Identification of Glu-D1 Alleles and Novel Marker–Trait Associations for Flour Quality and Grain Yield Traits under Heat-Stress Environments in Wheat Lines Derived from Diverse Accessions of Aegilops tauschii
Abstract
:1. Introduction
2. Results
2.1. Protein Content (%)
2.2. Dough Strength/Specific Sedimentation Values (SSVs) (mL/%)
2.3. Grain Yield (kg/ha)
2.4. Marker–Trait Associations for Protein Content
2.5. Marker–Trait Associations for Dough Strength (SSVs)
2.6. Marker–Trait Associations for Grain Yield
2.7. Marker–Trait Associations for Relative Performance (RP) of Dough Strength and Grain Yield
2.8. Concurrent/Pleiotropic Effect
2.9. Allele’s Contribution, Candidate Genes, and Gene Expression
3. Discussion
3.1. Quality Traits
3.2. Grain Yield
3.3. Relative Performance under Heat-Stress Conditions
3.4. Marker–Trait Associations for Quality Traits
3.5. Marker–Trait Associations for Grain Yield
3.6. Alleles’ Contribution
4. Materials and Methods
4.1. Plant Materials
4.2. The Experimental Sites and Field Management
4.3. Evaluation of Flour Quality
4.4. Genome-Wide Association Analysis (GWAS)
4.5. Candidate Genes and Gene Expression
4.6. Statistical Analysis
4.7. Relative Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, M.P.; Balota, M.; Delgado, M.I.B.; Amani, I.; Fischer, R.A. Physiological and Morphological Traits Associated with Spring Wheat Yield under Hot, Irrigated Conditions. Funct. Plant Biol. 1994, 21, 717–730. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, R.P.; Huerta-Espino, J.; Kehel, Z.; Autrique, E. Characterization of Heat-and Drought-Stress Tolerance in High-Yielding Spring Wheat. Crop Sci. 2015, 55, 1552–1562. [Google Scholar] [CrossRef]
- Wrigley, C.W.; Blumenthal, C.; Gras, P.W.; Barlow, E.W.R. Temperature Variation during Grain Filling and Changes in Wheat-Grain Quality. Funct. Plant Biol. 1994, 21, 875–885. [Google Scholar] [CrossRef]
- Blumenthal, C.; Bekes, F.; Gras, P.W.; Barlow, E.W.R.; Wrigley, C.W. Identification of Wheat Genotypes Tolerant to the Effects of Heat Stress on Grain Quality. Cereal Chem. 1995, 72, 539–544. [Google Scholar]
- Stone, P.J.; Gras, P.W.; Nicolas, M.E. The Influence of Recovery Temperature on the Effects of a Brief Heat Shock on Wheat. III. Grain Protein Composition and Dough Properties. J. Cereal Sci. 1997, 25, 129–141. [Google Scholar] [CrossRef]
- Spiertz, J.H.J.; Hamer, R.J.; Xu, H.; Primo-Martin, C.; Don, C.; Van Der Putten, P.E.L. Heat Stress in Wheat (Triticum aestivum L.): Effects on Grain Growth and Quality Traits. Eur. J. Agron. 2006, 25, 89–95. [Google Scholar] [CrossRef]
- Bushuk, W.; Zillman, R.R. Wheat Cultivar Identification by Gliadin Electrophoregrams. I. Apparatus, Method and Nomenclature. Can. J. Plant Sci. 1978, 58, 505–515. [Google Scholar] [CrossRef]
- Payne, P.I. Genetics of Wheat Storage Proteins and the Effect of Allelic Variation on Bread-Making Quality. Annu. Rev. Plant Physiol. 1987, 38, 141–153. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Yang, Y.; Liu, X.; Qin, H.; Dong, Z.; Zheng, S.; Zhang, K.; Wang, D. New Insight into the Function of Wheat Glutenin Proteins as Investigated with Two Series of Genetic Mutants. Sci. Rep. 2017, 7, 3428. [Google Scholar] [CrossRef]
- Tatham, A.S.; Miflin, B.J.; Shewry, P.R. The Beta-Turn Conformation in Wheat Gluten Proteins: Relationship to Gluten Elasticity. Cereal Chem. 1985, 62, 405–412. [Google Scholar]
- Shewry, P.R.; Halford, N.G.; Tatham, A.S.; Popineau, Y.; Lafiandra, D.; Belton, P.S. The High Molecular Weight Subunits of Wheat Glutenin and Their Role in Determining Wheat Processing Properties. Adv. Food Nutr. Res. 2003, 45, 221–302. [Google Scholar]
- Payne, P.I.; Holt, L.M.; Krattiger, A.F.; Carrillo, J.M. Relationships between Seed Quality Characteristics and HMW Glutenin Subunit Composition Determined Using Wheats Grown in Spain. J. Cereal Sci. 1988, 7, 229–235. [Google Scholar] [CrossRef]
