Asprosin in the Paraventricular Nucleus Induces Sympathetic Activation and Pressor Responses via cAMP-Dependent ROS Production
Abstract
1. Introduction
2. Results
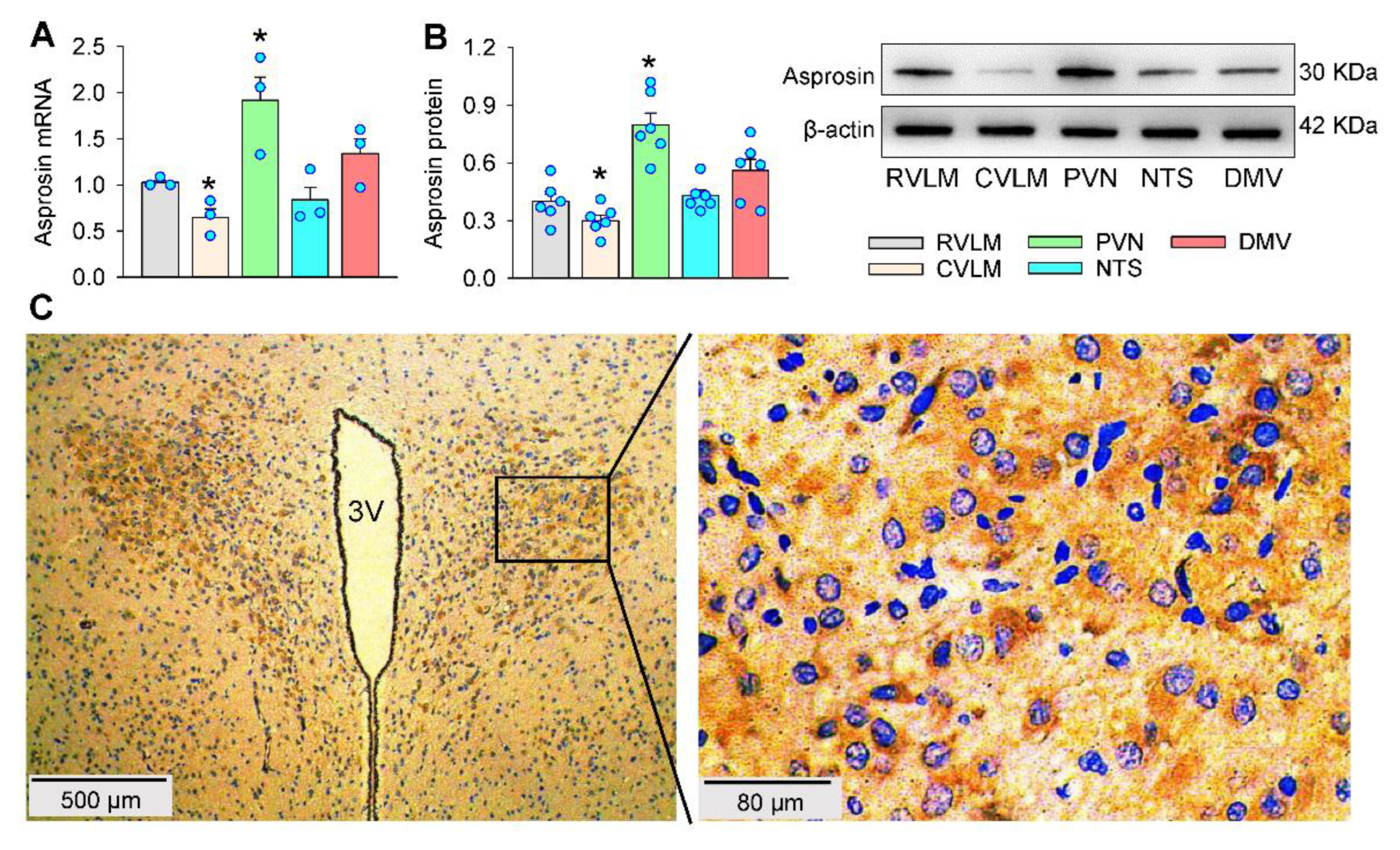
2.1. Asprosin Expression
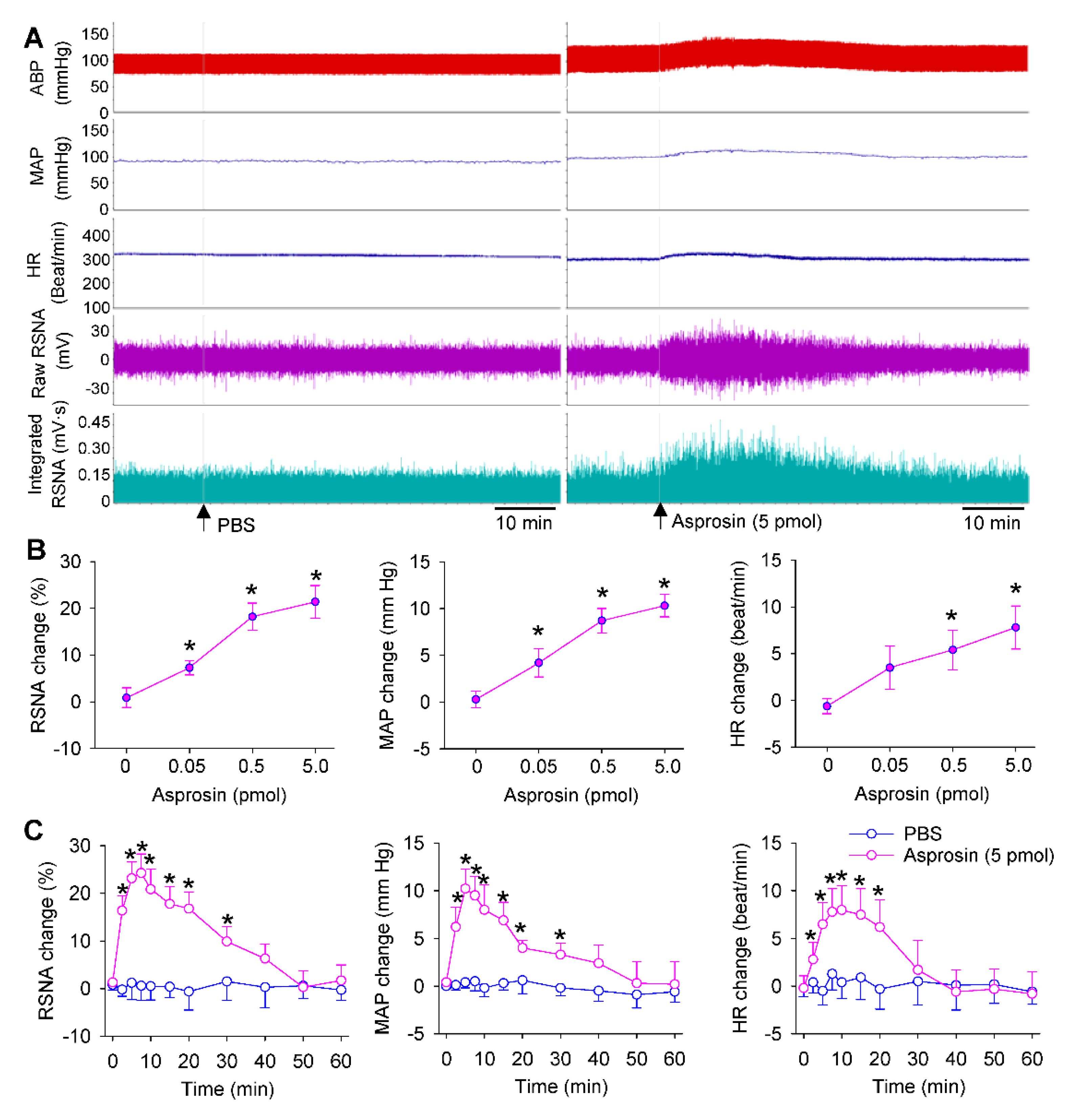
2.2. Dose Effects and Time Effects of Asprosin in PVN
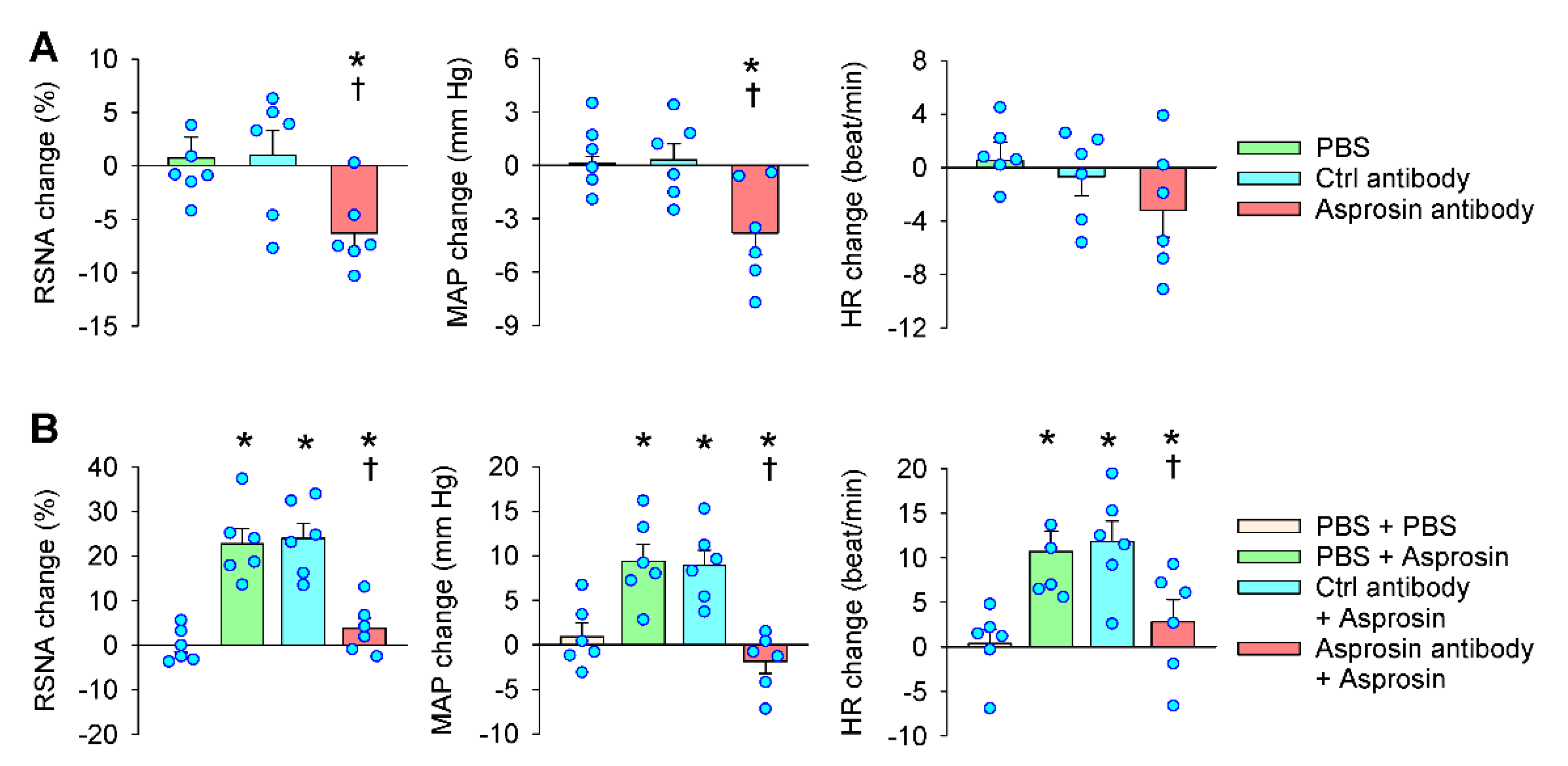
2.3. Effects of Asprosin Antibody in the PVN
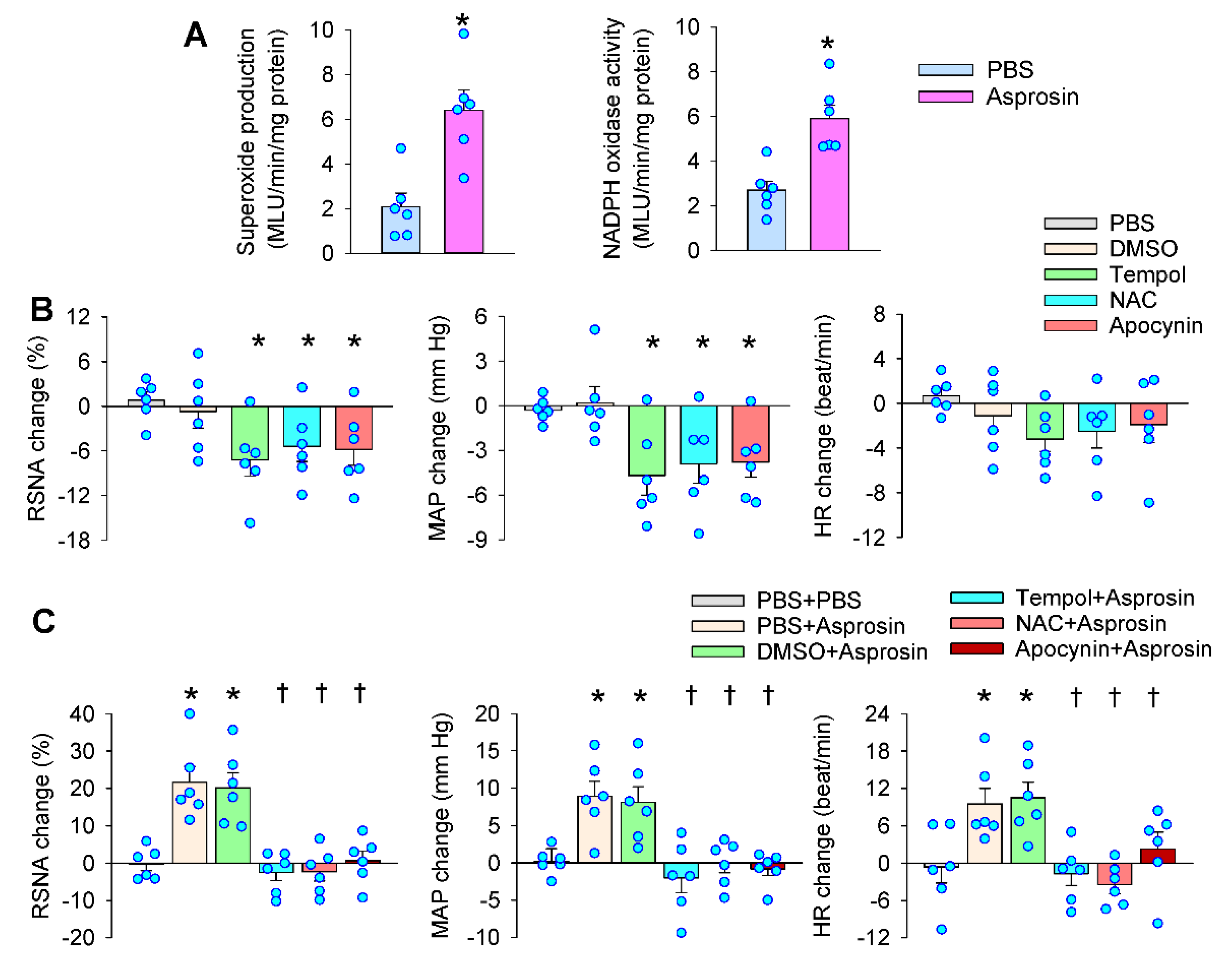
