Predicting the Recurrence of Gastric Cancer Using the Textural Features of Perigastric Adipose Tissue on [18F]FDG PET/CT
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
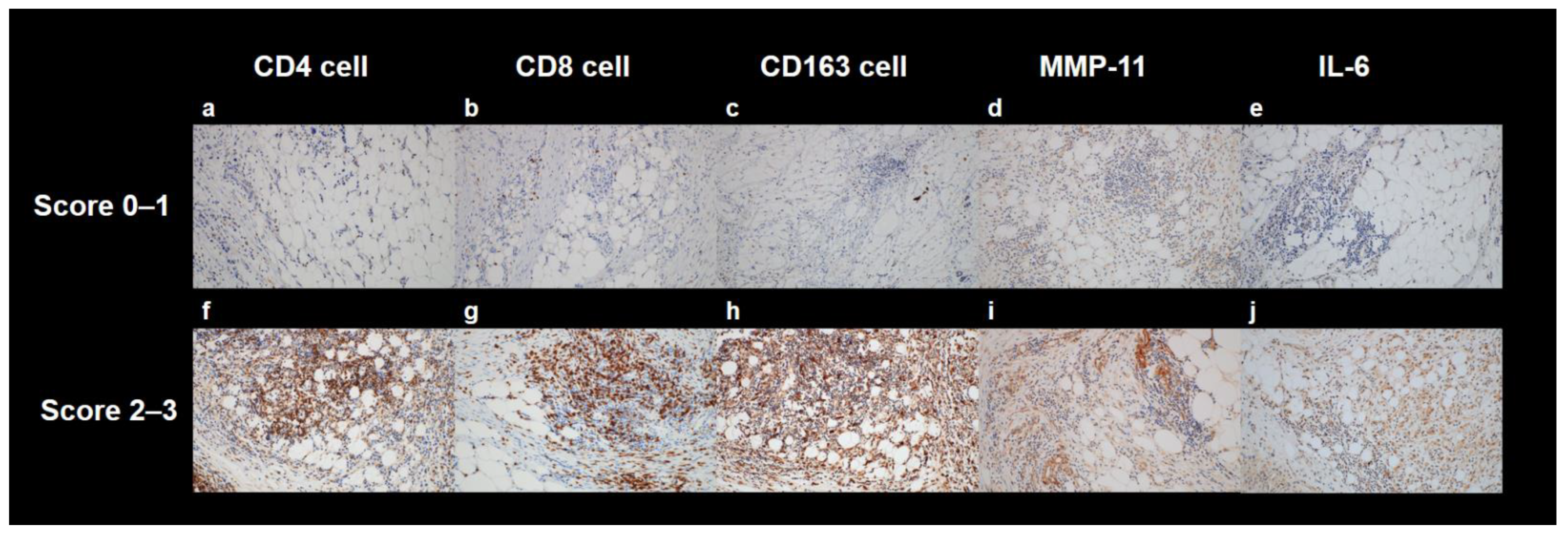
2.2. Correlation Analysis between PET Textural Features and Histopathological Results
2.3. Survival Analysis for RFS
3. Discussion
4. Materials and Methods
4.1. Patient Selection
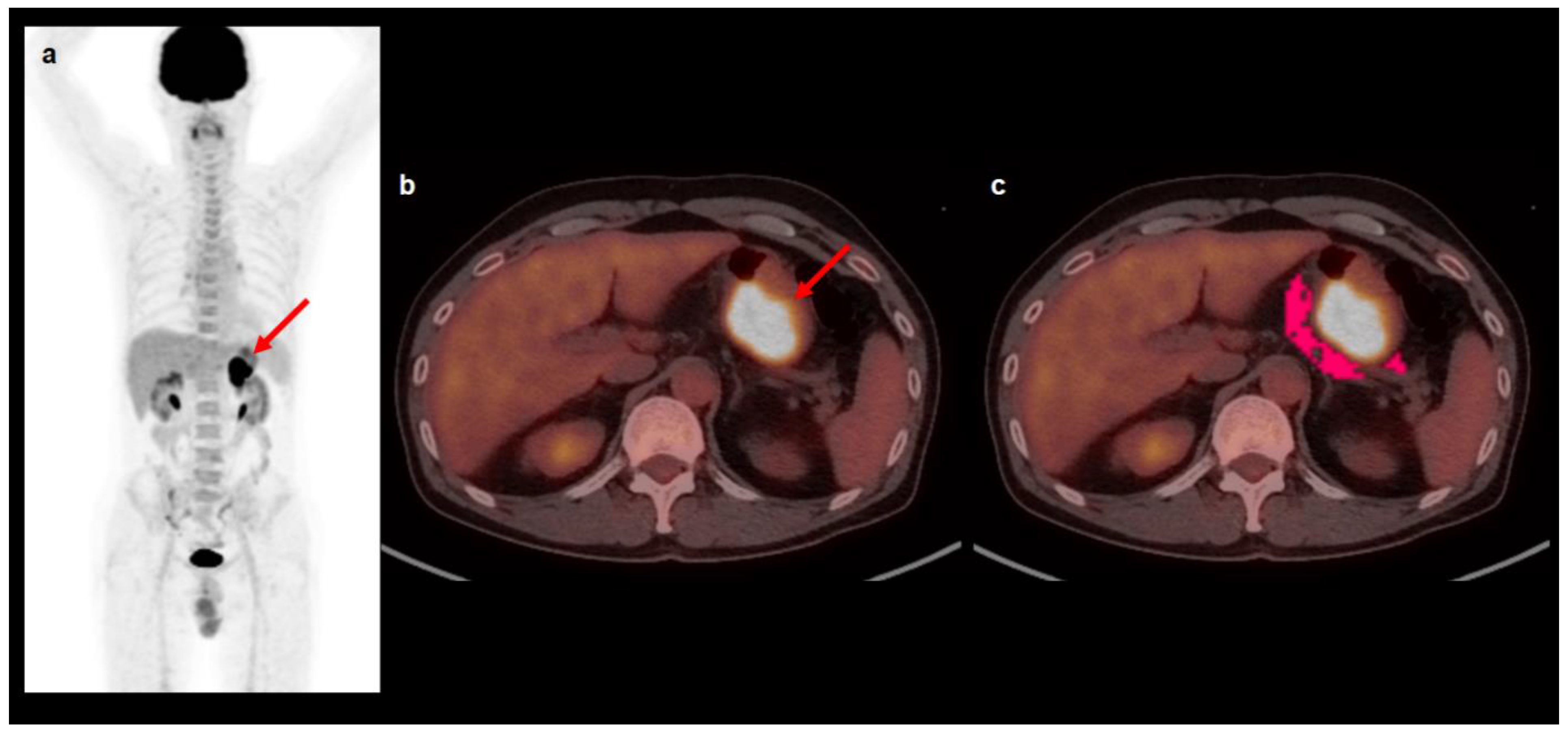
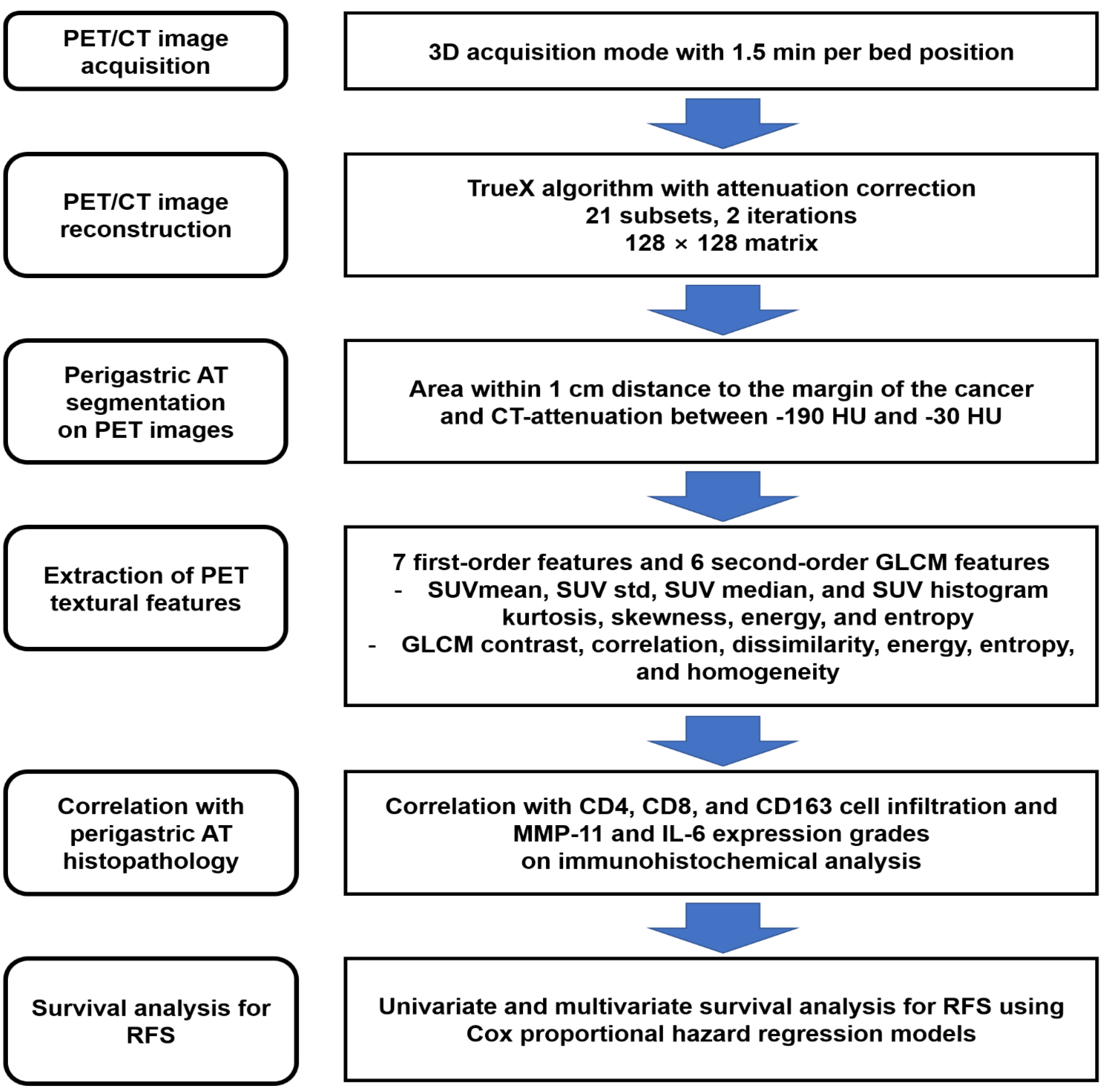
4.2. [18F]FDG PET/CT and Image Analysis
4.3. Histopathological Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Xu, A.; Sun, X.; Zhao, X.; Xia, Y.; Rao, H.; Zhang, Y.; Zhang, R.; Chen, L.; Zhang, T.; et al. Three-year outcomes of the randomized phase III SEIPLUS trial of extensive intraoperative peritoneal lavage for locally advanced gastric cancer. Nat. Commun. 2021, 12, 6598. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.-W.; Chen, Q.-Y.; Zheng, W.-Z.; He, Q.-C.; Huang, Z.-N.; Xie, J.-W.; Wang, J.-B.; Lin, J.-X.; Lu, J.; Cao, L.-L.; et al. Postoperative follow-up for gastric cancer needs to be individualized according to age, tumour recurrence pattern, and recurrence time. Eur. J. Surg. Oncol. 2022, 48, 1790–1798. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, S.M.; Lee, M.-S.; Shin, H.C. Role of 18F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur. J. Pediatr. 2012, 39, 1425–1434. [Google Scholar] [CrossRef]
- Nieman, K.M.; Romero, I.L.; Van Houten, B.; Lengyel, E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2013, 1831, 1533–1541. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; He, S. Multi-faceted role of cancer-associated adipocytes in the tumor microenvironment. Mol. Med. Rep. 2021, 24, 866. [Google Scholar] [CrossRef]
- Zoico, E.; Rizzatti, V.; Darra, E.; Budui, S.L.; Franceschetti, G.; Vinante, F.; Pedrazzani, C.; Guglielmi, A.; De Manzoni, G.; Mazzali, G.; et al. Morphological and Functional Changes in the Peritumoral Adipose Tissue of Colorectal Cancer Patients. Obesity 2017, 25, S87–S94. [Google Scholar] [CrossRef] [Green Version]