- Payne, P.I.; Holt, L.M.; Worland, A.J.; Law, C.N. Structural and Genetical Studies on the High-Molecular-Weight Subunits of Wheat Glutenin: Part 3. Telocentric Mapping of the Subunit Genes on the Long Arms of the Homoeologous Group 1 Chromosomes. Theor. Appl. Genet. 1982, 63, 129–138. [Google Scholar] [CrossRef]
- Payne, P.I.; Law, C.N.; Mudd, E.E. Control by Homoeologous Group 1 Chromosomes of the High-Molecular-Weight Subunits of Glutenin, a Major Protein of Wheat Endosperm. Theor. Appl. Genet. 1980, 58, 113–120. [Google Scholar] [CrossRef]
- Payne, P.I.; Nightingale, M.A.; Krattiger, A.F.; Holt, L.M. The Relationship between HMW Glutenin Subunit Composition and the Bread-Making Quality of British-Grown Wheat Varieties. J. Sci. Food Agric. 1987, 40, 51–65. [Google Scholar] [CrossRef]
- Kolster, P.; Van Eeuwijk, F.A.; Van Gelder, W.M.J. Additive and Epistatic Effects of Allelic Variation at the High Molecular Weight Glutenin Subunit Loci in Determining the Bread-Making Quality of Breeding Lines of Wheat. Euphytica 1991, 55, 277–285. [Google Scholar] [CrossRef]
- Mohamed, I.E.S.; Oe, H.; Kamal, N.M.; Mustafa, H.M.; Gorafi, Y.S.A.; Tahir, I.S.A.; Tsujimoto, H.; Tanaka, H. Enhancing Wheat Flour Quality through Introgression of High-Molecular-Weight Glutenin Subunits from Aegilops tauschii Accessions. Front. Sustain. Food Syst. 2022, 6, 887795. [Google Scholar] [CrossRef]
- Corbellini, M.; Canevar, M.G.; Mazza, L.; Ciaffi, M.; Lafiandra, D.; Borghi, B. Effect of the Duration and Intensity of Heat Shock during Grain Filling on Dry Matter and Protein Accumulation, Technological Quality and Protein Composition in Bread and Durum Wheat. Funct. Plant Biol. 1997, 24, 245–260. [Google Scholar] [CrossRef]
- Stone, P.J.; Nicolas, M.E. Comparison of Sudden Heat Stress with Gradual Exposure to High Temperature during Grain Filling in Two Wheat Varieties Differing in Heat Tolerance. II. Fractional Protein Accumulation. Funct. Plant Biol. 1998, 25, 1–11. [Google Scholar] [CrossRef]
- Tahir, I.S.A.; Nakata, N.; Ali, A.M.; Mustafa, H.M.; Saad, A.S.I.; Takata, K.; Ishikawa, N.; Abdalla, O.S. Genotypic and Temperature Effects on Wheat Grain Yield and Quality in a Hot Irrigated Environment. Plant Breed. 2006, 125, 323–330. [Google Scholar] [CrossRef]
- Tanaka, H.; Gorafi, Y.S.A.; Fujita, M.; Sasaki, H.; Tahir, I.S.A.; Tsujimoto, H. Expression of Seed Storage Proteins Responsible for Maintaining Kernel Traits and Wheat Flour Quality in Common Wheat under Heat Stress Conditions. Breed. Sci. 2021, 71, 184–192. [Google Scholar] [CrossRef]
- Randall, P.J.; Moss, H.J. Some Effects of Temperature Regime during Grain Filling on Wheat Quality. Aust. J. Agric. Res. 1990, 41, 603–617. [Google Scholar] [CrossRef]
- Daniel, C.; Triboi, E. Effects of Temperature and Nitrogen Nutrition on the Grain Composition of Winter Wheat: Effects on Gliadin Content and Composition. J. Cereal Sci. 2000, 32, 45–56. [Google Scholar] [CrossRef]
- Kulwal, P.; Kumar, N.; Kumar, A.; Gupta, R.K.; Balyan, H.S.; Gupta, P.K. Gene Networks in Hexaploid Wheat: Interacting Quantitative Trait Loci for Grain Protein Content. Funct. Integr. Genom. 2005, 5, 254–259. [Google Scholar] [CrossRef]
- Kumar, A.; Mantovani, E.E.; Simsek, S.; Jain, S.; Elias, E.M.; Mergoum, M. Genome Wide Genetic Dissection of Wheat Quality and Yield Related Traits and Their Relationship with Grain Shape and Size Traits in an Elite × Non-Adapted Bread Wheat Cross. PLoS ONE 2019, 14, e0221826. [Google Scholar] [CrossRef]
- Blanco, A.; De Giovanni, C.; Laddomada, B.; Sciancalepore, A.; Simeone, R.; Devos, K.M.; Gale, M.D. Quantitative Trait Loci Influencing Grain Protein Content in Tetraploid Wheats. Plant Breed. 1996, 115, 310–316. [Google Scholar] [CrossRef]
- Bogard, M.; Allard, V.; Martre, P.; Heumez, E.; Snape, J.W.; Orford, S.; Griffiths, S.; Gaju, O.; Foulkes, J.; Le Gouis, J. Identifying Wheat Genomic Regions for Improving Grain Protein Concentration Independently of Grain Yield Using Multiple Inter-Related Populations. Mol. Breed. 2013, 31, 587–599. [Google Scholar] [CrossRef]
