Radical in the Peroxide-Produced F-Type Ferryl Form of Bovine Cytochrome c Oxidase
Abstract
1. Introduction
2. Results
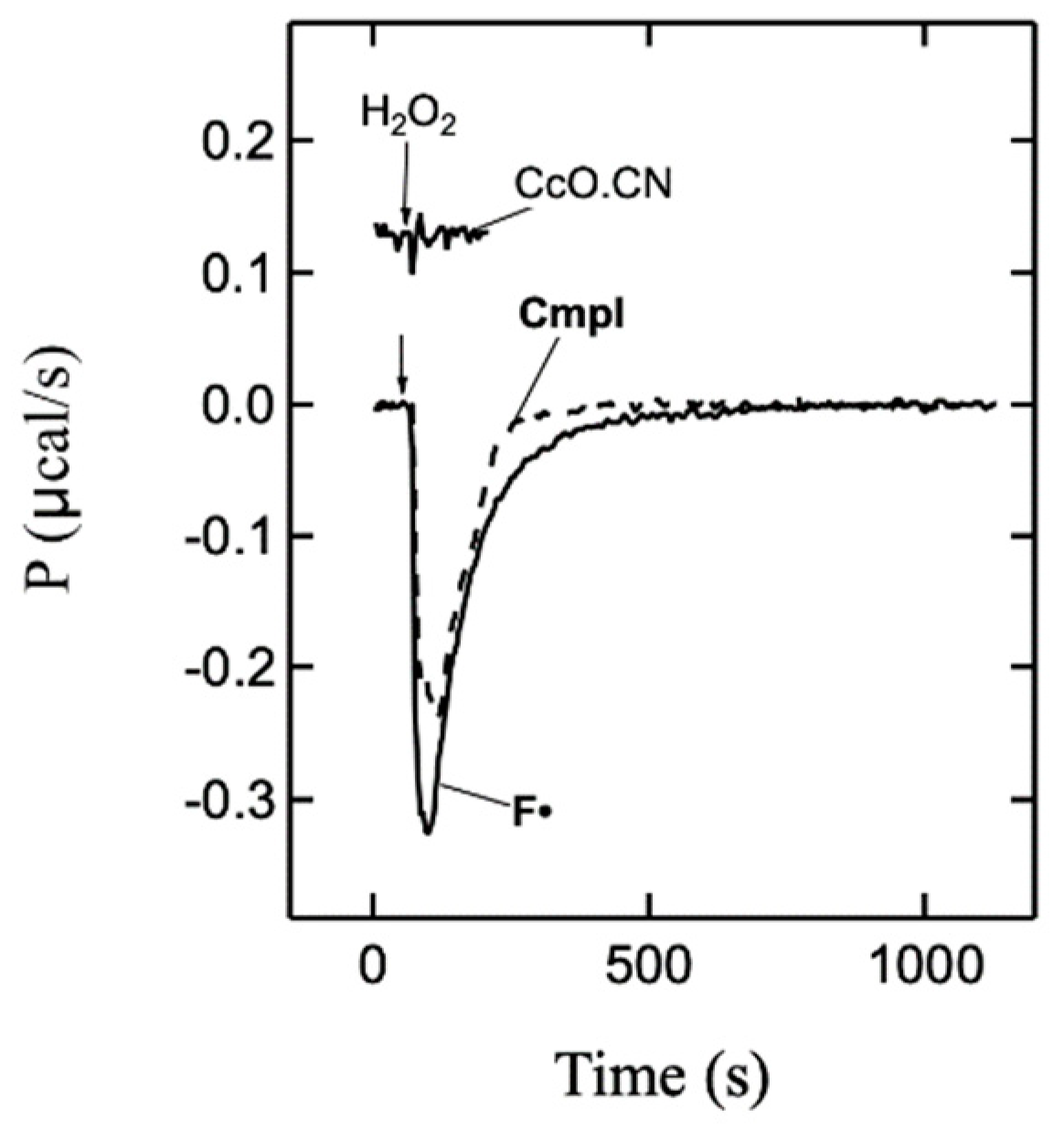
2.1. Formation and Decay of F• Form—UV-Vis and ITC Measurements
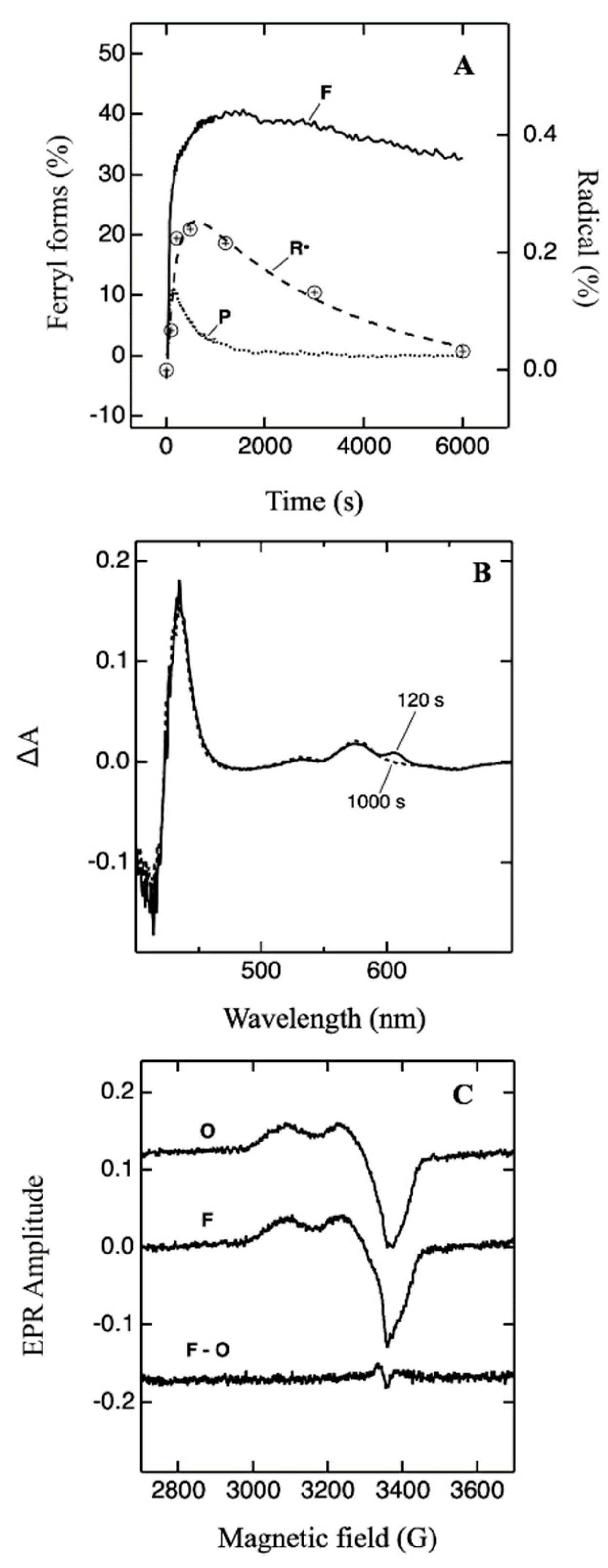
2.2. Formation of Radical in the F• State—UV-Vis and EPR Measurements
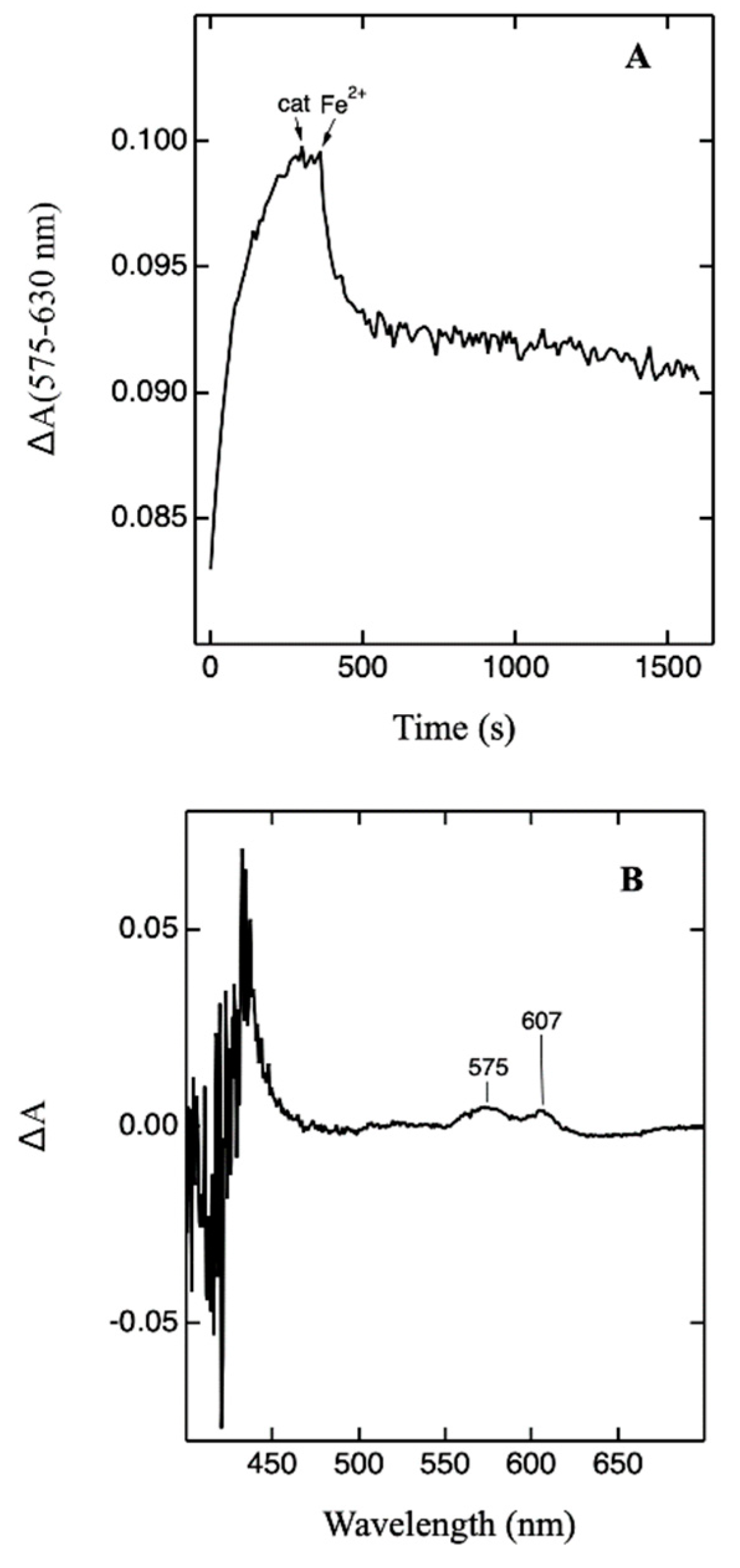
2.3. One-Electron Reduction of the F• Form
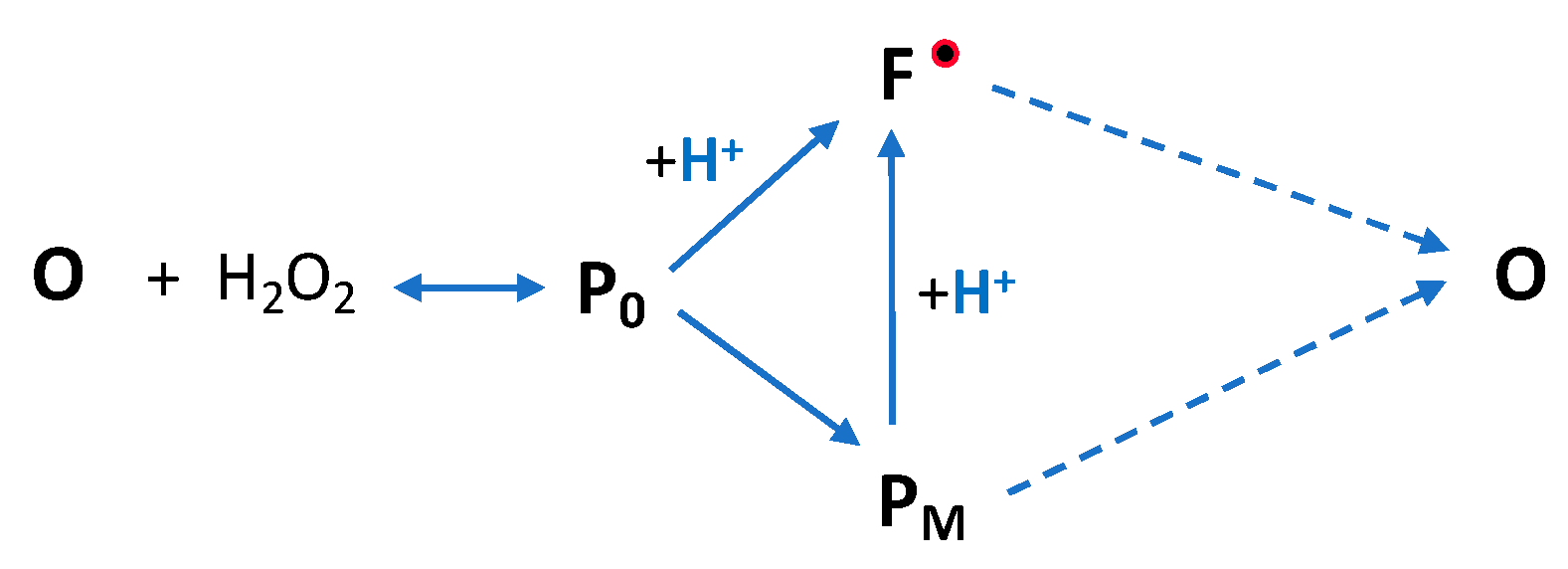
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Oxidized CcO
4.3. Isothermal Titration Calorimetry (ITC) Measurements
4.4. UV-Vis Absorption Spectroscopy Mesurements
4.5. EPR Spectroscopy
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sousa, F.L.; Alves, R.J.; Ribeiro, M.A.; Pereira-Leal, J.B.; Teixeira, M.; Manuela, M.; Pereira, M.M. The superfamily of heme–copper oxygen reductases:Types and evolutionary considerations. Biochim. Biophys. Acta 2012, 1817, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.P.; Hibdon, S.; Liu, R.Q.; Durham, B.; Millett, F. Intracomplex electron transfer between ruthenium-cytochrome c derivatives and cytochrome c oxidase. Biochemistry 1993, 32, 8492–8498. [Google Scholar] [CrossRef]
- Szundi, I.; Cappuccio, J.A.; Borovok, N.; Kotlyar, A.B.; Einarsdottir, O. Photoinduced electron transfer in the cytochrome c/cytochrome c oxidase complex using thiouredopyrenetrisulfonate-labeled cytochrome c optical multichannel detection. Biochemistry 2001, 40, 2186–2193. [Google Scholar] [CrossRef]
- Geren, L.; Durham, B.; Millett, F. Use of Ruthenium Photoreduction Techniques to Study Electron Transfer in Cytochrome Oxidase. Method Enzym. 2009, 456, 507–520. [Google Scholar]
- Ferguson-Miller, S.; Babcock, G.T. Heme/Copper Terminal Oxidases. Chem. Rev. 1996, 96, 2889–2908. [Google Scholar] [CrossRef]
- Belevich, I.; Verkhovsky, M.I. Molecular mechanism of proton translocation by cytochrome c oxidase. Antio. Redox Sig. 2008, 10, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Konstantinov, A.A. Cytochrome c oxidase: Intermediates of the catalytic cycle and their energy-coupled interconversion. FEBS Lett. 2012, 586, 630–639. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Shimada, A. Reaction mechanism of cytochrome c oxidase. Chem. Rev. 2015, 115, 1936–1989. [Google Scholar] [CrossRef]
- Rich, P.R. Mitochondrial cytochrome c oxidase: Catalysis, coupling and controversies. Biochem. Soc. Trans. 2017, 45, 813–829. [Google Scholar] [CrossRef]
- Wikstrom, M.; Krab, K.; Sharma, V. Oxygen Activation and Energy Conservation by Cytochrome c Oxidase. Chem. Rev. 2018, 118, 2469–2490. [Google Scholar] [CrossRef] [PubMed]
