SiMYB19 from Foxtail Millet (Setaria italica) Confers Transgenic Rice Tolerance to High Salt Stress in the Field
Abstract
:1. Introduction
2. Results
2.1. SiMYB19 Gene Structure and Phylogenetic Tree Analysis
2.2. SiMYB19 Expression Profile in Foxtail Millet under Different Treatments
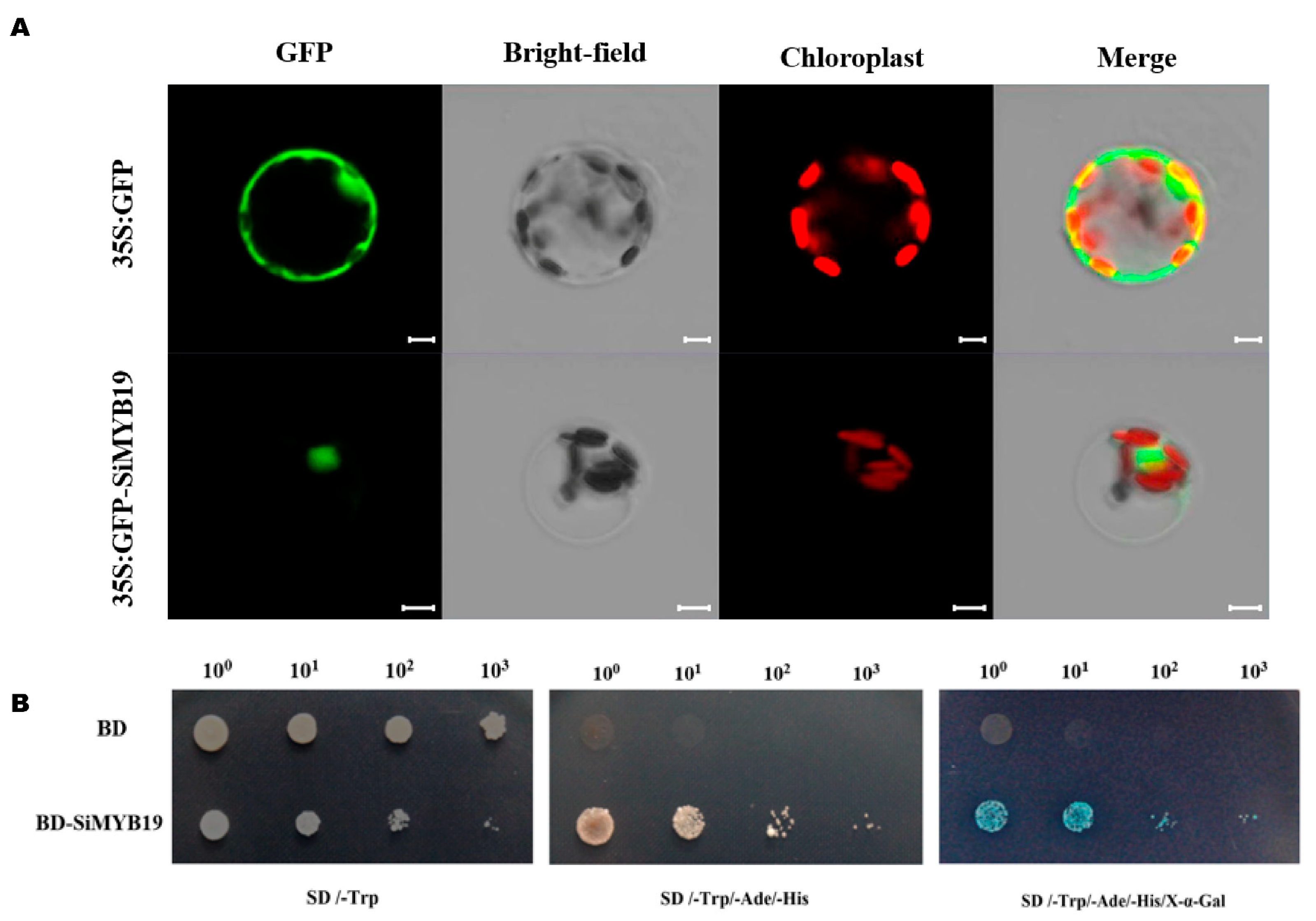
2.3. SiMYB19 Tentatively Localizes to The Nucleus and Activates Transcription
2.4. SiMYB19 Overexpression Enhanced Transgenic Rice Seedling Tolerance to Salt Stress
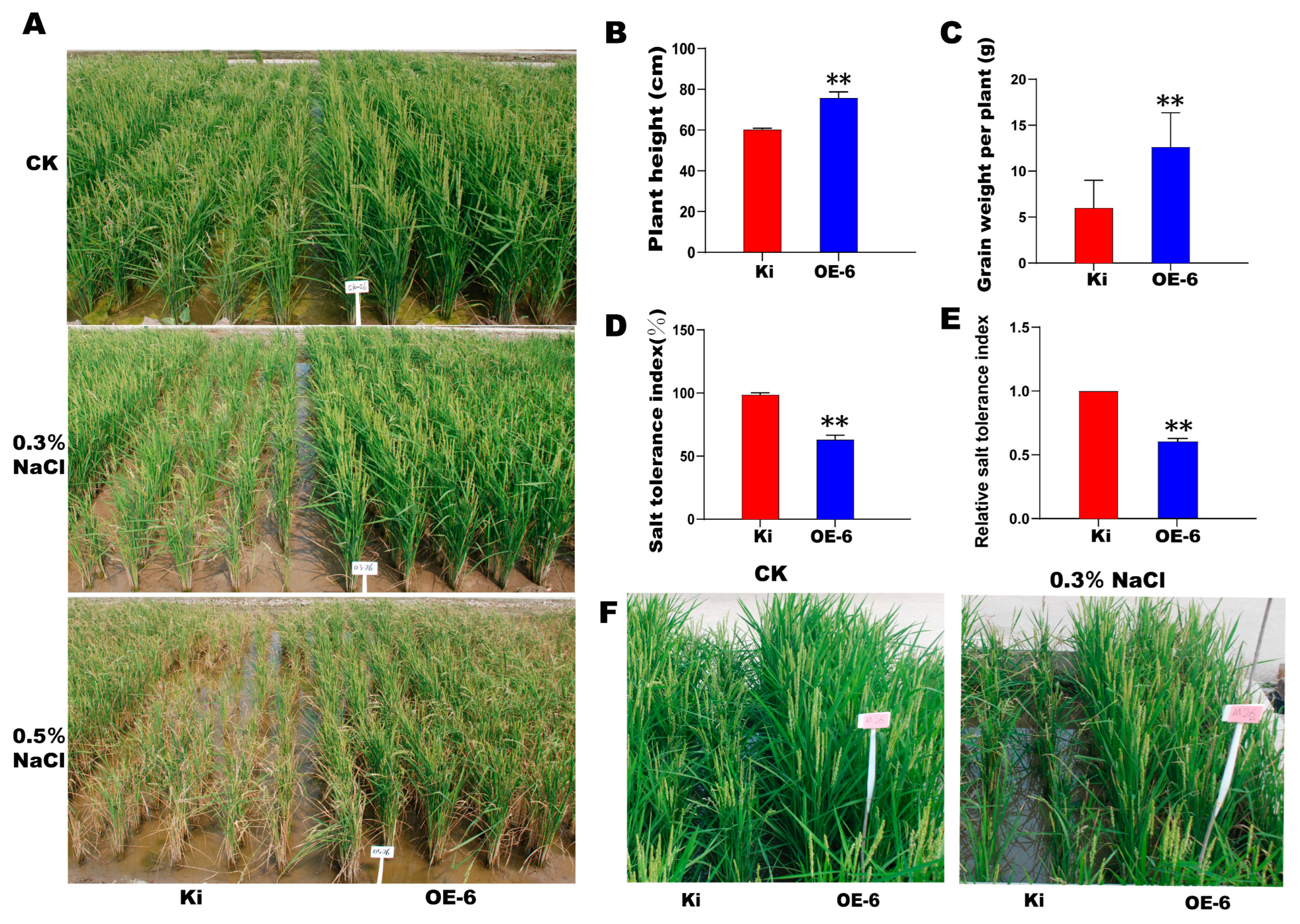
2.5. SiMYB19 Overexpression Increased the Yield of Field-Grown Transgenic Rice Subjected to High Salt Stress
2.6. Salt Tolerance, ABA, and Drought Stress Response in OE-6
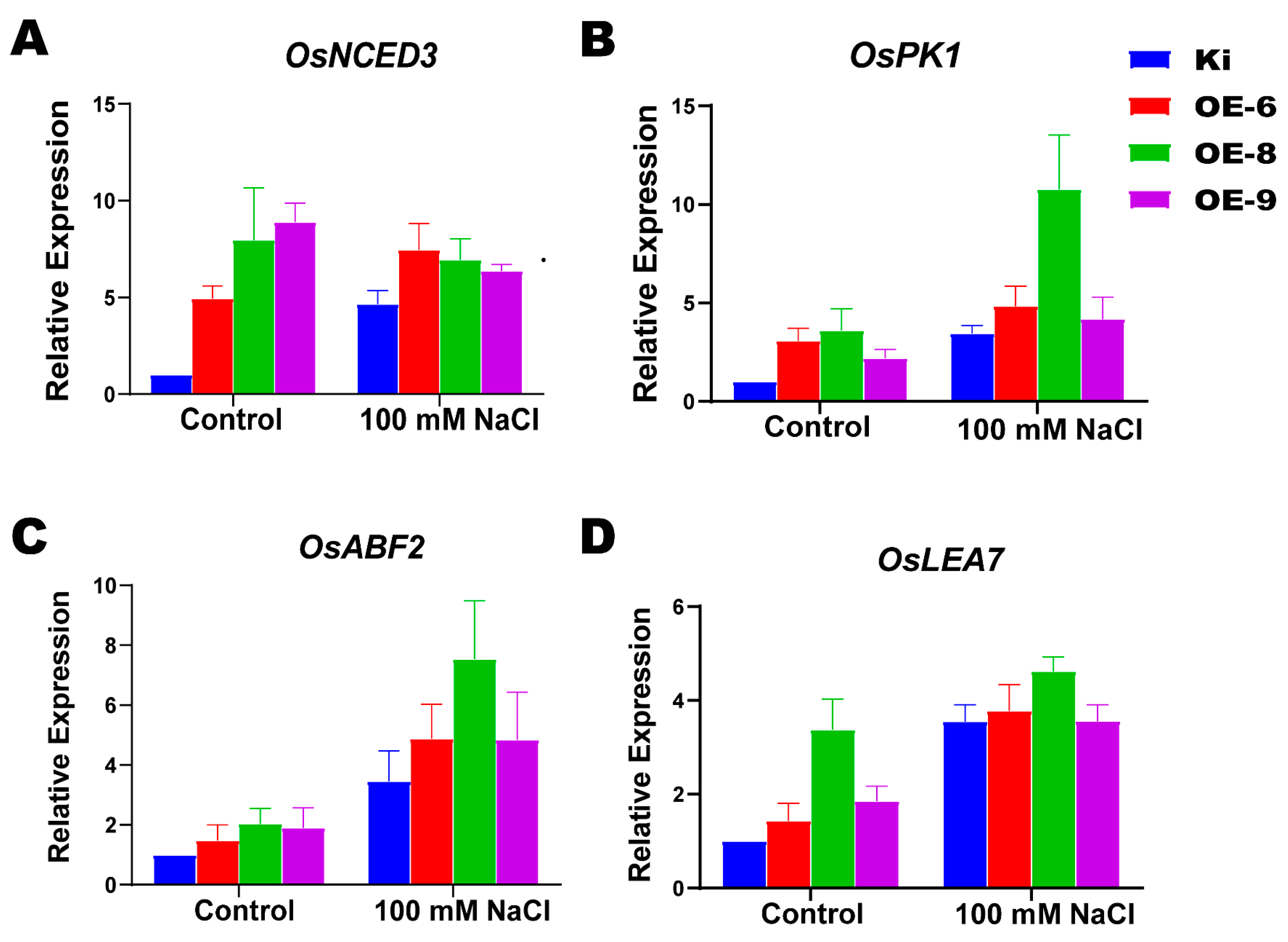
2.7. Expression Analysis of ABA- and Salt Stress-Related Genes in SiMYB19 Transgenic Rice
3. Discussion
3.1. SiMYB19 Is a Positive Regulator That Modulates Field Crop Salt Stress Tolerance
3.2. SiMYB19 Confers Salt Stress Tolerance through an Aba-Dependent Pathway
3.3. SiMYB19 Modulates Drought Stress
4. Methods
4.1. Plant Materials, Growth Conditions, and Stress Treatments
4.2. Sequence Alignment and Phylogenic Tree Construction
4.3. Rice Transformation and Subcellular Simyb19 Localization in Foxtail Millet
4.4. Transcription Activation Assay in Yeast
4.5. Salt Tolerance Analyses of SiMYB19 Transgenic Rice in the Field and Salt Ponds
4.6. Salt Tolerance Analysis of Germinating Transgenic Rice Seeds
4.7. RNA Extraction and qRT-PCRs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Muthamilarasan, M.; Prasad, M. Advances in Setaria genomics for genetic improvement of cereals and bioenergy grasses. Theor. Appl. Genet. 2015, 128, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.; Feng, B.; Yang, S.; Huang, Y.; Tang, Y. The R2R3-MYB transcription factor gene family in maize. PLoS ONE 2012, 7, e37463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, M.; Hao, Y.; Kapoor, A.; Dong, C.; Fujii, H.; Zheng, X.; Zhu, J. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J. Biol. Chem. 2006, 281, 37636–37645. [Google Scholar] [CrossRef] [Green Version]
- Woodger, F.; Millar, A.; Murray, F.; Jacobsen, J.; Gubler, F. The Role of GAMYB Transcription Factors in GA-Regulated Gene Expression. J. Plant Growth Regul. 2003, 22, 176–184. [Google Scholar] [CrossRef]
