSCs
2.1.3. Aging
2.2. Environmental Stresses
2.2.1. Loading
2.2.2. Microgravity
2.2.3. Hypoxia
2.2.4. Irradiation
2.2.5. PTH
3. Functional Heterogeneity of BMSC Subpopulations in Pathology
3.1. Fracture
3.2. Osteoporosis
3.3. Heterotopic Ossification
3.4. Obesity
3.5. Acute Myeloid Leukemia (AML)
4. Unresolved Questions, Challenges, and Potential Opportunities
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ono, N.; Balani, D.H.; Kronenberg, H.M. Stem and progenitor cells in skeletal development. Curr. Top Dev. Biol. 2019, 133, 1–24. [Google Scholar] [PubMed]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nombela-Arrieta, C.; Ritz, J.; Silberstein, L.E. The elusive nature and function of mesenchymal stem cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 126–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortinau, L.C.; Wang, H.; Lei, K.; Deveza, L.; Jeong, Y.; Hara, Y.; Grafe, I.; Rosenfeld, S.B.; Lee, D.; Lee, B.; et al. Identification of Functionally Distinct Mx1+ αSMA+ Periosteal Skeletal Stem Cells. Cell Stem Cell 2019, 25, 784–796.e5. [Google Scholar] [CrossRef]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.G.; Morrison, S.J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef] [Green Version]
- Dangl, J.L.; Lanier, L.L. Founding father of FACS: Professor Leonard A. Herzenberg (1931–2013). Proc. Natl. Acad. Sci. USA 2013, 110, 20848–20849. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Gronthos, S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone Miner. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Lange, C.; Schroeder, J.; Lioznov, M.V.; Zander, A.R. High-potential human mesenchymal stem cells. Stem Cells Dev. 2005, 14, 70–80. [Google Scholar] [CrossRef]
- Sorrentino, A.; Ferracin, M.; Castelli, G.; Biffoni, M.; Tomaselli, G.; Baiocchi, M.; Fatica, A.; Negrini, M.; Peschle, C.; Valtieri, M. Isolation and characterization of CD146+ multipotent mesenchymal stromal cells. Exp. Hematol. 2008, 36, 1035–1046. [Google Scholar] [CrossRef]
- Pinho, S.; Lacombe, J.; Hanoun, M.; Mizoguchi, T.; Bruns, I.; Kunisaki, Y.; Frenette, P.S. PDGFRα and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med. 2013, 210, 1351–1367. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, S.; Mabuchi, Y.; Kubota, Y.; Nagai, Y.; Niibe, K.; Hiratsu, E.; Suzuki, S.; Miyauchi-Hara, C.; Nagoshi, N.; Sunabori, T.; et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J. Exp. Med. 2009, 206, 2483–2496. [Google Scholar] [CrossRef]
- Koide, Y.; Morikawa, S.; Mabuchi, Y.; Muguruma, Y.; Hiratsu, E.; Hasegawa, K.; Kobayashi, M.; Ando, K.; Kinjo, K.; Okano, H.; et al. Two distinct stem cell lineages in murine bone marrow. Stem Cells 2007, 25, 1213–1221. [Google Scholar] [CrossRef]
- Chan, C.K.; Seo, E.Y.; Chen, J.Y.; Lo, D.; McArdle, A.; Sinha, R.; Tevlin, R.; Seita, J.; Vincent-Tompkins, J.; Wearda, T.; et al. Identification and specification of the mouse skeletal stem cell. Cell 2015, 160, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Ambrosi, T.H.; Longaker, M.T.; Chan, C.K.F. A Revised Perspective of Skeletal Stem Cell Biology. Front. Cell Dev. Biol. 2019, 7, 189. [Google Scholar] [CrossRef] [Green Version]
- Addo, R.K.; Heinrich, F.; Heinz, G.A.; Schulz, D.; Sercan-Alp, Ö.; Lehmann, K.; Tran, C.L.; Bardua, M.; Matz, M.; Löhning, M.; et al. Single-cell transcriptomes of murine bone marrow stromal cells reveal niche-associated heterogeneity. Eur. J. Immunol. 2019, 49, 1372–1379. [Google Scholar] [CrossRef] [Green Version]
- Baccin, C.; Al-Sabah, J.; Velten, L.; Helbling, P.M.; Grünschläger, F.; Hernández-Malmierca, P.; Nombela-Arrieta, C.; Steinmetz, L.M.; Trumpp, A.; Haas, S. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat. Cell Biol. 2020, 22, 38–48. [Google Scholar] [CrossRef]
- Tikhonova, A.N.; Dolgalev, I.; Hu, H.; Sivaraj, K.K.; Hoxha, E.; Cuesta-Domínguez, Á.; Pinho, S.; Akhmetzyanova, I.; Gao, J.; Witkowski, M.; et al. The bone marrow microenvironment at single-cell resolution. Nature 2019, 569, 222–228. [Google Scholar] [CrossRef]
- Wolock, S.L.; Krishnan, I.; Tenen, D.E.; Matkins, V.; Camacho, V.; Patel, S.; Agarwal, P.; Bhatia, R.; Tenen, D.G.; Klein, A.M.; et al. Mapping Distinct Bone Marrow Niche Populations and Their Differentiation Paths. Cell Rep. 2019, 28, 302–311.e5. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Yao, L.; Tower, R.J.; Wei, Y.; Miao, Z.; Park, J.; Shrestha, R.; Wang, L.; Yu, W.; Holdreith, N.; et al. Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. Elife 2020, 9, e54695. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Du, W.; Liang, L. New insights into the heterogeneity and functional diversity of human mesenchymal stem cells. Biomed. Mater Eng. 2017, 28, S29–S45. [Google Scholar] [CrossRef]