- Proshlyakov, D.A.; Pressler, M.A.; DeMaso, C.; Leykam, J.F.; DeWitt, D.L.; Babcock, G.T. Oxygen activation and reduction in respiration: Involvement of redox-active tyrosine 244. Science 2000, 290, 1588–1591. [Google Scholar] [CrossRef] [PubMed]
- Gorbikova, I.E.A.B.; Wikstrom, M.; Verkhovsky, M.I. The proton donor for OO bond scission by cytochrome c oxidase. Proc. Natl. Acad. Sci. USA. 2008, 105, 10733–10737. [Google Scholar] [CrossRef] [PubMed]
- Proshlyakov, D.A.; Pressler, M.A.; Babcock, G.T. Dioxygen activation and bond cleavage by mixed-valence cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 1998, 95, 8020–8025. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.; Wong, W.W.; Gennis, R.B.; Palmer, G. Mass spectrometric determination of dioxygen bond splitting in the “peroxy” intermediate of cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 1999, 96, 13114–13117. [Google Scholar] [CrossRef] [PubMed]
- Pinakoulaki, E.; Daskalakis, V.; Ohta, T.; Richter, O.M.; Budiman, K.; Kitagawa, T.; Ludwig, B.; Varotsis, C. The protein effect in the structure of two ferryl-oxo intermediates at the same oxidation level in the heme copper binuclear center of cytochrome c oxidase. J. Biol. Chem. 2013, 288, 20261–20266. [Google Scholar] [CrossRef]
- Morgan, J.E.; Verkhovsky, M.I.; Wikstrom, M. Observation and assignment of peroxy and ferryl intermediates in the reduction of dioxygen to water by cytochrome c oxidase. Biochemistry 1996, 35, 12235–12240. [Google Scholar] [CrossRef]
- Morgan, J.E.; Verkhovsky, M.I.; Palmer, G.; Wikstrom, M. Role of the P-R intermediate in the reaction of cytochrome c oxidase with O-2. Biochemistry 2001, 40, 6882–6892. [Google Scholar] [CrossRef]
- Ishigami, I.; Lewis-Ballester, A.; Echelmeier, A.; Brehm, G.; Zatsepin, N.A.; Grant, T.D.; Coe, J.D.; Lisova, S.; Nelson, G.; Zhang, S.; et al. Snapshot of an oxygen intermediate in the catalytic reaction of cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 2019, 116, 3572–3577. [Google Scholar] [CrossRef] [PubMed]
- Poiana, F.; von Ballmoos, C.; Gonska, N.; Blomberg, M.R.A.; Adelroth, P.; Brzezinski, P. Splitting of the O-O bond at the heme-copper catalytic site of respiratory oxidases. Sci. Adv. 2017, 3, e1700279. [Google Scholar] [CrossRef]
- Verkhovsky, J.E.M.M.I.; Wikstrom, M. Redox transitions between oxygen intermediates in cytochrome-c oxidase. Proc. Natl. Acad. Sci. USA 1996, 93, 12235–12239. [Google Scholar] [CrossRef]
- Siletsky, A.D.K.S.; Konstantinov, A.A. Resolution of Electrogenic Steps Coupled to Conversion of Cytochrome c Oxidase from the Peroxy to the Ferryl−Oxo State. Biochemistry 1999, 38, 4853–4861. [Google Scholar] [CrossRef] [PubMed]