- Yang, A.; Dai, X.; Zhang, W.-H. A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J. Exp. Bot. 2012, 63, 2541–2556. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Y.; Zheng, H.; Lu, W.; Wu, C.; Huang, J.; Yan, K.; Yang, G.; Zheng, C. Salt-induced transcription factor MYB74 is regulated by the RNA-directed DNA methylation pathway in Arabidopsis. J. Exp. Bot. 2015, 66, 5997–6008. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Jiang, Y.; Liang, Y.; Chen, L.; Chen, W.; Cheng, B. Expression of the maize MYB transcription factor ZmMYB3R enhances drought and salt stress tolerance in transgenic plants. Plant Physiol. Biochem. PPB 2019, 137, 179–188. [Google Scholar] [CrossRef]
- Dong, W.; Gao, T.; Wang, Q.; Chen, J.; Lv, J.; Song, Y. Salinity stress induces epigenetic alterations to the promoter of MsMYB4 encoding a salt-induced MYB transcription factor. Plant Physiol. Biochem. PPB 2020, 155, 709–715. [Google Scholar] [CrossRef]
- Fang, Q.; Jiang, T.; Xu, L.; Liu, H.; Mao, H.; Wang, X.; Jiao, B.; Duan, Y.; Wang, Q.; Dong, Q. A salt-stress-regulator from the Poplar R2R3 MYB family integrates the regulation of lateral root emergence and ABA signaling to mediate salt stress tolerance in Arabidopsis. Plant Physiol. Biochem. 2017, 114, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Chen, P.; Shen, X.; Zhang, Y.; Li, X.; Jiang, L.; Xie, Y.; Niu, C.; Zhang, J.; Huang, X.; et al. MdMYB88 and MdMYB124 Enhance Drought Tolerance by Modulating Root Vessels and Cell Walls in Apple. Plant Physiol. 2018, 178, 1296–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Tang, W.; Wang, C.; Ge, L.; Chen, M. SiMYB56 Confers Drought Stress Tolerance in Transgenic Rice by Regulating Lignin Biosynthesis and ABA Signaling Pathway. Front. Plant Sci. 2020, 11, 785. [Google Scholar] [CrossRef]
- Ge, L.; Dou, Y.; Li, M.; Qu, P.; He, Z.; Liu, Y.; Xu, Z.; Chen, J.; Chen, M.; Ma, Y. Setaria italicaSiMYB3 in Foxtail Millet (Setaria italica) Confers Tolerance to Low-Nitrogen Stress by Regulating Root Growth in Transgenic Plants. Int. J. Mol. Sci. 2019, 20, 5741. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yang, X.; He, K.; Liu, M.; Li, J.; Gao, Z.; Lin, Z.; Zhang, Y.; Wang, X.; Qiu, X.; et al. The MYB transcription factor superfamily of Arabidopsis: Expression analysis and phylogenetic comparison with the rice MYB family. Plant Mol. Biol. 2006, 60, 107–124. [Google Scholar] [CrossRef]