- Burlaka, A.; Ganusevich, I.; Vovk, A.; Gafurov, M.; Lukin, S. Redox state of adipose tissue for patients with gastric cancer and its connection with the body mass index and distance from the tumor. Obes. Res. Clin. Pr. 2019, 14, 34–38. [Google Scholar] [CrossRef]
- Natsume, M.; Shimura, T.; Iwasaki, H.; Okuda, Y.; Hayashi, K.; Takahashi, S.; Kataoka, H. Omental adipocytes promote peritoneal metastasis of gastric cancer through the CXCL2–VEGFA axis. Br. J. Cancer 2020, 123, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Akutagawa, T.; Aoki, S.; Yamamoto-Rikitake, M.; Iwakiri, R.; Fujimoto, K.; Toda, S. Cancer–adipose tissue interaction and fluid flow synergistically modulate cell kinetics, HER2 expression, and trastuzumab efficacy in gastric cancer. Gastric Cancer 2018, 21, 946–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch, K.D.; Chicklore, S.; Cook, G.J.; Davies, A.R.; Kelly, M.; Gossage, J.A.; Baker, C.R. Staging FDG PET-CT changes management in patients with gastric adenocarcinoma who are eligible for radical treatment. Eur. J. Pediatr. 2019, 47, 759–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Lee, S.M.; Son, M.W.; Lee, M.-S. Diagnostic performance of FDG PET/CT for surveillance in asymptomatic gastric cancer patients after curative surgical resection. Eur. J. Pediatr. 2015, 43, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Cho, Y.S.; Choi, J.Y. KSNM60 in Clinical Nuclear Oncology. Nucl. Med. Mol. Imaging 2021, 55, 210–224. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, J.W.; Lee, J.-H.; Jo, I.Y.; Jang, S.J. Prognostic Value of Dual-Time-Point [18F]FDG PET/CT for Predicting Distant Metastasis after Treatment in Patients with Non-Small Cell Lung Cancer. J. Pers. Med. 2022, 12, 592. [Google Scholar] [CrossRef]