- Verkhovsky, M.I.J.A.; Verkhovskaya, M.L.; Morgan, J.E.; Wikstrom, M. Proton translocation by cytochrome c oxidase. Nature 1999, 400, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Bloch, D.; Belevich, I.; Jasaitis, A.; Ribacka, C.; Puustinen, A.; Verkhovsky, M.I.; Wikstrom, M. The catalytic cycle of cytochrome c oxidase is not the sum of its two halves. Proc. Natl. Acad. Sci. USA 2004, 101, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Faxen, K.; Gilderson, G.; Adelroth, P.; Brzezinski, P. A mechanistic principle for proton pumping by cytochrome c oxidase. Nature 2005, 437, 286–289. [Google Scholar] [CrossRef]
- Belevich, I.V.; Wikstrom, M.I. Proton-coupled electron transfer drives the proton pump of cytochrome c oxidase. Nature 2006, 440, 829–832. [Google Scholar] [CrossRef]
- Siletsky, D.H.S.A.; Brand, S.; Morgan, J.E.; Fabian, M.; Geren, L.; Millett, F.; Durham, B.; Konstantinov, A.A.; Gennis, R.B. Single-electron photoreduction of the PM intermediate of cytochrome c oxidase. Biochim. Biophys. Acta 2006, 1757, 1122–1132. [Google Scholar] [CrossRef]
- Siletsky, S.A.; Gennis, R.B. Time-resolved electrometric: Study of the F→O transition in cytochrome c oxidase. The effect of Zn2+ ions on the positive side of the membrane. Biochemistry 2021, 86, 105–122. [Google Scholar]
- Zaslavsky, D.; Sadoski, R.C.; Rajagukguk, S.; Geren, L.; Millett, F.; Durham, B.; Gennis, R.B. Direct measurement of proton release by cytochrome c oxidase in solution during the F→O transition. Proc. Natl. Acad. Sci. USA 2004, 101, 10544–10547. [Google Scholar] [CrossRef]
- Zaslavsky, A.D.K.D.; Smirnova, I.A.; Vygodina, T.; Konstantinov, A.A. Flash-induced membrane potential generation by cytochrome c oxidase. FEBS Lett 1993, 336, 389–393. [Google Scholar]
- Ädelroth, M.S.E.P.; Mitchell, D.M.; Gennis, R.B.; Brzezinski, P. Glutamate 286 in Cytochrome aa3 from Rhodobacter sphaeroides Is Involved in Proton Uptake during the Reaction of the Fully-Reduced Enzyme with Dioxygen. Biochemistry 1997, 36, 13824–13829. [Google Scholar] [CrossRef]
- Verkhovsky, J.E.M.M.I.; Verkhovskaya, M.; Wikstrom, M. Translocation of electrical charge during a single turnover of cytochrome-c oxidase. Biochim. Biophys. Acta 1997, 1318, 6–10. [Google Scholar] [CrossRef]
- AÎdelroth, M.E.P.; Brzezinski, P. Factors determining electron-transfer rates in cytochromecoxidase: Investigation of the oxygen reaction in the R. Sphaeroides enzyme. Biochim. Biophys. Acta 1998, 1367, 107–117. [Google Scholar]
- Jasaitis, M.I.V.A.; Morgan, J.E.; Verkhovskaya, M.L.; Wikström, M. Assignment and Charge Translocation Stoichiometries of the Major Electrogenic Phases in the Reaction of Cytochrome c Oxidase with Dioxygen. Biochemistry 1999, 38, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Adelroth, M.K.P.; Gilderson, G.; Tomson, F.L.; Gennis, R.B.; Brzezinski, P. Proton transfer from glutamate 286 determines the transition rates between oxygen intermediates in cytochrome c oxidase. Biochim. Biophys. Acta 2000, 1459, 533–539. [Google Scholar] [CrossRef][Green Version]