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular Aspects of Seed Dormancy. Annu. Rev. Plant Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babu, R.C.; Zhang, J.; Blum, A.; Ho, T.H.D.; Wu, R.; Nguyen, H.T. HVA1, a LEA gene from barley confers dehydration tolerance in transgenic rice (Oryza sativa L.) via cell membrane protection. Plant Sci. 2004, 166, 855–862. [Google Scholar] [CrossRef]
- Kaneko, T.; Horie, T.; Nakahara, Y.; Tsuji, N.; Shibasaka, M.; Katsuhara, M. Dynamic regulation of the root hydraulic conductivity of barley plants in response to salinity/osmotic stress. Plant Cell Physiol. 2015, 56, 875–882. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Ghassemi, F.; Jakeman, A.J.; Nix, H.A. Salinisation of Land and Water Resources: Human Causes, Extent, Management and Case Studies; CAB International: Wallingford, UK, 1995; p. 544. [Google Scholar]
- Zhang, J. Salt-Affected Soil Resources in China; Springer: Berlin/Heidelberg, Germany, 2014; pp. 9–13. [Google Scholar]
- Wang, W.J.; He, H.S.; Zu, Y.G.; Guan, Y.; Liu, Z.G.; Zhang, Z.H.; Xu, H.N.; Yu, X.Y. Addition of HPMA affects seed germination, plant growth and properties of heavy saline-alkali soil in northeastern China: Comparison with other agents and determination of the mechanism. Plant Soil 2011, 339, 177–191. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2010, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Cheng, S.; Liu, X.; Du, H.; Dai, M.; Zhou, D.X.; Yang, W.; Zhao, Y. The R2R3-type MYB gene OsMYB91 has a function in coordinating plant growth and salt stress tolerance in rice. Plant Sci. Int. J. Exp. Plant Biol. 2015, 236, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Abdula, S.E.; Cho, Y.G. Overexpression of OsMLD Encoding MYB-like DNA Binding Domain Increases Tolerance to Salt Stress in Rice (Oryza sativa L.). Korean J. Breed. Sci. 2012, 44, 100–109. [Google Scholar]