- Rennerfeldt, D.A.; Van Vliet, K.J. Concise review: When colonies are not clones: Evidence and implications of intracolony heterogeneity in mesenchymal stem cells. Stem Cells 2016, 34, 1135–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.K.F.; Lindau, P.; Jiang, W.; Chen, J.Y.; Zhang, L.F.; Chen, C.-C.; Seita, J.; Sahoo, D.; Kim, J.-B.; Lee, A.; et al. Clonal precursor of bone, cartilage, and hematopoietic niche stromal cells. Proc. Natl. Acad. Sci. USA 2013, 110, 12643–12648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takashima, Y.; Era, T.; Nakao, K.; Kondo, S.; Kasuga, M.; Smith, A.G.; Nishikawa, S.-I. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell 2007, 129, 1377–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagoshi, N.; Shibata, S.; Kubota, Y.; Nakamura, M.; Nagai, Y.; Satoh, E.; Morikawa, S.; Okada, Y.; Mabuchi, Y.; Katoh, H.; et al. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell 2008, 2, 392–403. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Strecker, S.; Wang, L.; Kronenberg, M.S.; Wang, W.; Rowe, D.W.; Maye, P. Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS ONE. 2013, 8, e71318. [Google Scholar] [CrossRef] [Green Version]
- Isern, J.; García-García, A.; Martín, A.M.; Arranz, L.; Martín-Pérez, D.; Torroja, C.; Sanchez-Cabo, F.; Méndez-Ferrer, S. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife 2014, 3, e03696. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Pinho, S.; Ahmed, J.; Kunisaki, Y.; Hanoun, M.; Mendelson, A.; Ono, N.; Kronenberg, H.M.; Frenette, P.S. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev. Cell 2014, 29, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Ono, N.; Ono, W.; Nagasawa, T.; Kronenberg, H.M. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat. Cell Biol. 2014, 16, 1157–1167. [Google Scholar] [CrossRef]
- Chan, C.K.F.; Chen, C.-C.; Luppen, C.A.; Kim, J.-B.; DeBoer, A.T.; Wei, K.; Helms, J.A.; Kuo, C.J.; Kraft, D.L.; Weissman, I.L. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 2009, 457, 490–494. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, T.; Jeong, J.; Sheu, T.-J.; Hsu, W. Stem cells of the suture mesenchyme in craniofacial bone development, repair and regeneration. Nat. Commun. 2016, 7, 10526. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, J.; Ho, T.-V.; Grimes, W.; Urata, M.; Chai, Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat. Cell Biol. 2015, 17, 386–396. [Google Scholar] [CrossRef] [Green Version]
- Doro, D.H.; Grigoriadis, A.E.; Liu, K.J. Calvarial Suture-Derived Stem Cells and Their Contribution to Cranial Bone Repair. Front. Physiol. 2017, 8, 956. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, H.; Kim, J.-E.; Nakashima, K.; Balmes, G.; Iwai, N.; Deng, J.M.; Zhang, Z.; Martin, J.F.; Behringer, R.R.; Nakamura, T.; et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc. Natl. Acad. Sci. USA 2005, 102, 14665–14670. [Google Scholar] [CrossRef] [Green Version]
- Maes, C.; Kobayashi, T.; Selig, M.K.; Torrekens, S.; Roth, S.I.; Mackem, S.; Carmeliet, G.; Kronenberg, H.M. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 2010, 19, 329–344. [Google Scholar] [CrossRef] [Green Version]
- Cserjesi, P.; Lilly, B.; Bryson, L.; Wang, Y.; A Sassoon, D.; Olson, E.N. MHox: A mesodermally restricted homeodomain protein that binds an essential site in the muscle creatine kinase enhancer. Development 1992, 115, 1087–1101. [Google Scholar] [CrossRef]
- Logan, M.; Martin, J.F.; Nagy, A.; Lobe, C.; Olson, E.N.; Tabin, C.J. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 2002, 33, 77–80. [Google Scholar] [CrossRef]
- He, J.; Yan, J.; Wang, J.; Zhao, L.; Xin, Q.; Zeng, Y.; Sun, Y.; Zhang, H.; Bai, Z.; Li, Z.; et al. Dissecting human embryonic skeletal stem cell ontogeny by single-cell transcriptomic and functional analyses. Cell Res. 2021, 31, 742–757. [Google Scholar] [CrossRef]
- Omatsu, Y.; Sugiyama, T.; Kohara, H.; Kondoh, G.; Fujii, N.; Kohno, K.; Nagasawa, T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 2010, 33, 387–399. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Spencer, J.A.; Koh, B.I.; Kobayashi, T.; Fujisaki, J.; Clemens, T.L.; Lin, C.P.; Kronenberg, H.M.; Scadden, D.T. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 2012, 10, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.; Lira, S.A.; Scadden, D.T.; Ma’Ayan, A.; Enikolopov, G.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef]