- Siletsky, A.S.P.S.A.; Weiss, K.; Gennis, R.B.; Konstantinov, A.A. Transmembrane charge separation during the ferryl-oxo -> oxidized transition in a nonpumping mutant of cytochrome c oxidase. J. Biol. Chem. 2004, 279, 52558–52565. [Google Scholar] [CrossRef]
- Weng, L.C.; Baker, G.M. Reaction of hydrogen peroxide with the rapid form of resting cytochrome oxidase. Biochemistry 1991, 30, 5727–5733. [Google Scholar] [CrossRef]
- Fabian, M.; Palmer, G. The interaction of cytochrome oxidase with hydrogen peroxide: The relationship of compounds P and F. Biochemistry 1995, 34, 13802–13810. [Google Scholar] [CrossRef]
- Ksenzenko, M.; Vygodina, T.V.; Berka, V.; Ruuge, E.K.; Konstantinov, A.A. Cytochrome oxidase-catalyzed superoxide generation from hydrogen peroxide. FEBS Lett. 1992, 297, 63–66. [Google Scholar] [CrossRef]
- Junemann, S.; Heathcote, P.; Rich, P.R. The reactions of hydrogen peroxide with bovine cytochrome c oxidase. Biochim. Biophys. Acta 2000, 1456, 56–66. [Google Scholar] [CrossRef]
- Wrigglesworth, J.M. Formation and reduction of a ‘peroxy’ intermediate of cytochrome c oxidase by hydrogen peroxide. Biochem. J. 1984, 217, 715–719. [Google Scholar] [CrossRef]
- Vygodina, T.V.; Konstantinov, A.A. H2O2-induced conversion of cytochrome c oxidase peroxy complex to oxoferryl state. Ann. N. Y. Acad. Sci. 1988, 550, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Vygodina, T.; Konstantinov, A. Effect of pH on the spectrum of cytochrome c oxidase hydrogen peroxide complex. Biochim. Biophys. Acta 1989, 973, 390–398. [Google Scholar] [CrossRef]
- Pecoraro, C.; Gennis, R.B.; Vygodina, T.V.; Konstantinov, A.A. Role of the K-channel in the pH-dependence of the reaction of cytochrome c oxidase with hydrogen peroxide. Biochemistry 2001, 40, 9695–9708. [Google Scholar] [CrossRef] [PubMed]
- Brittain, T.; Little, R.H.; Greenwood, C.; Watmough, N.J. The reaction of Escherichia coli cytochrome bo with H2O2: Evidence for the formation of an oxyferryl species by two distinct routes. FEBS Lett. 1996, 399, 21–25. [Google Scholar] [CrossRef]
- Konstantinov, A.A.; Capitanio, N.; Vygodina, T.V.; Papa, S. pH changes associated with cytochrome c oxidase reaction with H2O2. Protonation state of the peroxy and oxoferryl intermediates. FEBS Lett. 1992, 312, 71–74. [Google Scholar] [CrossRef]
- Wikstrom, M. Active site intermediates in the reduction of O(2) by cytochrome oxidase, and their derivatives. Biochim. Biophys. Acta 2012, 1817, 468–475. [Google Scholar] [CrossRef]