- Ahn, H.; Lee, J.W.; Jang, S.-H.; Lee, H.J.; Lee, J.-H.; Oh, M.-H.; Lee, S.M. Prognostic significance of imaging features of peritumoral adipose tissue in FDG PET/CT of patients with colorectal cancer. Eur. J. Radiol. 2021, 145, 110047. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.Y.; Han, S.W.; Lee, J.E.; Hong, S.H.; Lee, S.M.; Jo, I.Y. Clinical Significance of Peritumoral Adipose Tissue PET/CT Imaging Features for Predicting Axillary Lymph Node Metastasis in Patients with Breast Cancer. J. Pers. Med. 2021, 11, 1029. [Google Scholar] [CrossRef]
- Lee, J.W.; Jeon, Y.S.; Kim, K.H.; Yang, H.J.; Lee, C.H.; Lee, S.M. Prognostic Value of CT-Attenuation and 18F-Fluorodeoxyglucose Uptake of Periprostatic Adipose Tissue in Patients with Prostate Cancer. J. Pers. Med. 2020, 10, 185. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, S.M. Radiomics in Oncological PET/CT: Clinical Applications. Nucl. Med. Mol. Imaging 2017, 52, 170–189. [Google Scholar] [CrossRef]
- Ahn, H.; Song, G.J.; Jang, S.-H.; Lee, H.J.; Lee, M.-S.; Lee, J.-H.; Oh, M.-H.; Jeong, G.C.; Lee, S.M.; Lee, J.W. Relationship of FDG PET/CT Textural Features with the Tumor Microenvironment and Recurrence Risks in Patients with Advanced Gastric Cancers. Cancers 2022, 14, 3936. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, Q.; Lv, W.; Xi, S.; Huang, W.; Sun, Z.; Chen, H.; Zhao, L.; Liu, W.; Hu, Y.; et al. Radiomic signature of 18F fluorodeoxyglucose PET/CT for prediction of gastric cancer survival and chemotherapeutic benefits. Theranostics 2018, 8, 5915–5928. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Kang, S.Y.; Yoon, J.; Kim, T.-Y.; Cheon, G.J.; Oh, D.-Y. Prospective evaluation of metabolic intratumoral heterogeneity in patients with advanced gastric cancer receiving palliative chemotherapy. Sci. Rep. 2021, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Son, M.W.; Chung, I.K.; Cho, Y.S.; Lee, M.-S.; Lee, S.M. Significance of CT attenuation and F-18 fluorodeoxyglucose uptake of visceral adipose tissue for predicting survival in gastric cancer patients after curative surgical resection. Gastric Cancer 2019, 23, 273–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, M.S.; White, A.; Perry, R.J.; Camporez, J.-P.; Hidalgo, J.; Shulman, G.I.; Davis, R.J. Regulation of adipose tissue inflammation by interleukin 6. Proc. Natl. Acad. Sci. USA 2020, 117, 2751–2760. [Google Scholar] [CrossRef] [Green Version]