- Kunisaki, Y.; Bruns, I.; Scheiermann, C.; Ahmed, J.; Pinho, S.; Zhang, D.; Mizoguchi, T.; Wei, Q.; Lucas, D.; Ito, K.; et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013, 502, 637–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, R.; Zhou, B.O.; Shimada, I.S.; Zhao, Z.; Morrison, S.J. Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow. Cell Stem Cell 2016, 18, 782–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, L.; Saunders, T.; Enikolopov, G.; Morrison, S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012, 481, 457–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenbaum, A.; Hsu, Y.-M.S.; Day, R.B.; Schuettpelz, L.G.; Christopher, M.J.; Borgerding, J.N.; Nagasawa, T.; Link, D.C. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 2013, 495, 227–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crane, G.; Jeffery, E.; Morrison, S. Adult haematopoietic stem cell niches. Nat. Rev. Immunol. 2017, 17, 573–590. [Google Scholar] [CrossRef]
- Ding, L.; Morrison, S.J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013, 495, 231–235. [Google Scholar] [CrossRef] [Green Version]
- Shu, H.S.; Liu, Y.L.; Tang, X.T.; Zhang, X.S.; Zhou, B.; Zou, W.; Zhou, B.O. Tracing the skeletal progenitor transition during postnatal bone formation. Cell Stem Cell 2021, 28, 2122–2136.e3. [Google Scholar] [CrossRef]
- Mizuhashi, K.; Ono, W.; Matsushita, Y.; Sakagami, N.; Takahashi, A.; Saunders, T.; Nagasawa, T.; Kronenberg, H.M.; Ono, N. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature 2018, 563, 254–258. [Google Scholar] [CrossRef]
- Baryawno, N.; Przybylski, D.; Kowalczyk, M.S.; Kfoury, Y.; Severe, N.; Gustafsson, K.; Kokkaliaris, K.D.; Mercier, F.; Tabaka, M.; Hofree, M.; et al. A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell 2019, 177, 1915–1932.e16. [Google Scholar] [CrossRef]
- Shi, Y.; He, G.; Lee, W.-C.; McKenzie, J.A.; Silva, M.J.; Long, F. Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat. Commun. 2017, 8, 2043. [Google Scholar] [CrossRef]
- Sivaraj, K.K.; Jeong, H.-W.; Dharmalingam, B.; Zeuschner, D.; Adams, S.; Potente, M.; Adams, R.H. Regional specialization and fate specification of bone stromal cells in skeletal development. Cell Rep. 2021, 36, 109352. [Google Scholar] [CrossRef]
- Marecic, O.; Tevlin, R.; McArdle, A.; Seo, E.Y.; Wearda, T.; Duldulao, C.; Walmsley, G.G.; Nguyen, A.; Weissman, I.L.; Chan, C.K.F.; et al. Identification and characterization of an injury-induced skeletal progenitor. Proc. Natl. Acad. Sci. USA 2015, 112, 9920–9925. [Google Scholar] [CrossRef] [Green Version]
- Sacchetti, B.; Funari, A.; Michienzi, S.; Di Cesare, S.; Piersanti, S.; Saggio, I.; Tagliafico, E.; Ferrari, S.; Robey, P.G.; Riminucci, M.; et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007, 131, 324–336. [Google Scholar] [CrossRef] [Green Version]
- Simmons, P.J.; Torok-Storb, B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood 1991, 78, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ghazanfari, R.; Zacharaki, D.; Ditzel, N.; Isern, J.; Ekblom, M.; Méndez-Ferrer, S.; Kassem, M.; Scheding, S. Low/negative expression of PDGFR-α identifies the candidate primary mesenchymal stromal cells in adult human bone marrow. Stem Cell Rep. 2014, 3, 965–974. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the Human Skeletal Stem Cell. Cell 2018, 175, 43–56.e21. [Google Scholar] [CrossRef] [Green Version]
- Gulati, G.S.; Murphy, M.P.; Marecic, O.; Lopez, M.; E Brewer, R.; Koepke, L.S.; Manjunath, A.; Ransom, R.; Salhotra, A.; Weissman, I.L.; et al. Isolation and functional assessment of mouse skeletal stem cell lineage. Nat. Protoc. 2018, 13, 1294–1309. [Google Scholar] [CrossRef]
- Liu, S.; Stroncek, D.F.; Zhao, Y.; Chen, V.; Shi, R.; Chen, J.; Ren, J.; Liu, H.; Bae, H.J.; Highfill, S.L.; et al. Single cell sequencing reveals gene expression signatures associated with bone marrow stromal cell subpopulations and time in culture. J. Transl. Med. 2019, 17, 23. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.Y.; Li, B.; Yin, F.; Liu, H. Single-cell transcriptome analysis of uncultured human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2021, 12, 25. [Google Scholar] [CrossRef]
- Liu, X.; Hou, M.; Zhang, S.; Zhao, Y.; Wang, Q.; Jiang, M.; Du, M.; Shao, Z.; Yuan, H. Neuroprotective effects of bone marrow Sca-1+ cells against age-related retinal degeneration in OPTN E50K mice. Cell Death Dis. 2021, 12, 613. [Google Scholar] [CrossRef]
- Yu, B.; Huo, L.; Liu, Y.; Deng, P.; Szymanski, J.; Li, J.; Luo, X.; Hong, C.; Lin, J.; Wang, C.Y. PGC-1α Controls Skeletal Stem Cell Fate and Bone-Fat Balance in Osteoporosis and Skeletal Aging by Inducing TAZ. Cell Stem Cell 2018, 23, 193–209.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Deng, P.; Liu, Y.; Wu, Y.; Chen, Y.; Guo, Y.; Zhang, S.; Zheng, X.; Zhou, L.; Liu, W.; et al. Alpha-ketoglutarate ameliorates age-related osteoporosis via regulating histone methylations. Nat. Commun. 2020, 11, 5596. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Guo, J.; Qin, J.; Zhang, X.; Sun, Y.; Wei, H.; Cao, D.; Zhang, Y.; Zhao, C.; Xiong, Y.; et al. Single-cell transcriptomics of LepR-positive skeletal cells reveals heterogeneous stress-dependent stem and progenitor pools. EMBO J. 2022, 41, e108415. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Gao, J.; Tang, C.; Xu, Z.; Sun, T. Single-Cell RNA Sequencing of Bone Marrow Mesenchymal Stem Cells from the Elderly People. Int. J. Stem Cells 2022, 15, 173–182. [Google Scholar] [CrossRef]