- Leonova, I.N.; Kiseleva, A.A.; Berezhnaya, A.A.; Stasyuk, A.I.; Likhenko, I.E.; Salina, E.A. Identification of QTLs for Grain Protein Content in Russian Spring Wheat Varieties. Plants 2022, 11, 437. [Google Scholar] [CrossRef]
- Liu, J.; Huang, L.; Wang, C.; Liu, Y.; Yan, Z.; Wang, Z.; Xiang, L.; Zhong, X.; Gong, F.; Zheng, Y.; et al. Genome-Wide Association Study Reveals Novel Genomic Regions Associated with High Grain Protein Content in Wheat Lines Derived from Wild Emmer Wheat. Front. Plant Sci. 2019, 10, 464. [Google Scholar] [CrossRef] [Green Version]
- Prasad, M.; Kumar, N.; Kulwal, P.L.; Röder, M.S.; Balyan, H.S.; Dhaliwal, H.S.; Gupta, P.K. QTL Analysis for Grain Protein Content Using SSR Markers and Validation Studies Using NILs in Bread Wheat. Theor. Appl. Genet. 2003, 106, 659–667. [Google Scholar] [CrossRef]
- Reif, J.C.; Zhang, P.; Dreisigacker, S.; Warburton, M.L.; van Ginkel, M.; Hoisington, D.; Bohn, M.; Melchinger, A.E. Wheat Genetic Diversity Trends during Domestication and Breeding. Theor. Appl. Genet. 2005, 110, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Shokat, S.; Novak, O.; Siroka, J.; Singh, S.; Gill, K.S.; Roitsch, T.; Großkinsky, D.K.; Liu, F. Elevated CO2 Modulates the Effect of Heat Stress Responses in Triticum aestivum by Differential Expression of an Isoflavone Reductase-like Gene. J. Exp. Bot. 2021, 72, 7594–7609. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, K.; Paulsen, G.M. Photosynthesis and Productivity during High-Temperature Stress of Wheat Genotypes from Major World Regions. Crop Sci. 1990, 30, 1127–1132. [Google Scholar] [CrossRef]
- Tahir, I.S.A.; Nakata, N. Remobilization of Nitrogen and Carbohydrate from Stems of Bread Wheat in Response to Heat Stress during Grain Filling. J. Agron. Crop Sci. 2005, 191, 106–115. [Google Scholar] [CrossRef]
- Tewolde, H.; Fernandez, C.J.; Erickson, C.A. Wheat Cultivars Adapted to Post-Heading High Temperature Stress. J. Agron. Crop Sci. 2006, 192, 111–120. [Google Scholar] [CrossRef]
- Asseng, S.; Foster, I.A.N.; Turner, N.C. The Impact of Temperature Variability on Wheat Yields. Glob. Chang. Biol. 2011, 17, 997–1012. [Google Scholar] [CrossRef]
- Ferris, R.; Ellis, R.H.; Wheeler, T.R.; Hadley, P. Effect of High Temperature Stress at Anthesis on Grain Yield and Biomass of Field-Grown Crops of Wheat. Ann. Bot. 1998, 82, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Lobell, D.B.; Sibley, A.; Ivan Ortiz-Monasterio, J. Extreme Heat Effects on Wheat Senescence in India. Nat. Clim. Chang. 2012, 2, 186–189. [Google Scholar] [CrossRef]
- Modarresi, M.; Mohammadi, V.; Zali, A.; Mardi, M. Response of Wheat Yield and Yield Related Traits to High Temperature. Cereal Res. Commun. 2010, 38, 23–31. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising Temperatures Reduce Global Wheat Production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Iizumi, T.; Ali-Babiker, I.-E.A.; Tsubo, M.; Tahir, I.S.A.; Kurosaki, Y.; Kim, W.; Gorafi, Y.S.A.; Idris, A.A.M.; Tsujimoto, H. Rising Temperatures and Increasing Demand Challenge Wheat Supply in Sudan. Nat. Food 2021, 2, 19–27. [Google Scholar] [CrossRef]
- Li, F.; Wen, W.; Liu, J.; Zhang, Y.; Cao, S.; He, Z.; Rasheed, A.; Jin, H. Genetic Architecture of Grain Yield in Bread Wheat Based on Genome-Wide Association Studies. BMC Plant Biol. 2019, 19, 168. [Google Scholar] [CrossRef]
- Id, M.G.; Eckermann, P.; Haefele, S.; Satija, S.; Sznajder, B.; Timmins, A.; Baumann, U.; Wolters, P.; Mather, D.E.; Fleury, D. Genome-Wide Association Mapping of Grain Yield in a Diverse Collection of Spring Wheat (Triticum aestivum L.) Evaluated in Southern Australia. PLoS ONE 2019, 14, e0211730. [Google Scholar] [CrossRef]
- Pinto, R.S.; Reynolds, M.P. Heat and Drought Adaptive QTL in a Wheat Population Designed to Minimize Confounding Agronomic Effects. Theor. Appl. Genet. 2010, 121, 1001–1021. [Google Scholar] [CrossRef] [Green Version]