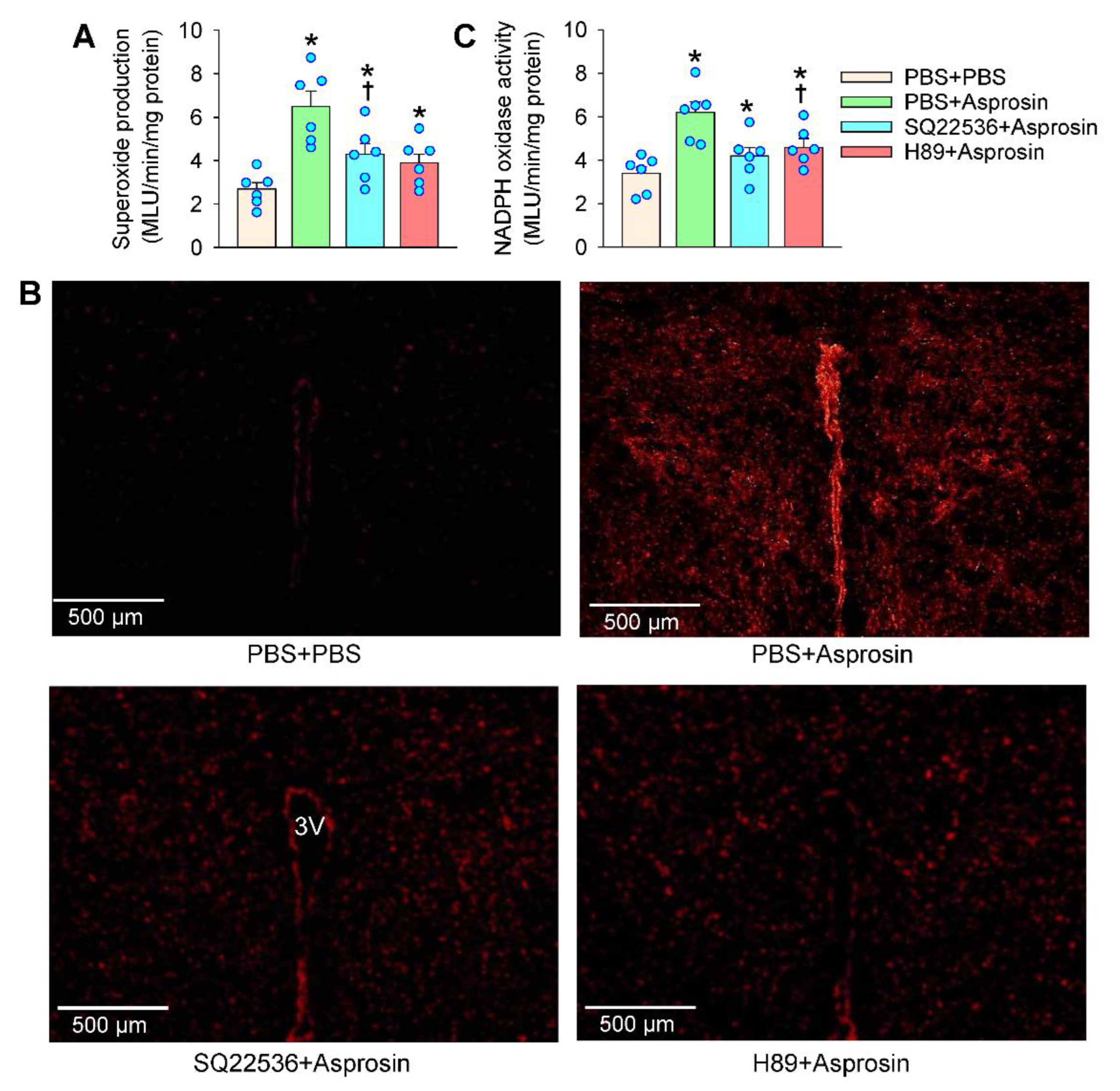
2.4. Roles of Superoxide Production Mediated the Effects of Asprosin
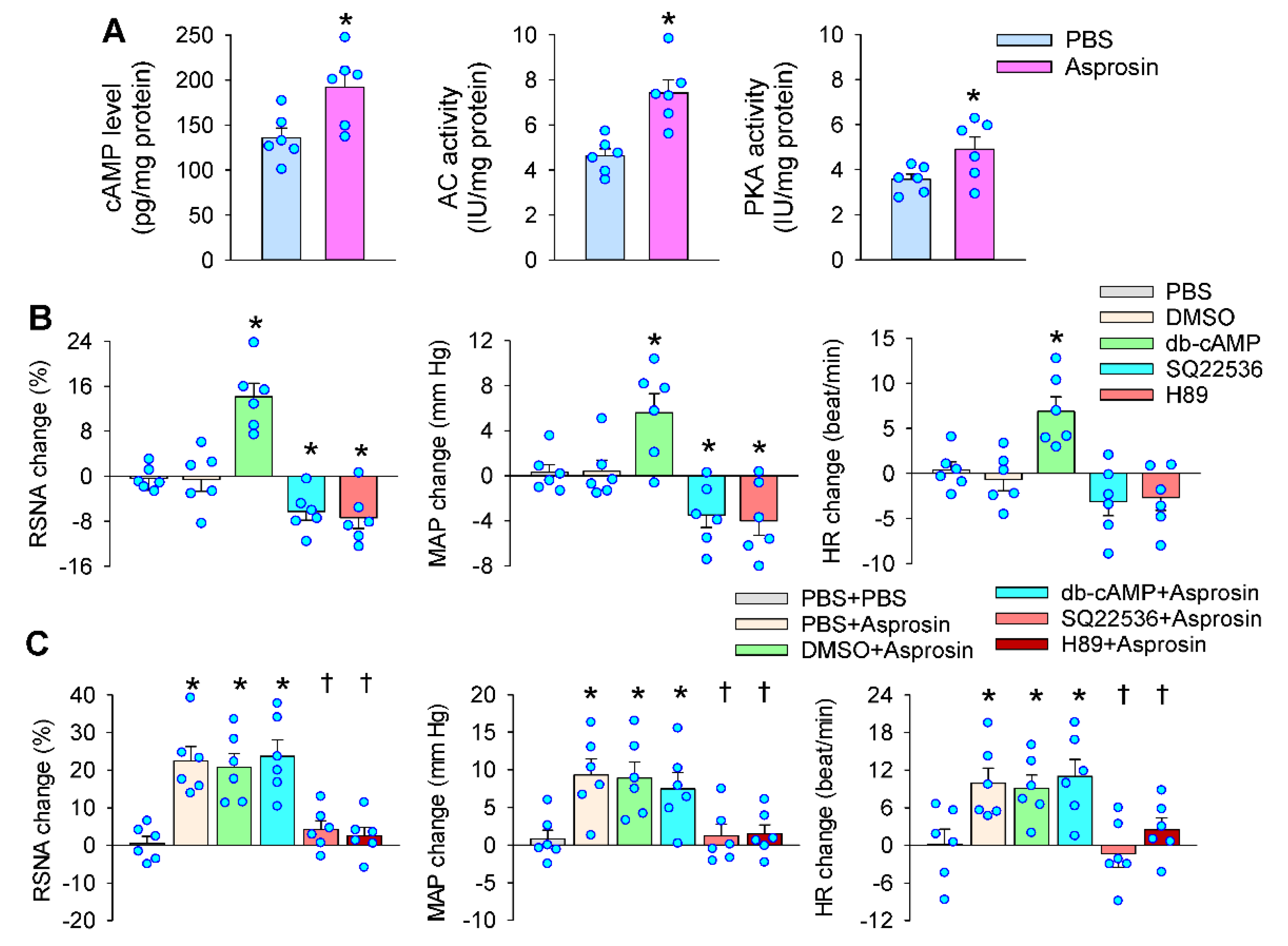
2.5. Roles of cAMP–PKA Signaling in Mediating the Effects of Asprosin in the PVN
2.6. cAMP–PKA Signaling Mediated the Effects of Asprosin on Superoxide Production
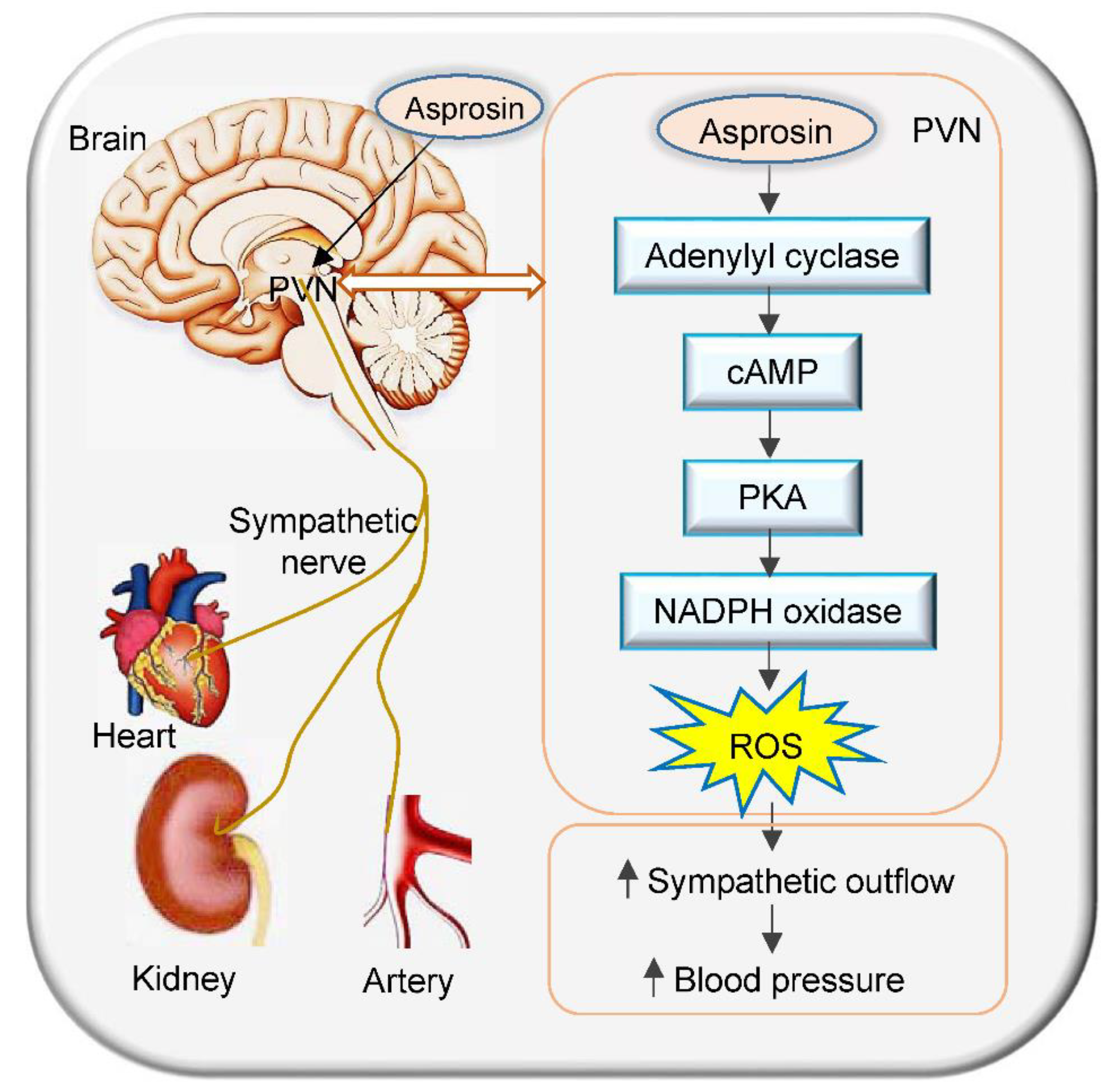
3. Discussion
4. Materials and Methods
4.1. Animals and General Procedures