- Wang, J.; Xia, C.; Pu, M.; Dai, B.; Yang, X.; Shang, R.; Yang, Z.; Zhang, R.; Tao, K.; Dou, K. Silencing of CDCA5 inhibits cancer progression and serves as a prognostic biomarker for hepatocellular carcinoma. Oncol. Rep. 2018, 40, 1875–1884. [Google Scholar] [CrossRef]
- Musa, J.; Aynaud, M.-M.; Mirabeau, O.; Delattre, O.; Grünewald, T.G. MYBL2 (B-Myb): A central regulator of cell proliferation, cell survival and differentiation involved in tumorigenesis. Cell Death Dis. 2017, 8, e2895. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Zheng, X.; Lu, S.; He, Z.; Miao, Y.; Huang, H.; Chu, X.; Cai, C.; Zou, F. Knockdown of FAM64A suppresses proliferation and migration of breast cancer cells. Breast Cancer 2019, 26, 835–845. [Google Scholar] [CrossRef]
- Giunta, S.; Funabiki, H. Integrity of the human centromere DNA repeats is protected by CENP-A, CENP-C, and CENP-T. Proc. Natl. Acad. Sci. USA 2017, 114, 1928–1933. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, R.; You, X.; Zhang, H.; Wei, S.; Cheng, T.; Cao, Q.; Wang, Z.; Chen, Y. The steady-state level of CDK4 protein is regulated by antagonistic actions between PAQR4 and SKP2 and involved in tumorigenesis. J. Mol. Cell Biol. 2017, 9, 409–421. [Google Scholar] [CrossRef] [Green Version]
- Soniat, M.; Cağatay, T.; Chook, Y.M. Recognition Elements in the Histone H3 and H4 Tails for Seven Different Importins. J. Biol. Chem. 2016, 291, 21171–21183. [Google Scholar] [CrossRef]
- Cheloufi, S.; Elling, U.; Hopfgartner, B.; Jung, Y.L.; Murn, J.; Ninova, M.; Hubmann, M.; Badeaux, A.I.; Ang, C.E.; Tenen, D.; et al. The histone chaperone CAF-1 safeguards somatic cell identity. Nature 2015, 528, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Kimura, A.; Matsuda, T.; Sakai, A.; Murao, N.; Nakashima, K. MGB2 expression is associated with transition from a quiescent to an activated state of adult neural stem cells. Dev. Dyn. 2018, 247, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Kanawa, M.; Igarashi, A.; Fujimoto, K.; Higashi, Y.; Kurihara, H.; Sugiyama, M.; Saskianti, T.; Kato, Y.; Kawamoto, T. Genetic Markers Can Predict Chondrogenic Differentiation Potential in Bone Marrow-Derived Mesenchymal Stromal Cells. Stem Cells Int. 2018, 2018, 9530932. [Google Scholar] [CrossRef] [Green Version]
- Narakornsak, S.; Aungsuchawan, S.; Pothacharoen, P.; Markmee, R.; Tancharoen, W.; Laowanitwattana, T.; Thaojamnong, C.; Peerapapong, L.; Boonma, N.; Tasuya, W.; et al. Sesamin encouraging effects on chondrogenic differentiation of human amniotic fluid-derived mesenchymal stem cells. Acta Histochem. 2017, 119, 451–461. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Li, D.; Zhou, X.; Chen, Z. LncRNA NEAT1/miR-29b-3p/BMP1 axis promotes osteogenic differentiation in human bone marrow-derived mesenchymal stem cells. Pathol. Res. Pract. 2019, 215, 525–531. [Google Scholar] [CrossRef]
- Longo, A.; Tobiasch, E.; Luparello, C. Type V collagen counteracts osteo-differentiation of human mesenchymal stem cells. Biologicals 2014, 42, 294–297. [Google Scholar] [CrossRef] [Green Version]
- Belambri, S.A.; Rolas, L.; Raad, H.; Hurtado-Nedelec, M.; Dang, P.M.-C.; El-Benna, J. NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12951. [Google Scholar] [CrossRef] [Green Version]
- Maffioli, E.; Nonnis, S.; Angioni, R.; Santagata, F.; Calì, B.; Zanotti, L.; Negri, A.; Viola, A.; Tedeschi, G. Proteomic analysis of the secretome of human bone marrow-derived mesenchymal stem cells primed by pro-inflammatory cytokines. J. Proteom. 2017, 166, 115–126. [Google Scholar] [CrossRef]
- Moraes, L.A.; Ampomah, P.B.; Lim, L.H.K. Annexin A1 in inflammation and breast cancer: A new axis in the tumor microenvironment. Cell Adhes. Migr. 2018, 12, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Akram, K.M.; Samad, S.; A Spiteri, M.; Forsyth, N.R. Mesenchymal stem cells promote alveolar epithelial cell wound repair in vitro through distinct migratory and paracrine mechanisms. Respir. Res. 2013, 14, 9. [Google Scholar] [CrossRef]