- Mmadi, M.; Dossa, K.; Wang, L.; Zhou, R.; Wang, Y.; Cisse, N.; Sy, M.; Zhang, X. Functional Characterization of the Versatile MYB Gene Family Uncovered Their Important Roles in Plant Development and Responses to Drought and Waterlogging in Sesame. Genes 2017, 8, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, T.; Rong, W.; Xu, H.; Du, L.; Liu, X.; Zhang, Z. The wheat R2R3-MYB transcription factor TaRIM1 participates in resistance response against the pathogen Rhizoctonia cerealis infection through regulating defense genes. Sci. Rep. 2016, 6, 28777. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Luo, Q.; Wang, R.; Zhang, F.; He, Y.; Zhang, Y.; Qiu, D.; Li, K.; Chang, J.; Yang, G.; et al. A Wheat R2R3-type MYB Transcription Factor TaODORANT1 Positively Regulates Drought and Salt Stress Responses in Transgenic Tobacco Plants. Front. Plant Sci. 2017, 8, 1374. [Google Scholar] [CrossRef] [Green Version]
- Shingote, P.R.; Kawar, P.G.; Pagariya, M.C.; Kuhikar, R.S.; Thorat, A.S.; Babu, K.H. SoMYB18, a sugarcane MYB transcription factor improves salt and dehydration tolerance in tobacco. Acta Physiol. Plant 2015, 37, 217. [Google Scholar] [CrossRef]
- Ding, Z.; Li, S.; An, X.; Xin, L.; Qin, H.; Wang, D. Transgenic expression of MYB15 confers enhanced sensitivity to abscisic acid and improved drought tolerance in Arabidopsis thaliana. J. Genet. Genom. 2009, 36, 17–29. [Google Scholar] [CrossRef]
- Lee, S.C.; Luan, S. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 2012, 35, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell Signaling during Cold, Drought, and Salt Stress. Plant Cell 2002, 14, 165–183. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [Green Version]
- Gosti, F.; Beaudoin, N.; Serizet, C.; Webb, A.A.; Vartanian, N.; Giraudat, J. ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell 1999, 11, 1897–1909. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Fung, P.; Nishimura, N.; Jensen, D.