- Shimada, A.; Etoh, Y.; Kitoh-Fujisawa, R.; Sasaki, A.; Shinzawa-Itoh, K.; Hiromoto, T.; Yamashita, E.; Muramoto, K.; Tsukihara, T.; Yoshikawa, S. X-ray structures of catalytic intermediates of cytochrome c oxidase provide insights into its O2 activation and unidirectional proton-pump mechanisms. J. Biol. Chem. 2020, 295, 5818–5833. [Google Scholar] [CrossRef]
- MacMillan, F.; Kannt, A.; Behr, J.; Prisner, T.; Michel, H. Direct evidence for a tyrosine radical in the reaction of cytochrome c oxidase with hydrogen peroxide. Biochemistry 1999, 38, 9179–9184. [Google Scholar] [CrossRef]
- Budiman, K.; Kannt, A.; Lyubenova, S.; Richter, O.M.; Ludwig, B.; Michel, H.; MacMillan, F. Tyrosine 167: The origin of the radical species observed in the reaction of cytochrome c oxidase with hydrogen peroxide in Paracoccus denitrificans. Biochemistry 2004, 43, 11709–11716. [Google Scholar] [CrossRef]
- von der Hocht, I.; van Wonderen, J.H.; Hilbers, F.; Angerer, H.; MacMillan, F.; Michel, H. Interconversions of P and F intermediates of cytochrome c oxidase from Paracoccus denitrificans. Proc. Natl. Acad. Sci. USA 2011, 108, 3964–3969. [Google Scholar] [CrossRef]
- Rich, P.R.; Rigby, S.E.; Heathcote, P. Radicals associated with the catalytic intermediates of bovine cytochrome c oxidase. Biochim. Biophys. Acta 2002, 1554, 137–146. [Google Scholar] [CrossRef]
- Rigby, S.E.; Junemann, S.; Rich, P.R.; Heathcote, P. Reaction of bovine cytochrome c oxidase with hydrogen peroxide produces a tryptophan cation radical and a porphyrin cation radical. Biochemistry 2000, 39, 5921–5928. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.A.; Egawa, T.; Shinzawa-Itoh, K.; Yoshikawa, S.; Guallar, V.; Yeh, S.R.; Rousseau, D.L.; Gerfen, G.J. Two tyrosyl radicals stabilize high oxidation states in cytochrome C oxidase for efficient energy conservation and proton translocation. J. Am. Chem. Soc. 2012, 134, 4753–4761. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.A.; Egawa, T.; Shinzawa-Itoh, K.; Yoshikawa, S.; Yeh, S.R.; Rousseau, D.L.; Gerfen, G.J. Radical formation in cytochrome c oxidase. Biochim. Biophys. Acta 2011, 1807, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Musatov, A.; Hebert, E.; Carroll, C.A.; Weintraub, S.T.; Robinson, N.C. Specific modification of two tryptophans within the nuclear-encoded subunits of bovine cytochrome c oxidase by hydrogen peroxide. Biochemistry 2004, 43, 1003–1009. [Google Scholar] [CrossRef]
- Musatov, A.; Robinson, N.C. Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radic. Res. 2012, 46, 1313–1326. [Google Scholar] [CrossRef]
- Lemma-Gray, P.; Weintraub, S.T.; Carroll, C.A.; Musatov, A.; Robinson, N.C. Tryptophan 334 oxidation in bovine cytochrome c oxidase subunit I involves free radical migration. FEBS Lett. 2007, 581, 437–442. [Google Scholar] [CrossRef]