- Sukumaran, S.; Dreisigacker, S.; Lopes, M.; Chavez, P.; Reynolds, M.P. Genome-Wide Association Study for Grain Yield and Related Traits in an Elite Spring Wheat Population Grown in Temperate Irrigated Environments. Theor. Appl. Genet. 2015, 128, 353–363. [Google Scholar] [CrossRef]
- Tadesse, W.; Suleiman, S.; Tahir, I.; Jighly, A.; Hagras, A.; Thabet, S.; Baum, M.; Tadesse, W.; Jighly, A.; Baum, M. Heat-Tolerant QTLs Associated with Grain Yield and Its Components in Spring Bread Wheat under Heat-Stressed Environments of Sudan and Egypt. Crop Sci. 2019, 211, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Suliman, S.; Alemu, A.; Abdelmula, A.A.; Badawi, G.H.; Al-Abdallat, A.; Tadesse, W. Genome-Wide Association Analysis Uncovers Stable QTLs for Yield and Quality Traits of Spring Bread Wheat (Triticum aestivum) across Contrasting Environments. Plant Gene 2021, 25, 100269. [Google Scholar] [CrossRef]
- Elahmadi, A.B. Review of Wheat Breeding in the Sudan. In Wheat Production and Improvement in the Sudan, Proceedings of the National Research Review Workshop; ICARDA: Aleppo, Syria, 1995; pp. 27–30. [Google Scholar]
- Tahir, I.S.A.; Elbashier, E.M.E.; Ibrahim, M.A.S.; Saad, A.S.I.; Abdalla, O.S. Genetic Gain in Wheat Grain Yield and Nitrogen Use Efficiency at Different Nitrogen Levels in an Irrigated Hot Environment. Int. J. Agron. 2020, 2020, 9024671. [Google Scholar] [CrossRef]
- Ogbonnaya, F.C.; Abdalla, O.; Mujeeb-Kazi, A.; Kazi, A.G.; Xu, S.S.; Gosman, N.; Lagudah, E.S.; Bonnett, D.; Sorrells, M.E.; Tsujimoto, H.; et al. Synthetic Hexaploids: Harnessing Species of the Primary Gene Pool for Wheat Improvement. Plant Breed. Rev 2013, 37, 35–122. [Google Scholar]
- Singh, S.; Vikram, P.; Sehgal, D.; Burgueño, J.; Sharma, A.; Singh, S.K.; Sansaloni, C.P.; Joynson, R.; Brabbs, T.; Ortiz, C.; et al. Harnessing Genetic Potential of Wheat Germplasm Banks through Impact-Oriented-Prebreeding for Future Food and Nutritional Security. Sci. Rep. 2018, 8, 12527. [Google Scholar] [CrossRef] [Green Version]
- Kishii, M. An Update of Recent Use of Aegilops Species in Wheat Breeding. Front. Plant Sci. 2019, 10, 585. [Google Scholar] [CrossRef] [Green Version]
- Cox, T.; Wu, J.; Wang, S.; Cai, J.; Zhong, Q.; Fu, B. Comparing Two Approaches for Introgression of Germplasm from Aegilops Tauschii into Common Wheat. Crop J. 2017, 5, 355–362. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Sohail, Q.; Matsuoka, Y. Broadening the Genetic Diversity of Common and Durum Wheat for Abiotic Stress Tolerance Breeding. In Advances in Wheat Genetics: From Genome to Field; Springer: Tokyo, Japan, 2015; pp. 233–238. [Google Scholar]
- Gorafi, Y.S.A.; Kim, J.S.; Elbashir, A.A.E.; Tsujimoto, H. A Population of Wheat Multiple Synthetic Derivatives: An Effective Platform to Explore, Harness and Utilize Genetic Diversity of Aegilops Tauschii for Wheat Improvement. Theor. Appl. Genet. 2018, 131, 1615–1626. [Google Scholar] [CrossRef]
- Elbashir, A.A.E.; Gorafi, Y.S.A.; Tahir, I.S.A.; Kim, J.-S.; Tsujimoto, H. Wheat Multiple Synthetic Derivatives: A New Source for Heat Stress Tolerance Adaptive Traits. Breed. Sci. 2017, 67, 248–256. [Google Scholar] [CrossRef]
- Itam, M.O.; Gorafi, Y.S.A.; Tahir, I.S.A.; Tsujimoto, H. Genetic Variation in Drought Resilience-Related Traits among Wheat Multiple Synthetic Derivative Lines: Insights for Climate Resilience Breeding. Breed. Sci. 2021, 71, 435–443. [Google Scholar] [CrossRef]
- Itam, M.O.; Mega, R.; Gorafi, Y.S.A.; Yamasaki, Y.; Tahir, I.S.A.; Akashi, K.; Tsujimoto, H. Genomic Analysis for Heat and Combined Heat–Drought Resilience in Bread Wheat under Field Conditions. Theor. Appl. Genet. 2021, 135, 337–350. [Google Scholar] [CrossRef]
- Elhadi, G.M.I.; Kamal, N.M.; Gorafi, Y.S.A.; Yamasaki, Y.; Takata, K.; Tahir, I.S.A.; Itam, M.O.; Tanaka, H.; Tsujimoto, H. Exploitation of Tolerance of Wheat Kernel Weight and Shape-Related Traits from Aegilops Tauschii under Heat and Combined Heat-Drought Stresses. Int. J. Mol. Sci. 2021, 22, 1830. [Google Scholar] [CrossRef]