- Feliciano, E.M.C.; Winkels, R.M.; A Meyerhardt, J.; Prado, C.M.; A Afman, L.; Caan, B.J. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am. J. Clin. Nutr. 2021, 114, 1917–1924. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, S.; Lee, H.S.; Park, E.J.; Baik, S.H.; Jeon, T.J.; Lee, K.Y.; Ryu, Y.H.; Kang, J. Different prognostic impact of glucose uptake in visceral adipose tissue according to sex in patients with colorectal cancer. Sci. Rep. 2021, 11, 21556. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Chung, Y. Prognostic value of CT attenuation and FDG uptake of adipose tissue in patients with pancreatic adenocarcinoma. Clin. Radiol. 2018, 73, 1056.e1–1056.e10. [Google Scholar] [CrossRef]
- Tamal, M. A Phantom Study to Investigate Robustness and Reproducibility of Grey Level Co-Occurrence Matrix (GLCM)-Based Radiomics Features for PET. Appl. Sci. 2021, 11, 535. [Google Scholar] [CrossRef]
- Piao, H.; Fu, L.; Wang, Y.; Liu, Y.; Wang, Y.; Meng, X.; Yang, D.; Xiao, X.; Zhang, J. A positive feedback loop between gastric cancer cells and tumor-associated macrophage induces malignancy progression. J. Exp. Clin. Cancer Res. 2022, 41, 174. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Wu, F.; Zhou, Y.; Bao, Z.; Li, H.; Zheng, P.; Zhao, S. Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Dis. 2019, 10, 918. [Google Scholar] [CrossRef]
- Yang, E.; Chua, W.; Ng, W.; Roberts, T.L. Peripheral Cytokine Levels as a Prognostic Indicator in Gastric Cancer: A Review of Existing Literature. Biomedicines 2021, 9, 1916. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Zhang, H.; Zhou, Z.; Chen, M.; Wang, Q. Tumor-associated macrophages induce PD-L1 expression in gastric cancer cells through IL-6 and TNF-ɑ signaling. Exp. Cell Res. 2020, 396, 112315. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, T.; Okada, R.; Suzuki, Y.; Takagi, M.; Yamazaki, T.; Sumi, T.; Aoki, T.; Ohnuma, S.; Aoki, T. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: Role of IL-6 as a prognostic factor. Gastric Cancer 2005, 8, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, M.C.; Svensson, M.; Nodin, B.; Borg, D.; Hedner, C.; Hjalmarsson, C.; Leandersson, K.; Jirström, K. High Infiltration of CD68+/CD163− Macrophages Is an Adverse Prognostic Factor after Neoadjuvant Chemotherapy in Esophageal and Gastric Adenocarcinoma. J. Innate Immun. 2022, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Glass, E.B.; Hoover, A.A.; Bullock, K.K.; Madden, M.Z.; Reinfeld, B.I.; Harris, W.; Parker, D.; Hufnagel, D.H.; Crispens, M.A.; Khabele, D.; et al. Stimulating TAM-mediated anti-tumor immunity with mannose-decorated nanoparticles in ovarian cancer. BMC Cancer 2022, 22, 497. [Google Scholar] [CrossRef]
- Bailly, C.; Bodet-Milin, C.; Couespel, S.; Necib, H.; Kraeber-Bodéré, F.; Ansquer, C.; Carlier, T. Revisiting the Robustness of PET-Based Textural Features in the Context of Multi-Centric Trials. PLoS ONE 2016, 11, e0159984. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-F.; Li, J.-L.; Wang, Y.-H.; Tai, X.-H.; Liu, L.; Zhang, X.-X.; An, Y.-W.; Li, H.-L. The Correlation between 18F-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography Semiquantitative Parameters and the Clinical Features and Pathological Biological Indexes of Gastric Cancer. Cancer Biother. Radiopharm. 2021; online ahead of print. [Google Scholar] [CrossRef]
- Chen, R.; Chen, Y.; Huang, G.; Liu, J. Relationship between PD-L1 expression and 18F-FDG uptake in gastric cancer. Aging 2019, 11, 12270–12277. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [Green Version]