- Krishnaswamy, V.R.; Balaguru, U.M.; Chatterjee, S.; Korrapati, P.S. Dermatopontin augments angiogenesis and modulates the expression of transforming growth factor beta 1 and integrin alpha 3 beta 1 in endothelial cells. Eur. J. Cell Biol. 2017, 96, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Harayama, M.; Kanemura, S.; Sitia, R.; Inaba, K. Structural basis of pH-dependent client binding by ERp44, a key regulator of protein secretion at the ER-Golgi interface. Proc. Natl. Acad. Sci. USA 2017, 114, E3224–E3232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Lee, J.; Liem, D.; Ping, P. HSPA5 Gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum. Gene 2017, 618, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Cabahug-Zuckerman, P.; Liu, C.; Cai, C.; Mahaffey, I.; Norman, S.C.; Cole, W.; Castillo, A.B. Site-Specific Load-Induced Expansion of Sca-1+ Prrx1+ and Sca-1−Prrx1+ Cells in Adult Mouse Long Bone Is Attenuated With Age. JBMR Plus 2019, 3, e10199. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Cabahug-Zuckerman, P.; Stubbs, C.; Pendola, M.; Cai, C.; A Mann, K.; Castillo, A.B. Mechanical Loading Promotes the Expansion of Primitive Osteoprogenitors and Organizes Matrix and Vascular Morphology in Long Bone Defects. J. Bone Miner. Res. 2019, 34, 896–910. [Google Scholar] [CrossRef]
- Moore, E.R.; Zhu, Y.X.; Ryu, H.S.; Jacobs, C.R. Periosteal progenitors contribute to load-induced bone formation in adult mice and require primary cilia to sense mechanical stimulation. Stem Cell Res. Ther. 2018, 9, 190. [Google Scholar] [CrossRef] [Green Version]
- Zannit, H.M.; Silva, M.J. Proliferation and Activation of Osterix-Lineage Cells Contribute to Loading-Induced Periosteal Bone Formation in Mice. JBMR Plus 2019, 3, e10227. [Google Scholar] [CrossRef] [Green Version]
- Leucht, P.; Temiyasathit, S.; Russell, A.; Arguello, J.F.; Jacobs, C.R.; Helms, J.A.; Castillo, A.B. CXCR4 antagonism attenuates load-induced periosteal bone formation in mice. J. Orthop. Res. 2013, 31, 1828–1838. [Google Scholar] [CrossRef]
- Shen, B.; Tasdogan, A.; Ubellacker, J.M.; Zhang, J.; Nosyreva, E.D.; Du, L.; Murphy, M.M.; Hu, S.; Yi, Y.; Kara, N.; et al. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature 2021, 591, 438–444. [Google Scholar] [CrossRef]
- Ozcivici, E.; Luu, Y.K.; Rubin, C.T.; Judex, S. Low-level vibrations retain bone marrow’s osteogenic potential and augment recovery of trabecular bone during reambulation. PLoS ONE 2010, 5, e11178. [Google Scholar] [CrossRef]
- Booker, C.N.; Haga, C.L.; Boregowda, S.V.; Strivelli, J.; Phinney, D.G. Transcriptional responses of skeletal stem/progenitor cells to hindlimb unloading and recovery correlate with localized but not systemic multi-systems impacts. NPJ Microgravity 2021, 7, 49. [Google Scholar] [CrossRef]
- Adesida, A.B.; Mulet-Sierra, A.; Jomha, N.M. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res. Ther. 2012, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Spiller, K.V.; Tang, J.; Karner, C.M.; Hilton, M.J.; Wu, C. Hypoxia depletes contaminating CD45+ hematopoietic cells from murine bone marrow stromal cell (BMSC) cultures: Methods for BMSC culture purification. Stem Cell Res. 2021, 53, 102317. [Google Scholar] [CrossRef]
- Regan, J.N.; Lim, J.; Shi, Y.; Joeng, K.S.; Arbeit, J.M.; Shohet, R.V.; Long, F. Up-regulation of glycolytic metabolism is required for HIF1α-driven bone formation. Proc. Natl. Acad. Sci. USA 2014, 111, 8673–8678. [Google Scholar] [CrossRef] [Green Version]
- van Gastel, N.; Stegen, S.; Eelen, G.; Schoors, S.; Carlier, A.; Daniëls, V.W.; Baryawno, N.; Przybylski, D.; Depypere, M.; Stiers, P.J.; et al. Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature 2020, 579, 111–117. [Google Scholar] [CrossRef]
- Severe, N.; Karabacak, N.M.; Gustafsson, K.; Baryawno, N.; Courties, G.; Kfoury, Y.; Kokkaliaris, K.D.; Rhee, C.; Lee, D.; Scadden, E.W.; et al. Stress-Induced Changes in Bone Marrow Stromal Cell Populations Revealed through Single-Cell Protein Expression Mapping. Cell Stem Cell 2018, 25, 570–583.e7. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, C.; Wu, J.; Quan, W.; Cheng, C.; Zhou, J.; Chen, L.; Xiang, L.; Li, F.; Zhang, K.; et al. In vitro expansion affects the response of human bone marrow stromal cells to irradiation. Stem Cell Res. Ther. 2019, 10, 82. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zhang, S.; Wang, J.; Li, Q.; Xue, H.; Sheng, R.; Xiong, Q.; Qi, X.; Wen, J.; Fan, Y.; et al. LepR-Expressing Stem Cells Are Essential for Alveolar Bone Regeneration. J. Dent. Res. 2020, 99, 1279–1286. [Google Scholar] [CrossRef]
- Yang, M.; Arai, A.; Udagawa, N.; Zhao, L.; Nishida, D.; Murakami, K.; Hiraga, T.; Takao-Kawabata, R.; Matsuo, K.; Komori, T.; et al. Parathyroid Hormone Shifts Cell Fate of a Leptin Receptor-Marked Stromal Population from Adipogenic to Osteoblastic Lineage. J. Bone Miner. Res. 2019, 34, 1952–1963. [Google Scholar] [CrossRef]
- Zhang, J.; Cohen, A.; Shen, B.; Du, L.; Tasdogan, A.; Zhao, Z.; Shane, E.J.; Morrison, S.J. The effect of parathyroid hormone on osteogenesis is mediated partly by osteolectin. Proc. Natl. Acad. Sci. USA 2021, 118, e2026176118. [Google Scholar] [CrossRef]
- Caire, R.; Roche, B.; Picot, T.; Aanei, C.-M.; He, Z.; Campos, L.; Thomas, M.; Malaval, L.; Vico, L.; Lafage-Proust, M.-H. Parathyroid Hormone Remodels Bone Transitional Vessels and the Leptin Receptor-Positive Pericyte Network in Mice. J. Bone Miner. Res. 2019, 34, 1487–1501. [Google Scholar] [CrossRef]