- Elhadi, G.M.I.; Kamal, N.M.; Gorafi, Y.S.A.; Yamasaki, Y.; Ban, Y.; Kato, K.; Tahir, I.S.A.; Ishii, T.; Tanaka, H.; Tsujimoto, H. Novel Loci for Kernel Hardness Appeared as a Response to Heat and Combined Heat-Drought Conditions in Wheat Harboring Aegilops Tauschii Diversity. Agronomy 2021, 11, 1061. [Google Scholar] [CrossRef]
- Borrill, P.; Ramirez-Gonzalez, R.; Uauy, C. ExpVIP: A Customizable RNA-Seq Data Analysis and Visualization Platform. Plant Physiol. 2016, 170, 2172–2186. [Google Scholar] [CrossRef] [Green Version]
- Elbashir, A.A.E.; Gorafi, Y.S.A.; Tahir, I.S.A.; Elhashimi, A.M.A.; Abdalla, M.G.A.; Tsujimoto, H. Genetic Variation in Heat Tolerance-Related Traits in a Population of Wheat Multiple Synthetic Derivatives. Breed. Sci. 2017, 67, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Alvarado, G.; López, M.; Vargas, M.; Pacheco, A.; Rodríguez, F.; Burgueño, J.; Crossa, J. META-R (Multi Environment Trial Analysis with R for Windows) Version 6.01. Hdl: 11529/10201. CIMMYT Res. Data Softw. Repos. Netw. 2016, 20, 2017. [Google Scholar]
- Stone, P.J.; Savin, R. Grain Quality and Its Physiological Determinants. In Wheat: Ecology and Physiology of Yield Determination; Haworth Press Inc.: New York, NY, USA, 1999; pp. 85–120. [Google Scholar]
- Shewry, P.R.; Underwood, C.; Wan, Y.; Lovegrove, A.; Bhandari, D.; Toole, G.; Mills, E.N.C.; Denyer, K.; Mitchell, R.A.C. Storage Product Synthesis and Accumulation in Developing Grains of Wheat. J. Cereal Sci. 2009, 50, 106–112. [Google Scholar] [CrossRef]
- Don, C.; Lookhart, G.; Naeem, H.; MacRitchie, F.; Hamer, R.J. Heat Stress and Genotype Affect the Glutenin Particles of the Glutenin Macropolymer-Gel Fraction. J. Cereal Sci. 2005, 42, 69–80. [Google Scholar] [CrossRef]
- Irmak, S.; Naeem, H.A.; Lookhart, G.L.; MacRitchie, F. Effect of Heat Stress on Wheat Proteins during Kernel Development in Wheat Near-Isogenic Lines Differing at Glu-D1. J. Cereal Sci. 2008, 48, 513–516. [Google Scholar] [CrossRef]
- Uthayakumaran, S.; Tanner, R.I.; Dai, S.; Qi, F.; Newberry, M.; Wrigley, C.; Copeland, L. Genotype-Based Stability of Dough Quality in Wheat from Different Growth Environments. J. Agric. Sci. 2012, 4, 41–50. [Google Scholar] [CrossRef]
- Blumenthal, C.S.; Batey, I.L.; Bekes, F.; Wrigley, C.W.; Barlow, E.W.R. Seasonal Changes in Wheat-Grain Quality Associated with High Temperatures during Grain Filling. Aust. J. Agric. Res. 1991, 42, 21–30. [Google Scholar] [CrossRef]
- Ciaffi, M.; Tozzi, L.; Borghi, B.; Corbellini, M.; Lafiandra, D. Effect of Heat Shock during Grain Filling on the Gluten Protein Composition of Bread Wheat. J. Cereal Sci. 1996, 24, 91–100. [Google Scholar] [CrossRef]
- Wardlaw, I.F.; Sofield, I.; Cartwright, P.M. Factors Limiting the Rate of Dry Matter Accumulation in the Grain of Wheat Grown at High Temperature. Funct. Plant Biol. 1980, 7, 387–400. [Google Scholar] [CrossRef]
- Pfeifer, M.; Kugler, K.G.; Sandve, S.R.; Zhan, B.; Rudi, H.; Hvidsten, T.R.; Mayer, K.F.X.; Olsen, O.A.; Rogers, J.; Doležel, J.; et al. Genome Interplay in the Grain Transcriptome of Hexaploid Bread Wheat. Science 2014, 345, 1250091. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Jain, S.; Elias, E.M.; Ibrahim, M.; Sharma, L.K. An Overview of QTL Identification and Marker-Assisted Selection for Grain Protein Content in Wheat. In Eco-Friendly Agro-Biological Techniques for Enhancing Crop Productivity; Springer: Singapore, 2018; pp. 245–274. [Google Scholar]
- Liu, H.; Zhou, X.; Dong, N.; Liu, X.; Zhang, H.; Zhang, Z. Expression of a Wheat MYB Gene in Transgenic Tobacco Enhances Resistance to Ralstonia Solanacearum, and to Drought and Salt Stresses. Funct. Integr. Genom. 2011, 11, 431–443. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, M.; Tian, Y.; He, W.; Han, L.; Xia, G. Over-Expression of TaMYB33 Encoding a Novel Wheat MYB Transcription Factor Increases Salt and Drought Tolerance in Arabidopsis. Mol. Biol. Rep. 2012, 39, 7183–7192. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, X.; Liu, X.; Wu, H.; Bi, H.; Xu, H. The Wheat MYB Transcription Factor TaMYB 31 Is Involved in Drought Stress Responses in Arabidopsis. Front. Plant Sci. 2018, 9, 1426. [Google Scholar] [CrossRef]