- Zwanenburg, A. Radiomics in nuclear medicine: Robustness, reproducibility, standardization, and how to avoid data analysis traps and replication crisis. Eur. J. Pediatr. 2019, 46, 2638–2655. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ersen, A.; Unlu, M.S.; Akman, T.; Sagol, O.; Oztop, I.; Atila, K.; Bora, S.; Ellidokuz, H.; Sarioglu, S. Tumor deposits in gastric carcinomas. Pathol.-Res. Pract. 2014, 210, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-C.; Lin, G.; Yeh, T.-S. Visceral-to-subcutaneous fat ratio independently predicts the prognosis of locally advanced gastric cancer—Highlighting the role of adiponectin receptors and PPARα, β/δ, ɤ. Eur. J. Surg. Oncol. 2021, 47, 3064–3073. [Google Scholar] [CrossRef] [PubMed]

| Variables | Number of Patients (%) | |
|---|---|---|
| Age (years) | 60 (34–80) * | |
| Sex | Men | 39 (56.5%) |
| Women | 30 (43.5%) | |
| Tumor location | Upper | 7 (10.1%) |
| Middle | 28 (40.6%) | |
| Lower | 34 (49.3%) | |
| Histopathological classification | PAC/TAC | 43 (62.3%) |
| PDAC | 16 (23.2%) | |
| Mucinous carcinoma/SRC | 10 (14.5%) | |
| Lauren classification | Intestinal | 30 (43.5%) |
| Non-intestinal | 39 (56.5%) | |
| pT stage | T1 stage | 9 (13.0%) |
| T2 stage | 17 (24.6%) | |
| T3 stage | 23 (33.3%) | |
| T4 stage | 20 (29.0%) | |
| pN stage | N0 stage | 29 (42.0%) |
| N1−N3 stages | 40 (58.0%) | |
| TNM stage | Stage I | 18 (26.1%) |
| Stage II | 19 (27.5%) | |
| Stage III | 32 (46.4%) | |
| Adjuvant treatment | Yes | 41 (59.4%) |
| No | 28 (40.6%) | |
| CD4 cell infiltration | Grade 0 | 17 (24.6%) |
| Grade 1 | 17 (24.6%) | |
| Grade 2 | 23 (33.3%) | |
| Grade 3 | 12 (17.4%) | |
| CD8 cell infiltration | Grade 0 | 16 (23.2%) |
| Grade 1 | 17 (24.6%) | |
| Grade 2 | 19 (27.5%) | |
| Grade 3 | 17 (24.6%) | |
| CD163 cell infiltration | Grade 0 | 10 (14.5%) |
| Grade 1 | 20 (29.0%) | |
| Grade 2 | 23 (33.3%) | |
| Grade 3 | 16 (23.2%) | |
| MMP-11 expression | Grade 0 | 14 (20.3%) |
| Grade 1 | 22 (31.9%) | |
| Grade 2 | 23 (33.3%) | |
| Grade 3 | 10 (14.5%) | |
| IL-6 expression | Grade 0 | 28 (40.6%) |
| Grade 1 | 24 (34.8%) | |
| Grade 2 | 12 (17.4%) | |
| Grade 3 | 5 (7.2%) | |
| Variables | CD4 Cell Infiltration | CD8 Cell Infiltration | CD163 Cell Infiltration | MMP-11 Expression | IL-6 Expression |