- Yang, M.; Arai, A.; Udagawa, N.; Hiraga, T.; Lijuan, Z.; Ito, S.; Komori, T.; Moriishi, T.; Matsuo, K.; Shimoda, K.; et al. Osteogenic Factor Runx2 Marks a Subset of Leptin Receptor-Positive Cells that Sit Atop the Bone Marrow Stromal Cell Hierarchy. Sci. Rep. 2017, 7, 4928. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozcivici, E. Effects of spaceflight on cells of bone marrow origin. Turk. J. Haematol. 2013, 30, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveiras, O.; Nardi, V.; Wenzel, P.L.; Hauschka, P.V.; Fahey, F.H.; Daley, G.Q. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 2009, 460, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esbrit, P.; Alcaraz, M.J. Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies. Biochem. Pharmacol. 2013, 85, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- de Lageneste, O.D.; Julien, A.; Abou-Khalil, R.; Frangi, G.; Carvalho, C.; Cagnard, N.; Cordier, C.; Conway, S.J.; Colnot, C. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat. Commun. 2018, 9, 773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debnath, S.; Yallowitz, A.R.; McCormick, J.; Lalani, S.; Zhang, T.; Xu, R.; Li, N.; Liu, Y.; Yang, Y.S.; Eiseman, M.; et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 2018, 562, 133–139. [Google Scholar] [CrossRef]
- Buettmann, E.G.; A McKenzie, J.; Migotsky, N.; Sykes, D.A.; Hu, P.; Yoneda, S.; Silva, M.J. VEGFA From Early Osteoblast Lineage Cells (Osterix+) Is Required in Mice for Fracture Healing. J. Bone Miner. Res. 2019, 34, 1690–1706. [Google Scholar] [CrossRef]
- Collette, N.; Yee, C.S.; Hum, N.; Murugesh, D.K.; Christiansen, B.; Xie, L.; Economides, A.; Manilay, J.O.; Robling, A.G.; Loots, G.G. Sostdc1 deficiency accelerates fracture healing by promoting the expansion of periosteal mesenchymal stem cells. Bone 2016, 88, 20–30. [Google Scholar] [CrossRef]
- Zhou, X.; Von Der Mark, K.; Henry, S.; Norton, W.; Adams, H.; De Crombrugghe, B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 2014, 10, e1004820. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-J.; Cheng, P.; Liang, M.-K.; Chen, Y.-S.; Lu, Q.; Wang, J.-Y.; Xia, Z.-Y.; Zhou, H.-D.; Cao, X.; Xie, H.; et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J. Clin. Investig. 2015, 125, 1509–1522. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Qu, M.; Gong, W.; Zhou, C.; Lai, Y.; Xiao, G. Kindlin-2 deletion in osteoprogenitors causes severe chondrodysplasia and low-turnover osteopenia in mice. J. Orthop. Translat. 2021, 32, 41–48. [Google Scholar] [CrossRef]
- Kim, H.-N.; Ponte, F.; Warren, A.; Ring, R.; Iyer, S.; Han, L.; Almeida, M. A decrease in NAD+ contributes to the loss of osteoprogenitors and bone mass with aging. NPJ Aging Mech. Dis. 2021, 7, 8. [Google Scholar] [CrossRef]
- Liu, G.; Xie, Y.; Su, J.; Qin, H.; Wu, H.; Li, K.; Yu, B.; Zhang, X. The role of EGFR signaling in age-related osteoporosis in mouse cortical bone. FASEB J. 2019, 33, 11137–11147. [Google Scholar] [CrossRef] [Green Version]
- Lerbs, T.; Cui, L.; Muscat, C.; Saleem, A.; van Neste, C.; Domizi, P.; Chan, C.; Wernig, G. Expansion of Bone Precursors through Jun as a Novel Treatment for Osteoporosis-Associated Fractures. Stem Cell Rep. 2020, 14, 603–613. [Google Scholar] [CrossRef]
- Wang, T.; Yang, L.; Liang, Z.; Bai, L.; Pei, H.; Zhang, T.; Wu, L.; Wang, L.; Wang, X.; You, X.; et al. Pulsed electromagnetic fields attenuate glucocorticoid-induced bone loss by targeting senescent LepR+ bone marrow mesenchymal stromal cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 133, 112635. [Google Scholar] [CrossRef]
- Pagani, C.A.; Huber, A.K.; Hwang, C.; Marini, S.; Padmanabhan, K.; Livingston, N.; Nunez, J.; Sun, Y.; Edwards, N.; Cheng, Y.-H.; et al. Novel Lineage-Tracing System to Identify Site-Specific Ectopic Bone Precursor Cells. Stem Cell Rep. 2021, 16, 626–640. [Google Scholar] [CrossRef]
- Agarwal, S.; Loder, S.; Brownley, C.; Cholok, D.; Mangiavini, L.; Li, J.; Breuler, C.; Sung, H.H.; Li, S.; Ranganathan, K.; et al. Inhibition of Hif1α prevents both trauma-induced and genetic heterotopic ossification. Proc. Natl. Acad. Sci. USA 2016, 113, E338–E347. [Google Scholar] [CrossRef] [Green Version]
- Hwang, C.; Marini, S.; Huber, A.K.; Stepien, D.M.; Sorkin, M.; Loder, S.; Pagani, C.; Li, J.; Visser, N.D.; Vasquez, K.; et al. Mesenchymal VEGFA induces aberrant differentiation in heterotopic ossification. Bone Res. 2019, 7, 36. [Google Scholar] [CrossRef]