- Gunupuru, L.R.; Arunachalam, C.; Malla, K.B.; Kahla, A.; Perochon, A.; Jia, J.; Thapa, G.; Doohan, F.M. A Wheat Cytochrome P450 Enhances Both Resistance to Deoxynivalenol and Grain Yield. PLoS ONE 2018, 13, e0204992. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Liu, X.; Mao, W.; Zhang, X.; Chen, S.; Zhan, K.; Bi, H.; Xu, H. Genome-Wide Identification and Analysis of HAK/KUP/KT Potassium Transporters Gene Family in Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2018, 19, 3969. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Liu, Y.; Li, N.; Liu, Y.; Zhao, D.; Wei, B.; Wen, X. Effects of the Foliar Application of Potassium Fertilizer on the Grain Protein and Dough Quality of Wheat. Agronomy 2021, 11, 1749. [Google Scholar] [CrossRef]
- Wang, J.; Tian, W.; Tao, F.; Wang, J.; Shang, H.; Chen, X.; Xu, X.; Hu, X. TaRPM1 Positively Regulates Wheat High-Temperature Seedling-Plant Resistance to Puccinia Striiformis f. Sp. Tritici. Front. Plant Sci. 2020, 10, 1679. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wang, W.; Wang, W.; Zhang, G.; Liu, Y.; Wang, Y.; Wang, W. Wheat F-Box Protein Gene TaFBA1 Is Involved in Plant Tolerance to Heat Stress. Front. Plant Sci. 2018, 9, 521. [Google Scholar] [CrossRef] [Green Version]
- Azadi, A.; Mardi, M.; Hervan, E.M.; Mohammadi, S.A.; Moradi, F.; Tabatabaee, M.T.; Pirseyedi, S.M.; Ebrahimi, M.; Fayaz, F.; Kazemi, M.; et al. QTL Mapping of Yield and Yield Components under Normal and Salt-Stress Conditions in Bread Wheat (Triticum aestivum L.). Plant Mol. Biol. Rep. 2015, 33, 102–120. [Google Scholar] [CrossRef]
- Németh, E.; Nagy, Z.; Pécsváradi, A. Chloroplast Glutamine Synthetase, the Key Regulator of Nitrogen Metabolism in Wheat, Performs Its Role by Fine Regulation of Enzyme Activity via Negative Cooperativity of Its Subunits. Front. Plant Sci. 2018, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Xiong, S.; Wei, Y.; Meng, X.; Wang, X.; Ma, X. The Role of Glutamine Synthetase Isozymes in Enhancing Nitrogen Use Efficiency of N-Efficient Winter Wheat. Sci. Rep. 2017, 7, 1000. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Sun, M.; Liu, S.; Teng, Q.; Li, S.; Jiang, Y. Functions of Ppr Proteins in Plant Growth and Development. Int. J. Mol. Sci. 2021, 22, 11274. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Nasuda, S. Durum Wheat as a Candidate for the Unknown Female Progenitor of Bread Wheat: An Empirical Study with a Highly Fertile F1 Hybrid with Aegilops Tauschii Coss. Theor. Appl. Genet. 2004, 109, 1710–1717. [Google Scholar] [CrossRef]
- Kajimura, T.; Murai, K.; Takumi, S. Distinct Genetic Regulation of Flowering Time and Grain-Filling Period Based on Empirical Study of D-Genome Diversity in Synthetic Hexaploid Wheat Lines. Breed. Sci. 2011, 61, 130–141. [Google Scholar] [CrossRef] [Green Version]
- Gbegbelegbe, S.; Cammarano, D.; Asseng, S.; Robertson, R.; Chung, U.; Adam, M.; Abdalla, O.; Payne, T.; Reynolds, M.; Sonder, K.; et al. Baseline Simulation for Global Wheat Production with CIMMYT Mega-Environment Specific Cultivars. F. Crop. Res. 2017, 202, 122–135. [Google Scholar] [CrossRef]
- Takata, K.; Yamauchi, H.; Iriki, N.; Kuwabara, T. Prediction of Bread-Making Quality by Prolonged Swelling SDS-Sedimentation Test [in Soft Wheat]. Breed. Sci. 1999, 49, 221–223. [Google Scholar] [CrossRef]
- Axtord, D.W.; McDermott, E.E.; Redman, D.G. Note on the Sodium Dodecyl Sulfate Test of Bread Making Quality: Comparision with Pelschenke and Zeleny Test. Cereal Chem. 1979, 56, 582–584. [Google Scholar]