|---|---|---|---|---|---|
| Maximum SUV of primary tumor | 0.078 | 0.149 | 0.006 | 0.458 | 0.094 |
| First-order PET features of perigastric AT | |||||
| SUV mean | 0.163 | 0.072 | 0.037 | 0.099 | 0.042 |
| SUV std | 0.469 | 0.949 | 0.402 | 0.583 | 0.343 |
| SUV median | 0.189 | 0.062 | 0.095 | 0.122 | 0.025 |
| SUV histogram kurtosis | 0.699 | 0.911 | 0.330 | 0.714 | 0.869 |
| SUV histogram skewness | 0.470 | 0.387 | 0.496 | 0.226 | 0.170 |
| SUV histogram energy | 0.191 | 0.661 | 0.095 | 0.596 | 0.261 |
| SUV histogram entropy | 0.320 | 0.537 | 0.030 | 0.392 | 0.493 |
| Second-order PET features of perigastric AT | |||||
| GLCM contrast | 0.700 | 0.556 | 0.216 | 0.687 | 0.638 |
| GLCM correlation | 0.386 | 0.072 | 0.356 | 0.469 | 0.292 |
| GLCM dissimilarity | 0.648 | 0.622 | 0.097 | 0.721 | 0.830 |
| GLCM energy | 0.122 | 0.145 | 0.023 | 0.752 | 0.066 |
| GLCM entropy | 0.106 | 0.192 | 0.035 | 0.296 | 0.097 |
| GLCM homogeneity | 0.612 | 0.325 | 0.115 | 0.721 | 0.927 |
| Variables | p-Value | Hazard Ratio (95% CI) | |
|---|---|---|---|
| Age (for 1-year increase) | 0.997 | 1.00 (0.97–1.04) | |
| Sex (women vs. men) | 0.295 | 1.63 (0.65–4.10) | |
| Histopathological classification (PAC/TAC vs.) | PDAC | 0.234 | 1.71 (0.71–4.13) |
| Mucinous/SRC | 0.196 | 1.42 (0.86–4.38) | |
| Lauren classification (intestinal vs. non-intestinal) | 0.553 | 1.27 (0.57–2.84) | |
| pT stage (T1–T2 vs. T3–T4) | 0.003 | 20.95 (2.83–155.14) | |
| pN stage (N0 vs. N1–3) | <0.001 | 12.36 (2.90–52.61) | |
| TNM stage (stages I−II vs. stage III) | <0.001 | 14.88 (4.41–50.20) | |
| Maximum SUV of primary tumor (for 1.0 increase) | 0.001 | 1.10 (1.04–1.16) | |
| First-order PET features of perigastric AT (for a 0.10 increase) | SUV mean | <0.001 | 1.27 (1.12–1.46) |
| SUV std | 0.464 | 1.17 (0.77–1.75) | |
| SUV median | 0.001 | 1.29 (1.13–1.47) | |
| SUV histogram kurtosis | 0.785 | 1.00 (0.98–1.03) | |
| SUV histogram skewness | 0.157 | 0.94 (0.86–1.02) | |
| SUV histogram energy | 0.017 | 0.61 (0.41–0.91) | |