- Chen, Y.R.; Gunther, M.R.; Mason, R.P. An electron spin resonance spin-trapping investigation of the free radicals formed by the reaction of mitochondrial cytochrome c oxidase with H2O2. J. Biol. Chem. 1999, 274, 3308–3314. [Google Scholar] [CrossRef]
- Chen, Y.R.; Mason, R.P. Mechanism in the reaction of cytochrome c oxidase with organic hydroperoxides: An ESR spin-trapping investigation. Biochem. J. 2002, 365, 461–469. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yamazaki, I. The oxidation-reduction potentials of compound I/compound II and compound II/ferric couples of horseradish peroxidases A2 and C. J. Biol. Chem. 1979, 254, 9101–9106. [Google Scholar] [CrossRef]
- Hewson, W.D.; Hager, L.P. Oxidation of horseradish peroxidase compound II to compound I. J. Biol. Chem. 1979, 254, 3182–3186. [Google Scholar] [CrossRef]
- Musatov, A. Contribution of peroxidized cardiolipin to inactivation of bovine heart cytochrome c oxidase. Free Radic. Biol. Med. 2006, 41, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Jancura, D.; Stanicova, J.; Palmer, G.; Fabian, M. How hydrogen peroxide is metabolized by oxidized cytochrome c oxidase. Biochemistry 2014, 53, 3564–3575. [Google Scholar] [CrossRef]
- Khan, K.K.; Mondal, M.S.; Padhy, L.; Mitra, S. The role of distal histidine in peroxidase activity of myoglobin—Transient-kinetics study of the reaction of H2O2 with wild-type and distal-histidine-mutanted recombinant human myoglobin. Eur. J. Biochem. 1998, 257, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.S.; Mitra, S. Kinetic studies of the two-step reactions of H2O2 with manganese-reconstituted myoglobin. Biochim. Biophys. Acta 1996, 1296, 174–180. [Google Scholar] [CrossRef]
- Baek, H.K.; Van Wart, H.E. Elementary steps in the formation of horseradish peroxidase compound I: Direct observation of compound 0, a new intermediate with a hyperporphyrin spectrum. Biochemistry 1989, 28, 5714–5719. [Google Scholar] [CrossRef]
- Khan, K.K.; Mondal, M.S.; Mitra, S. Kinetic studies of the reaction of hydrogen peroxide with manganesereconstituted horseradish peroxidase. J. Chem. Soc. Dalton Trans. 1996, 1059–1062. [Google Scholar] [CrossRef]
- Farhangrazi, Z.S.; Copeland, B.R.; Nakayama, T.; Amachi, T.; Yamazaki, I.; Powers, L.S. Oxidation-reduction properties of compounds I and II of Arthromyces ramosus peroxidase. Biochemistry 1994, 33, 5647–5652. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, M.R.A. Active Site Midpoint Potentials in Different Cytochrome c Oxidase Families: A Computational Comparison. Biochemistry 2019, 58, 2028–2038. [Google Scholar] [CrossRef]
- Siegbahn, P.E.M.; Blomberg, M.R.A. A Systematic DFT Approach for Studying Mechanisms of Redox Active Enzymes. Front. Chem. 2018, 6, 644. [Google Scholar] [CrossRef]