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.; et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, L.; Soon, F.; Zhou, X.; West, G.; Kovach, A.; Suino-Powell, K.; Chalmers, M.; Li, J.; Yong, E.; Zhu, J.; et al. Structural basis for basal activity and autoactivation of abscisic acid (ABA) signaling SnRK2 kinases. Proc. Natl. Acad. Sci. USA 2011, 108, 21259–21264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soon, F.; Ng, L.; Zhou, X.; West, G.; Kovach, A.; Tan, M.; Suino-Powell, K.; He, Y.; Xu, Y.; Chalmers, M.; et al. Molecular mimicry regulates ABA signaling by SnRK2 kinases and PP2C phosphatases. Science 2012, 335, 85–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, P.J.; Xiang, F.; Qiao, M.; Park, J.Y.; Park, C.M. The MYB96 Transcription Factor Mediates Abscisic Acid Signaling during Drought Stress Response in Arabidopsis. Plant Physiol. 2009, 151, 275–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, C.; Seo, J.; Han, S.; Koo, Y.; Kim, C.; Song, S.; Nahm, B.; Choi, Y.; Cheong, J. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol. 2008, 146, 623–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Bao, X.; Zhi, Y.; Wu, Q.; Guo, Y.; Yin, X.; Zeng, L.; Li, J.; Zhang, J.; He, W.; et al. Overexpression of a MYB Family Gene, OsMYB6, Increases Drought and Salinity Stress Tolerance in Transgenic Rice. Front. Plant Sci. 2019, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liu, G.; Zhao, G.; Xia, C.; Jia, J.; Liu, X.; Kong, X. Characterization of a wheat R2R3-MYB transcription factor gene, TaMYB19, involved in enhanced abiotic stresses in Arabidopsis. Plant Cell Physiol. 2014, 10, 1802. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi-Shinozaki, S.K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirayama, T. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef]
- Nakashima, K.; Tran, L.S.P.; Nguyen, D.V.; Fujita, M.; Yamaguchi-Shinozaki, K. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. 2010, 51, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Langridge, P. Breeding Technologies to Increase Crop Production in a Changing World. Science 2010, 327, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yu, B.; Wu, Q.; Min, Q.; Zeng, R.; Xie, Z.; Huang, J. OsMADS23 phosphorylated by SAPK9 confers drought and salt tolerance by regulating ABA biosynthesis in rice. PLoS Genet. 2021, 17, e1009699. [Google Scholar] [CrossRef]
- Sudhir, K.; Glen, S.; Koichiro, T. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870. [Google Scholar]