- Moonen, J.H.E.; Scheepstra, A.; Graveland, A. Use of the SDS-Sedimentation Test and SDS-Polyacrylamidegel Electrophoresis for Screening Breeder’s Samples of Wheat for Bread-Making Quality. Euphytica 1982, 31, 677–690. [Google Scholar] [CrossRef]
- Tanaka, H.; Tsujimoto, H. Positive or Negative Effects on Dough Strength in Large-Scale Group-1 Chromosome Deletion Lines of Common Wheat (Triticum aestivum L.). Euphytica 2012, 186, 57–65. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for Association Mapping of Complex Traits in Diverse Samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, H.; Tang, Z.; Xu, J.; Yin, D.; Zhang, Z.; Yuan, X.; Zhu, M.; Zhao, S.; Li, X.; et al. RMVP: A Memory-Efficient, Visualization-Enhanced, and Parallel-Accelerated Tool for Genome-Wide Association Study. Genom. Proteom. Bioinform. 2021, 19, 619–628. [Google Scholar] [CrossRef]
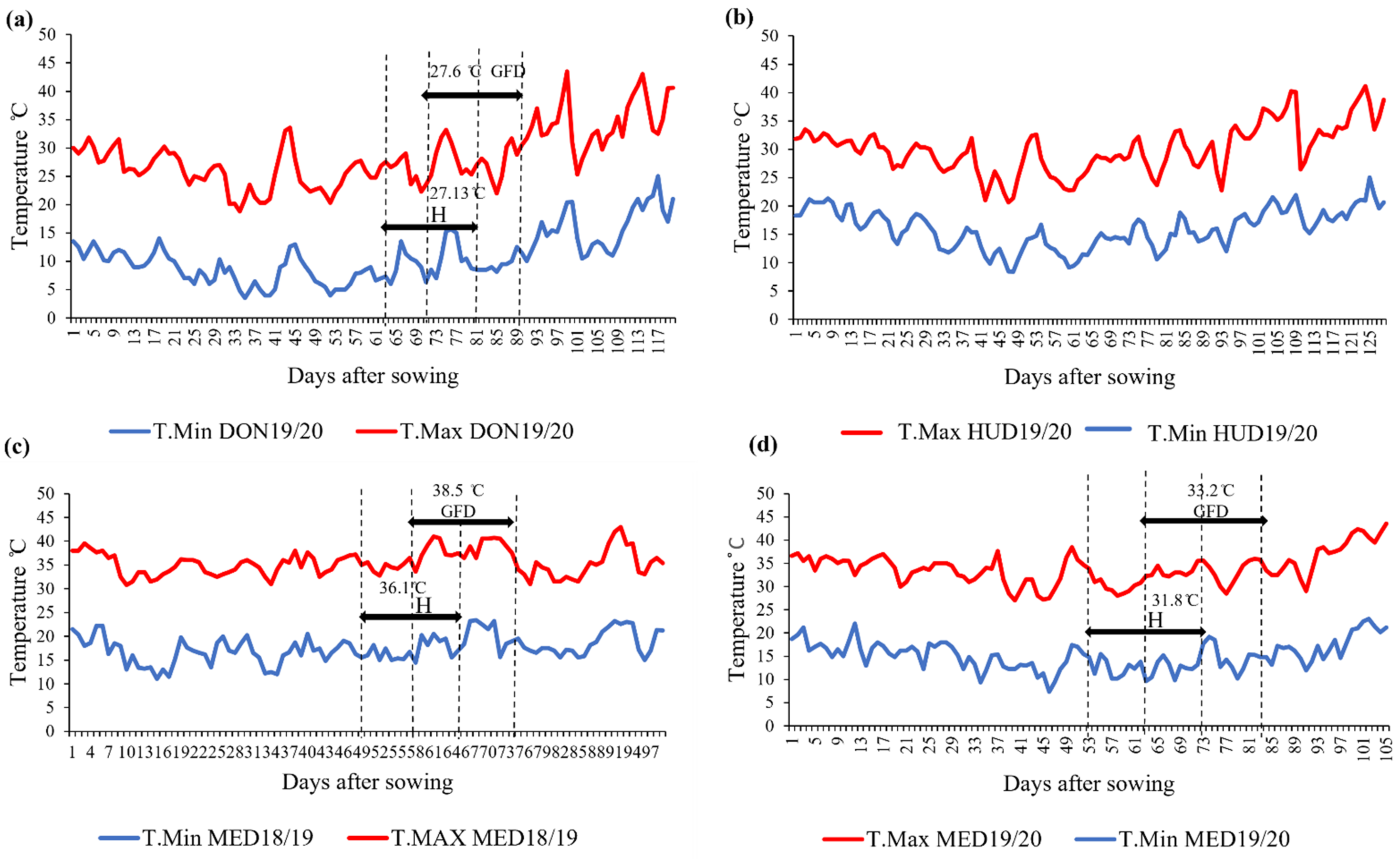


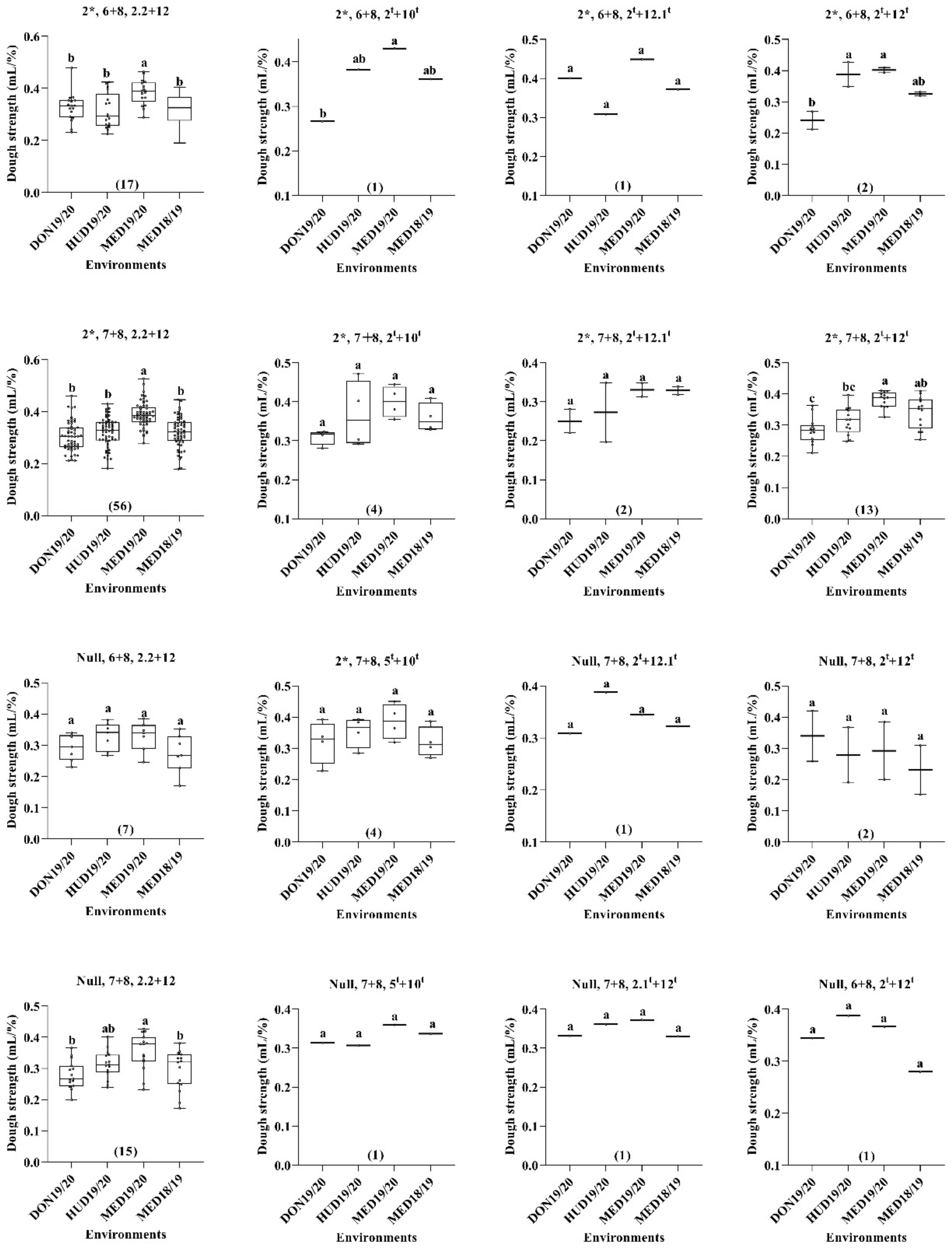
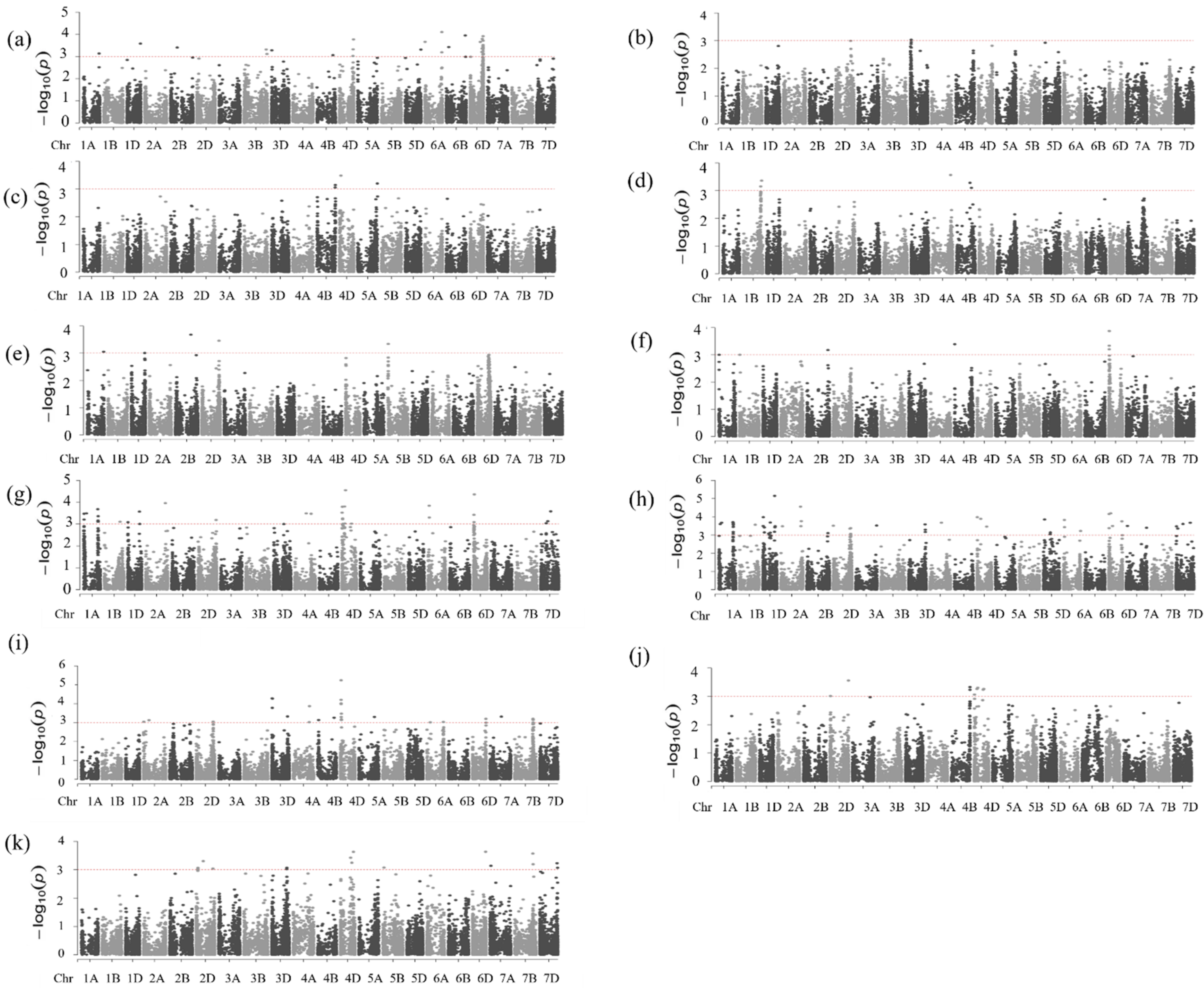
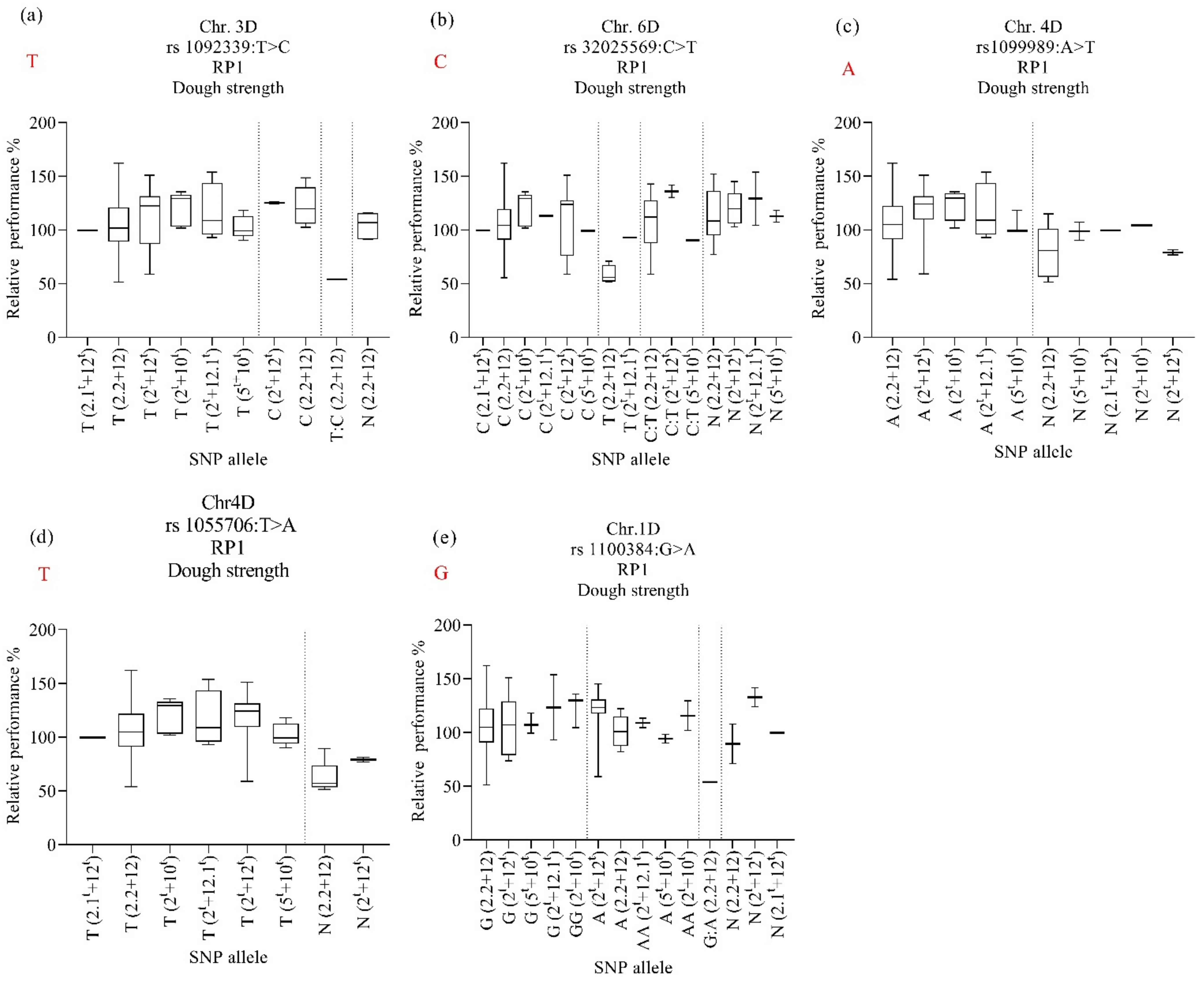


| Protein Content (%) | Dough Strength (mL/%) | Grain Yield kg/ha−1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DON19/20 | HUD19/20 | MED18/19 | MED19/20 | C | DON19/20 | HUD19/20 | MED18/19 | MED19/20 | C | DON19/20 | MED18/19 | MED19/20 | C | |
| G | <0.001 | 0.103 | <0.001 | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| E | - | - | - | <0.001 | - | - | - | - | <0.001 | - | - | - | <0.001 | |
| G × E | - | - | - | 0.56 | - | - | - | - | <0.001 | - | - | - | <0.001 | |
| Mean | 13.6 | 15.1 | 17 | 15.5 | 15.5 | 0.30 | 0.32 | 0.32 | 0.38 | 0.33 | 4485 | 2733 | 3133 | 3433 |
| Range | 10.2–16.3 | 12.2–18.3 | 14.4–20.5 | 13.2–18.1 | 13.8–17.6 | 0.20–0.48 | 0.18–0.47 | 0.15–0.45 | 0.20–0.53 | 0.20–0.44 | 1942–7218 | 1030–5084 | 1150–4764 | 2154–5395 |
| CV% | 4.2 | 7.4 | 4.9 | 3.5 | 8.5 | 15.5 | 12.7 | 8.9 | 10.0 | 14.0 | 21.34 | 22.21 | 18.76 | 25.2 |
| LSD | 1.25 | 2.58 | 1.63 | 1.12 | 2.85 | 0.104 | 0.093 | 0.056 | 0.079 | 0.101 | 1850 | 1175.4 | 1132 | 1711 |
| SE± | 0.57 | 1.12 | 0.83 | 0.54 | 1.31 | 0.047 | 0.041 | 0.028 | 0.038 | 0.046 | 934.5 | 593.8 | 571.9 | 870.4 |
| h2 | - | - | - | - | 0.68 | - | - | - | - | 0.86 | - | - | - | 0.58 |
| Goumria | 14.6 | 15.8 | 17.0 | 16.1 | 15.9 | 0.33 | 0.37 | 0.42 | 0.42 | 0.39 | 6438 | 2188 | 4166 | 4258 |
| Imam | 12.9 | 15.6 | 14.6 | 13.2 | 14.2 | 0.39 | 0.35 | 0.45 | 0.47 | 0.41 | 6969 | 3594 | 4387 | 4965 |
| Norin 61 | 13.5 | 16.4 | 15.5 | 14.8 | 15.1 | 0.41 | 0.38 | 0.39 | 0.43 | 0.40 | 4579 | 2636 | 3277 | 3494 |
| Environments | |||||||
|---|---|---|---|---|---|---|---|
| Marker | Chr | Pos | DON19/20 | HUD19/20 | MED18/19 | MED19/20 | R2 |
| 1204551|F|0-57 | 1A | 500.253 | (SSVs) | (SSVs) | 11.2–14.0 | ||
| 1094315|F|0-45 | 1A | 506.846 | (SSVs) | (SSVs) | 9.8–11.6 | ||
| 3959168|F|0-15 | 1A | 508.265 | (SSVs) | (SSVs) | 9.8–11.6 | ||
| 1210578|F|0-9 | 1A | 510.293 | (SSVs) | (SSVs) | 9.9–12.0 | ||
| 3947627|F|0-33 | 1A | 510.911 | (SSVs) | (SSVs) | 12.7–14.1 | ||
| 4910833|F|0-62 | 1A | 511.070 | (SSVs) | (SSVs) | 9.8–11.6 | ||
| 2303774|F|0-6 | 1A | 513.884 | (SSVs) | (SSVs) | 10.9–12.1 | ||
| 3953635|F|0-16 | 1A | 97.919 | (SSVs) | (SSVs) | 15.1–17.3 | ||
| 996849|F|0-11 | 1B | 559.059 | (SSVs) | (SSVs) | 9.8–11.6 | ||
| 1092278|F|0-29 | 1D | 412.338 | (SSVs) | (SSVs) | 12.7–22.3 | ||
| 1696345|F|0-38 | 1D | 421.872 | (SSVs) | (SSVs) | 12.1–13.8 | ||
| 994055|F|0-66 | 2A | 697.354 | (SSVs) | (SSVs) | 17.2–20.2 | ||
| 1088439|F|0-52 | 3D | 14.283 | (GY) | (RP2.GY) | 13.2–15.4 | ||
| 1042486|F|0-52 | 4A | 577.563 | (GY) | (SSVs), (RP1.SSVs) | 13.1–15.5 | ||
| 3953635|F|0-25 | 4A | 403.756 | (SSVs) | (SSVs) | 15.1–17.3 | ||
| 1134011|F|0-55 | 4B | 585.751 | (GY) | (RP1.GY) | 12.0–13.1 | ||
| 1201923|F|0-38 | 4D | 23.837 | (GY) | (SSVs) | 10.5–18.3 | ||
| 1062681|F|0-26 | 4D | 23.470 | (GY) | (SSVs) | 13.1–17.6 | ||
| 1051116|F|0-23 | 4D | 335.228 | (SSVs), (GY), (RP1.SSVs) | 9.4–10.2 | |||
| 1055706|F|0-65 | 4D | 123.018 | (SSVs), (GY) | (SSVs), (RP2.SSVs) | 13.2–18.9 | ||