- Gray, H.B.; Winkler, J.R. Hole hopping through tyrosine/tryptophan chains protects proteins from oxidative damage. Proc. Natl. Acad. Sci. USA 2015, 112, 10920–10925. [Google Scholar] [CrossRef]
- Watmough, N.J.; Cheesman, M.R.; Greenwood, C.; Thomson, A.J. Cytochrome bo from Escherichia coli: Reaction of the oxidized enzyme with hydrogen peroxide. Biochem. J. 1994, 300, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Parul, D.; Palmer, G.; Fabian, M. Proton interactions with hemes a and a3 in bovine heart cytochrome c oxidase. Biochemistry 2005, 44, 4562–4571. [Google Scholar] [CrossRef] [PubMed]
- Mikulova, L.; Pechova, I.; Jancura, D.; Stupak, M.; Fabian, M. Thermodynamics of the P-type Ferryl Form of Bovine Cytochrome c Oxidase. Biochemistry 2021, 86, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Sztachova, T.; Pechova, I.; Mikulova, L.; Stupak, M.; Jancura, D.; Fabian, M. Peroxide stimulated transition between the ferryl intermediates of bovine cytochrome c oxidase. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148447. [Google Scholar] [CrossRef] [PubMed]
- King, N.K.; Winfield, M.E. The mechanism of metmyoglobin oxidation. J. Biol. Chem. 1963, 238, 1520–1528. [Google Scholar] [CrossRef]
- Wilks, A.; Ortiz de Montellano, P.R. Intramolecular translocation of the protein radical formed in the reaction of recombinant sperm whale myoglobin with H2O2. J. Biol. Chem. 1992, 267, 8827–8833. [Google Scholar] [CrossRef]
- Tew, D.; Ortiz de Montellano, P.R. The myoglobin protein radical. Coupling of Tyr-103 to Tyr-151 in the H2O2-mediated cross-linking of sperm whale myoglobin. J. Biol. Chem. 1988, 263, 17880–17886. [Google Scholar] [CrossRef]
- Svistunenko, D.A. An EPR study of the peroxyl radicals induced by hydrogen peroxide in the haem proteins. Biochim. Biophys. Acta 2001, 1546, 365–378. [Google Scholar] [CrossRef]
- Svistunenko, D.A.; Dunne, J.; Fryer, M.; Nicholls, P.; Reeder, B.J.; Wilson, M.T.; Bigotti, M.G.; Cutruzzola, F.; Cooper, C.E. Comparative study of tyrosine radicals in hemoglobin and myoglobins treated with hydrogen peroxide. Biophys. J. 2002, 83, 2845–2855. [Google Scholar] [CrossRef]
- Witting, P.K.; Douglas, D.J.; Mauk, A.G. Reaction of human myoglobin and H2O2. Involvement of a thiyl radical produced at cysteine 110. J. Biol. Chem. 2000, 275, 20391–20398. [Google Scholar] [CrossRef] [PubMed]
- Reeder, B.J.; Svistunenko, D.A.; Cooper, C.E.; Wilson, M.T. The radical and redox chemistry of myoglobin and hemoglobin: From in vitro studies to human pathology. Antioxid. Redox Signal. 2004, 6, 954–966. [Google Scholar] [PubMed]
- Erman, J.E.; Yonetani, T. A kinetic study of the endogenous reduction of the oxidized sites in the primary cytochrome c peroxidase-hydrogen peroxide compound. Biochim. Biophys. Acta 1975, 393, 350–357. [Google Scholar] [CrossRef]