- Komari, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. Cell Mol. Biol. 2010, 6, 271–282. [Google Scholar]
- Yoo, S.; Cho, Y.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, C.; Bartel, P.; Sternglanz, R.; Fields, S. The two-hybrid system: A method to identify and clone genes for proteins that interact with a protein of interest. Proc. Natl. Acad. Sci. USA 1991, 88, 9578–9582. [Google Scholar] [CrossRef] [Green Version]
- Fields, S.; Sternglanz, R. The two-hybrid system: An assay for protein-protein interactions. Trends Genet. 1994, 10, 286. [Google Scholar] [CrossRef]
- Abbas, M.K.; Ali, A.S.; Hasan, H.H.; Ghal, R.H. Salt Tolerance Study of Six Cultivars of Rice (Oryza sativa L.) During Germination and Early Seedling Growth. J. Agric. Sci. 2012, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.J.; Fu, J.D.; Tang, Y.M.; Yu, T.F.; Yin, Z.G.; Chen, J.; Zhou, Y.B.; Chen, M.; Xu, Z.S.; Ma, Y.Z. GmNFYA13 Improves Salt and Drought Tolerance in Transgenic Soybean Plants. Front. Plant Sci. 2020, 11, 587244. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Skinner, J.; Bennett, J.E. Evaluation of reference genes for real-time quantitative PCR studies in Candida glabrata following azole treatment. BMC Mol. Biol. 2012, 13, 22. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Luo, M.; Sun, X.; Yan, J.; Shi, H.; Yan, H.; Yan, R.; Wang, S.; Tang, W.; Zhou, Y.; et al. SiMYB19 from Foxtail Millet (Setaria italica) Confers Transgenic Rice Tolerance to High Salt Stress in the Field. Int. J. Mol. Sci. 2022, 23, 756. https://doi.org/10.3390/ijms23020756
Xu C, Luo M, Sun X, Yan J, Shi H, Yan H, Yan R, Wang S, Tang W, Zhou Y, et al. SiMYB19 from Foxtail Millet (Setaria italica) Confers Transgenic Rice Tolerance to High Salt Stress in the Field. International Journal of Molecular Sciences. 2022; 23(2):756. https://doi.org/10.3390/ijms23020756
Chicago/Turabian StyleXu, Chengjie, Mingzhao Luo, Xianjun Sun, Jiji Yan, Huawei Shi, Huishu Yan, Rongyue Yan, Shuguang Wang, Wensi Tang, Yongbin Zhou, and et al. 2022. "SiMYB19 from Foxtail Millet (Setaria italica) Confers Transgenic Rice Tolerance to High Salt Stress in the Field" International Journal of Molecular Sciences 23, no. 2: 756. https://doi.org/10.3390/ijms23020756