- Tencerova, M.; Frost, M.; Figeac, F.; Nielsen, T.K.; Ali, D.; Lauterlein, J.-J.L.; Andersen, T.L.; Haakonsson, A.K.; Rauch, A.; Madsen, J.S.; et al. Obesity-Associated Hypermetabolism and Accelerated Senescence of Bone Marrow Stromal Stem Cells Suggest a Potential Mechanism for Bone Fragility. Cell Rep. 2019, 27, 2050–2062.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picke, A.-K.; Campbell, G.M.; Blüher, M.; Krügel, U.; Schmidt, F.N.; Tsourdi, E.; Winzer, M.; Rauner, M.; Vukicevic, V.; Busse, B.; et al. Thy-1 (CD90) promotes bone formation and protects against obesity. Sci. Transl. Med. 2018, 10, eaao6806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosi, T.H.; Scialdone, A.; Graja, A.; Gohlke, S.; Jank, A.-M.; Bocian, C.; Woelk, L.; Fan, H.; Logan, D.W.; Schürmann, A.; et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 2017, 20, 771–784.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanoun, M.; Zhang, D.; Toshihide, M.; Piho, S.; Pierce, H.; Kunisaki, Y.; Lacombe, J.; Armstrong, S.A.; Dührsen, U.; Frenette, P.S. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell 2014, 15, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Forte, D.; García-Fernández, M.; Sánchez-Aguilera, A.; Stavropoulou, V.; Fielding, C.; Martín-Pérez, D.; López, J.A.; Costa, A.S.; Tronci, L.; Nikitopoulou, E.; et al. Bone Marrow Mesenchymal Stem Cells Support Acute Myeloid Leukemia Bioenergetics and Enhance Antioxidant Defense and Escape from Chemotherapy. Cell Metab. 2020, 32, 829–843.e9. [Google Scholar] [CrossRef]
- Schepers, K.; Pietras, E.M.; Reynaud, D.; Flach, J.; Binnewies, M.; Garg, T.; Wagers, A.J.; Hsiao, E.C.; Passegué, E. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell 2013, 13, 285–299. [Google Scholar] [CrossRef] [Green Version]
- van Gastel, N.; Spinelli, J.B.; Sharda, A.; Schajnovitz, A.; Baryawno, N.; Rhee, C.; Oki, T.; Grace, E.; Soled, H.J.; Milosevic, J.; et al. Induction of a Timed Metabolic Collapse to Overcome Cancer Chemoresistance. Cell Metab. 2020, 32, 391–403.e6. [Google Scholar] [CrossRef]
- Yuan, B.; Ly, S.; Nguyen, K.; Tran, V.; Maldonado, K.; Kinglsey, C.; Burks, J.K.; Zhou, X.; Decrombrugghe, B.; Andreeff, M.; et al. Acute Myeloid Leukemia Expands Osteoprogenitor Rich Niche in the Bone Marrow but Resorbs Mature Bone Causing Osteopenia/Osteoporosis in Animal Models. Blood 2018, 132, 86. [Google Scholar] [CrossRef]
- Chen, Y.; Hoffmeister, L.M.; Zaun, Y.; Arnold, L.; Schmid, K.W.; Giebel, B.; Klein-Hitpass, L.; Hanenberg, H.; Squire, A.; Reinhardt, H.C.; et al. Acute myeloid leukemia-induced remodeling of the human bone marrow niche predicts clinical outcome. Blood Adv. 2020, 4, 5257–5268. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, P.; Zhang, X.; Lv, L.; Zhou, Y. Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis. Cell Prolif. 2021, 54, e12956. [Google Scholar] [CrossRef]
- Pagnotti, G.M.; Styner, M.; Uzer, G.; Patel, V.S.; Wright, L.E.; Ness, K.K.; Guise, T.A.; Rubin, J.; Rubin, C.T. Combating osteoporosis and obesity with exercise: Leveraging cell mechanosensitivity. Nat. Rev. Endocrinol. 2019, 15, 339–355. [Google Scholar] [CrossRef]
- Lounev, V.Y.; Ramachandran, R.; Wosczyna, M.N.; Yamamoto, M.; DA Maidment, A.; Shore, E.M.; Glaser, D.L.; Goldhamer, D.J.; Kaplan, F.S. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J. Bone Jt. Surg. Am. 2009, 91, 652–663. [Google Scholar] [CrossRef] [Green Version]
- Medici, D.; Shore, E.M.; Lounev, V.Y.; Kaplan, F.S.; Kalluri, R.; Olsen, B.R. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat. Med. 2010, 16, 1400–1406. [Google Scholar] [CrossRef]
- Prados, B.; Del Toro, R.; MacGrogan, D.; Gómez-Apiñániz, P.; Papoutsi, T.; Muñoz-Cánoves, P.; Méndez-Ferrer, S.; de la Pompa, J.L. Heterotopic ossification in mice overexpressing Bmp2 in Tie2+ lineages. Cell Death Dis. 2021, 12, 729. [Google Scholar] [CrossRef]
- Agarwal, S.; Loder, S.J.; Cholok, D.; Peterson, J.; Li, J.; Breuler, C.; Brownley, R.C.; Sung, H.H.; Chung, M.T.; Kamiya, N.; et al. Scleraxis-Lineage Cells Contribute to Ectopic Bone Formation in Muscle and Tendon. Stem Cells 2017, 35, 705–710. [Google Scholar] [CrossRef] [Green Version]
- Wosczyna, M.N.; A Biswas, A.; A Cogswell, C.; Goldhamer, D.J. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J. Bone Miner. Res. 2012, 27, 1004–1017. [Google Scholar] [CrossRef] [Green Version]
- Dey, D.; Bagarova, J.; Hatsell, S.J.; Armstrong, K.A.; Huang, L.; Ermann, J.; Vonner, A.J.; Shen, Y.; Mohedas, A.H.; Lee, A.; et al. Two tissue-resident progenitor lineages drive distinct phenotypes of heterotopic ossification. Sci. Transl. Med. 2016, 8, 366ra163. [Google Scholar] [CrossRef]
- Stricker, S.; Mathia, S.; Haupt, J.; Seemann, P.; Meier, J.; Mundlos, S. Odd-skipped related genes regulate differentiation of embryonic limb mesenchyme and bone marrow mesenchymal stromal cells. Stem Cells Dev. 2012, 21, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Moore, A.L.; Desjardins-Park, H.E.; Duoto, B.A.; Mascharak, S.; Murphy, M.P.; Irizarry, D.M.; Foster, D.S.; Jones, R.E.; Barnes, L.A.; Marshall, C.D.; et al. Doxycycline Reduces Scar Thickness and Improves Collagen Architecture. Ann. Surg. 2020, 272, 183–193. [Google Scholar] [CrossRef]