- Hiner, A.N.; Martinez, J.I.; Arnao, M.B.; Acosta, M.; Turner, D.D.; Lloyd Raven, E.; Rodriguez-Lopez, J.N. Detection of a tryptophan radical in the reaction of ascorbate peroxidase with hydrogen peroxide. Eur. J. Biochem. 2001, 268, 3091–3098. [Google Scholar] [CrossRef]
- Wu, G.; Rogge, C.E.; Wang, J.S.; Kulmacz, R.J.; Palmer, G.; Tsai, A.L. Oxyferryl heme and not tyrosyl radical is the likely culprit in prostaglandin H synthase-1 peroxidase inactivation. Biochemistry 2007, 46, 534–542. [Google Scholar] [CrossRef][Green Version]
- Soulimane, T.; Buse, G. Integral Cytochrome-C-Oxidase—Preparation and Progress Towards a 3-Dimensional Crystallization. Eur. J. Biochem. 1995, 227, 588–595. [Google Scholar] [CrossRef]
- Liao, G.L.; Palmer, G. The reduced minus oxidized difference spectra of cytochromes a and a(3). Bba-Bioenergetics 1996, 1274, 109–111. [Google Scholar] [CrossRef]
- Wikstrom, M.; Morgan, J.E. The dioxygen cycle. Spectral, kinetic, and thermodynamic characteristics of ferryl and peroxy intermediates observed by reversal of the cytochrome oxidase reaction. J. Biol. Chem. 1992, 267, 10266–10273. [Google Scholar] [CrossRef]
- Bergmayer, H.U.; Gawehn, K.; Grassl, M. Methoden der Enzymatischen Analyze. Bergmayer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1970; p. 440. [Google Scholar]
- Appleby, C.A.; Morton, R.K. Lactic dehydrogenase and cytochrome b2 of baker’s yeast; purification and crystallization. Biochem. J. 1959, 71, 492–499. [Google Scholar] [CrossRef] [PubMed]



| Reaction | τ (s) |
|---|---|
| rapid formation of F• | 26 |
| formation of PM | 22 |
| loss of PM | 330 |
| slow formation of F• | 330 |
| formation of the radical | 200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sztachova, T.; Tomkova, A.; Cizmar, E.; Jancura, D.; Fabian, M. Radical in the Peroxide-Produced F-Type Ferryl Form of Bovine Cytochrome c Oxidase. Int. J. Mol. Sci. 2022, 23, 12580. https://doi.org/10.3390/ijms232012580
Sztachova T, Tomkova A, Cizmar E, Jancura D, Fabian M. Radical in the Peroxide-Produced F-Type Ferryl Form of Bovine Cytochrome c Oxidase. International Journal of Molecular Sciences. 2022; 23(20):12580. https://doi.org/10.3390/ijms232012580
Chicago/Turabian StyleSztachova, Tereza, Adriana Tomkova, Erik Cizmar, Daniel Jancura, and Marian Fabian. 2022. "Radical in the Peroxide-Produced F-Type Ferryl Form of Bovine Cytochrome c Oxidase" International Journal of Molecular Sciences 23, no. 20: 12580. https://doi.org/10.3390/ijms232012580
APA StyleSztachova, T., Tomkova, A., Cizmar, E., Jancura, D., & Fabian, M. (2022). Radical in the Peroxide-Produced F-Type Ferryl Form of Bovine Cytochrome c Oxidase. International Journal of Molecular Sciences, 23(20), 12580. https://doi.org/10.3390/ijms232012580