| SUV histogram entropy | 0.019 | 1.11 (1.02–1.21) | |
| Second-order PET features of perigastric AT (for a 0.10 increase) | GLCM contrast | 0.004 | 1.13 (1.04–1.23) |
| GLCM correlation | 0.087 | 0.82 (0.65–1.03) | |
| GLCM dissimilarity | 0.007 | 1.36 (1.14–1.62) | |
| GLCM energy | 0.022 | 0.58 (0.36–0.92) | |
| GLCM entropy | <0.001 | 1.12 (1.05–1.20) | |
| GLCM homogeneity | 0.003 | 0.40 (0.24–0.65) | |
| Variables | p-Value | Hazard Ratio (95% CI) | |
|---|---|---|---|
| First-order PET features of perigastric AT (for a 0.10 increase) | SUV mean | 0.147 | |
| SUV median | 0.090 | ||
| SUV histogram energy | 0.222 | ||
| SUV histogram entropy | 0.409 | ||
| Second-order PET features of perigastric AT (for 0.10 increase) | GLCM contrast | 0.464 | |
| GLCM dissimilarity | 0.013 | 1.31 (1.06–1.62) | |
| GLCM energy | 0.099 | ||
| GLCM entropy | 0.019 | 1.07 (1.02–1.15) | |
| GLCM homogeneity | 0.012 | 0.42 (0.21–0.82) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, H.; Song, G.J.; Jang, S.-H.; Son, M.W.; Lee, H.J.; Lee, M.-S.; Lee, J.-H.; Oh, M.-H.; Jeong, G.C.; Yun, J.H.; et al. Predicting the Recurrence of Gastric Cancer Using the Textural Features of Perigastric Adipose Tissue on [18F]FDG PET/CT. Int. J. Mol. Sci. 2022, 23, 11985. https://doi.org/10.3390/ijms231911985
Ahn H, Song GJ, Jang S-H, Son MW, Lee HJ, Lee M-S, Lee J-H, Oh M-H, Jeong GC, Yun JH, et al. Predicting the Recurrence of Gastric Cancer Using the Textural Features of Perigastric Adipose Tissue on [18F]FDG PET/CT. International Journal of Molecular Sciences. 2022; 23(19):11985. https://doi.org/10.3390/ijms231911985
Chicago/Turabian StyleAhn, Hyein, Geum Jong Song, Si-Hyong Jang, Myoung Won Son, Hyun Ju Lee, Moon-Soo Lee, Ji-Hye Lee, Mee-Hye Oh, Geum Cheol Jeong, Jong Hyuk Yun, and et al. 2022. "Predicting the Recurrence of Gastric Cancer Using the Textural Features of Perigastric Adipose Tissue on [18F]FDG PET/CT" International Journal of Molecular Sciences 23, no. 19: 11985. https://doi.org/10.3390/ijms231911985