- Kfoury, Y.; Scadden, D.T. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell 2015, 16, 239–253. [Google Scholar] [CrossRef]
- Dong, L.; Yu, W.-M.; Zheng, H.; Loh, M.L.; Bunting, S.T.; Pauly, M.; Huang, G.; Zhou, M.; Broxmeyer, H.E.; Scadden, D.T.; et al. Leukaemogenic effects of Ptpn11 activating mutations in the stem cell microenvironment. Nature 2016, 539, 304–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kode, A.; Manavalan, J.S.; Mosialou, I.; Bhagat, G.; Rathinam, C.V.; Luo, N.; Khiabanian, H.; Lee, A.; Murty, V.V.; Friedman, R.; et al. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature 2014, 506, 240–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raaijmakers, M.H.G.P.; Mukherjee, S.; Guo, S.; Zhang, S.; Kobayashi, T.; Schoonmaker, J.A.; Ebert, B.L.; Al-Shahrour, F.; Hasserjian, R.P.; Scadden, E.O.; et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 2010, 464, 852–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siveen, K.S.; Uddin, S.; Mohammad, R.M. Targeting acute myeloid leukemia stem cell signaling by natural products. Mol. Cancer 2017, 16, 13. [Google Scholar] [CrossRef] [Green Version]
- O’Reilly, E.; Zeinabad, H.A.; Szegezdi, E. Hematopoietic versus leukemic stem cell quiescence: Challenges and therapeutic opportunities. Blood Rev. 2021, 50, 100850. [Google Scholar] [CrossRef]
- Battula, V.L.; Le, P.M.; Sun, J.C.; Nguyen, K.; Yuan, B.; Zhou, X.; Sonnylal, S.; McQueen, T.; Ruvolo, V.; Michel, K.A.; et al. AML-induced osteogenic differentiation in mesenchymal stromal cells supports leukemia growth. JCI Insight 2017, 2, e90036. [Google Scholar] [CrossRef]
- Lange, M.; Bergen, V.; Klein, M.; Setty, M.; Reuter, B.; Bakhti, M.; Lickert, H.; Ansari, M.; Schniering, J.; Schiller, H.B.; et al. CellRank for directed single-cell fate mapping. Nat. Methods 2022, 19, 159–170. [Google Scholar] [CrossRef]
- Ning, K.; Liu, S.; Yang, B.; Wang, R.; Man, G.; Wang, D.-E.; Xu, H. Update on the effects of energy metabolism in bone marrow mesenchymal stem cells differentiation. Mol. Metab. 2022, 58, 101450. [Google Scholar] [CrossRef]
- Joffin, N.; Paschoal, V.A.; Gliniak, C.M.; Crewe, C.; Elnwasany, A.; Szweda, L.I.; Zhang, Q.; Hepler, C.; Kusminski, C.M.; Gordillo, R.; et al. Mitochondrial metabolism is a key regulator of the fibro-inflammatory and adipogenic stromal subpopulations in white adipose tissue. Cell Stem Cell 2021, 28, 702–717.e8. [Google Scholar] [CrossRef]
| Pathological Conditions | BMSC Subpopulations | Functions | References |
|---|---|---|---|
| Fracture | Mx1+ aSMA+, Grem1+, LepR+, Periostin+, and CTSK+, Osx+, Osx+ Sostdc1(−/−) | Expands and actives osteogenesis in response to bone fracture | [4,16,27,107,108,109,110,111] |
| Osteoporosis | Prx1+, Osx+, Thy+/6c3− and LepR+ | Exhibits lower osteogenic potential: Prx1+, Osx+ | [61,112,113,114,115,116] |
| Actives Thy+ /6c3−, LepR+ can treat osteoporosis | [116,117] | ||
| Heterotopic ossification | Prx1+, PDGFRα+, Hoxa11+ | Upregulates Hif1α or increases VEGFA secretion to promote endochondral ossification to form extraskeletal bone | [118,119,120] |
| Obesity | IR+ and LepR+ in obese bone marrow; CD45− Sca-1+, Sca1+ CD24+ | Exhibits lower osteogenesis potential and dysregulated metabolism; | [121] |
| Impairs osteogenesis capacity through dowregulating Thy-1 expression or upregulating DPP4 level | [122,123] | ||
| AML | Nes+, CD45− Ter119− CD31− LepR+, Osx+, DAPI− CD45− CD235a− CD31− CD146low/+ CD271+ | Decreases BMSCs number, altered cell shape, and impairs multi-potential | [124,125,126,127,128,129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, K.; Yang, B.; Chen, M.; Man, G.; Liu, S.; Wang, D.-e.; Xu, H. Functional Heterogeneity of Bone Marrow Mesenchymal Stem Cell Subpopulations in Physiology and Pathology. Int. J. Mol. Sci. 2022, 23, 11928. https://doi.org/10.3390/ijms231911928
Ning K, Yang B, Chen M, Man G, Liu S, Wang D-e, Xu H. Functional Heterogeneity of Bone Marrow Mesenchymal Stem Cell Subpopulations in Physiology and Pathology. International Journal of Molecular Sciences. 2022; 23(19):11928. https://doi.org/10.3390/ijms231911928
Chicago/Turabian StyleNing, Kaiting, Baoqiang Yang, Meng Chen, Guigui Man, Shuaiting Liu, Dong-en Wang, and Huiyun Xu. 2022. "Functional Heterogeneity of Bone Marrow Mesenchymal Stem Cell Subpopulations in Physiology and Pathology" International Journal of Molecular Sciences 23, no. 19: 11928. https://doi.org/10.3390/ijms231911928
APA StyleNing, K., Yang, B., Chen, M., Man, G., Liu, S., Wang, D.-e., & Xu, H. (2022). Functional Heterogeneity of Bone Marrow Mesenchymal Stem Cell Subpopulations in Physiology and Pathology. International Journal of Molecular Sciences, 23(19), 11928. https://doi.org/10.3390/ijms231911928