| 7352852|F|0-19 | 4D | 4.891 | (SSVs) | (SSVs) | 10.0–13.6 | ||
| 1079306|F|0-62 | 4D | 25.702 | (GY) | (RP1.SSVs) | 13.1 | ||
| 998809|F|0-7 | 4D | 98.475 | (SSVs), (GY) | 9.5–10.2 | |||
| 3534425|F|0-23 | 6A | 37.593 | (SSVs) | (SSVs) | 10.4–13.9 | ||
| 3940208|F|0-6 | 6A | 39.432 | (SSVs) | (SSVs) | 12.2–12.3 | ||
| 1091824|F|0-36 | 7D | 245.790 | (SSVs) | (SSVs) | 9.9–11.8 | ||
| Marker | Environment | Trait | Ch. | R2 | Gene | Protein | Function |
|---|---|---|---|---|---|---|---|
| 1105119|F|0-22 | DON19/20 | SSVs | 2B | 0.15 | TraesCS2B02G387800 | MYB transcription factor | Drought stress response in wheat |
| 4262010|F|0-9 | DON19/20 | SSVs | 2D | 0.14 | TraesCS2D02G521800 | Cytochrome P450 family protein | Enhanced biotic stress resistance and grain development in wheat |
| 1201923|F|0-5 | DON19/20 | GY | 4D | 0.20 | TraesCS4D02G047400 | Glutamine synthase | Regulated nitrogen metabolism in wheat |
| 1240703|F|0-26 | HUD19/20 | SSVs | 6D | 0.13 | TraesCS6D02G035600 | High-affinity nitrate transporter | Improved nitrogen uptake, root growth, and grain yield in wheat |
| 1668806|F|0-24 | MED18/19 | SSVs | 4D | 0.19 | TraesCS4D02G048800 | Protein kinase | Regulated plant development and stress tolerance in wheat |
| 3026863|F|0-12 | MED18/19 | GY | 2D | 0.14 | TraesCS2D02G588900 | Pentatricopeptide repeat-containing family protein | Regulated plant growth, development, cytoplasmic male sterility, stress responses, and seed development |
| 1092278|F|0-29 | MED19/20 MED18/19 | SSVs | 1D | 0.22 | TraesCS1D02G321000 | F-box family protein | Enhanced tolerance to oxidative stress in wheat |
| TraesCS1D02G320200 | Potassium transporter | Regulated potassium uptake in wheat. HMW-GL levels were regulated by potassium availability | |||||
| 1055706|F|0-65 | MED18/19 | SSVs GY | 4D | 0.18 | TraesCS4D02G136900 | NBS-LRR disease resistance protein, putative, expressed | TaRPM1 is a type of CC-NBS-LRR that positively regulated wheat at high temperatures |
| MED19/20 | SSVs RP2.SSVs | ||||||
| 32025569|F|0-33 | RP1.SSVs RP2.SSVs | 6D | 0.19 | TraesCS6D02G056300 | F-box and associated interaction domains-containing protein TE | Enhance heat-stress tolerance in wheat through improve enzymatic antioxidant | |
| 1100384|F|0-67 | RP1.SSVs | 1D | 0.13 | TraesCS1D02G159700 | Protease inhibitor/seed storage/lipid transfer family protein | Seed storage protein regulated elasticity and extensibility of dough that determine the processing qualities of various end-products |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, I.E.S.; Kamal, N.M.; Mustafa, H.M.; Abdalla, M.G.A.; Elhashimi, A.M.A.; Gorafi, Y.S.A.; Tahir, I.S.A.; Tsujimoto, H.; Tanaka, H. Identification of Glu-D1 Alleles and Novel Marker–Trait Associations for Flour Quality and Grain Yield Traits under Heat-Stress Environments in Wheat Lines Derived from Diverse Accessions of Aegilops tauschii. Int. J. Mol. Sci. 2022, 23, 12034. https://doi.org/10.3390/ijms231912034
Mohamed IES, Kamal NM, Mustafa HM, Abdalla MGA, Elhashimi AMA, Gorafi YSA, Tahir ISA, Tsujimoto H, Tanaka H. Identification of Glu-D1 Alleles and Novel Marker–Trait Associations for Flour Quality and Grain Yield Traits under Heat-Stress Environments in Wheat Lines Derived from Diverse Accessions of Aegilops tauschii. International Journal of Molecular Sciences. 2022; 23(19):12034. https://doi.org/10.3390/ijms231912034
Chicago/Turabian StyleMohamed, Ikram Elsadig Suliman, Nasrein Mohamed Kamal, Hala Mohamed Mustafa, Modather Galal Abdeldaim Abdalla, Ashraf. M. A. Elhashimi, Yasir Serag Alnor Gorafi, Izzat Sidahmed Ali Tahir, Hisashi Tsujimoto, and Hiroyuki Tanaka. 2022. "Identification of Glu-D1 Alleles and Novel Marker–Trait Associations for Flour Quality and Grain Yield Traits under Heat-Stress Environments in Wheat Lines Derived from Diverse Accessions of Aegilops tauschii" International Journal of Molecular Sciences 23, no. 19: 12034. https://doi.org/10.3390/ijms231912034