4.2. RSNA Recording
4.3. PVN Microinjection
4.4. In Situ Detection of the Superoxide Level in the PVN
4.5. Measurements of Superoxide Production and NADPH Oxidase Activity
4.6. Quantitative PCR
4.7. Western Blot
4.8. Measurements with Commercial Kits
4.9. Immunohistochemistry
4.10. Chemicals
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grassi, G.; Ram, V.S. Evidence for a critical role of the sympathetic nervous system in hypertension. J. Am. Soc. Hypertens. 2016, 10, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Hering, D.; Schlaich, M. The Role of Central Nervous System Mechanisms in Resistant Hypertension. Curr. Hypertens. Rep. 2015, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.R.; Jang, H.S.; Kim, J.; Padanilam, B.J. Renal Sympathetic Nerve-Derived Signaling in Acute and Chronic kidney Diseases. Int. J. Mol. Sci. 2020, 21, 1647. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; Borges, J.I.; Cora, N.; Sizova, A. Sympatholytic Mechanisms for the Beneficial Cardiovascular Effects of SGLT2 Inhibitors: A Research Hypothesis for Dapagliflozin’s Effects in the Adrenal Gland. Int. J. Mol. Sci. 2021, 22, 7684. [Google Scholar] [CrossRef]
- DeLalio, L.J.; Sved, A.F.; Stocker, S.D. Sympathetic Nervous System Contributions to Hypertension: Updates and Therapeutic Relevance. Can. J. Cardiol. 2020, 36, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Dampney, R.A.; Michelini, L.C.; Li, D.P.; Pan, H.L. Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1200–H1214. [Google Scholar] [CrossRef]
- Chen, W.W.; Xiong, X.Q.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. Cardiac sympathetic afferent reflex and its implications for sympathetic activation in chronic heart failure and hypertension. Acta. Physiol. 2015, 213, 778–794. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Zhang, Y.; Li, X.Z.; Du, X.L.; Gao, Y.; Wang, J.X.; Wang, X.L.; Chen, Q.; Li, Y.H.; Zhu, G.Q.; et al. Impact of Selective Renal Afferent Denervation on Oxidative Stress and Vascular Remodeling in Spontaneously Hypertensive Rats. Antioxidants 2022, 11, 1003. [Google Scholar] [CrossRef] [PubMed]
- Romere, C.; Duerrschmid, C.; Bournat, J.; Constable, P.; Jain, M.; Xia, F.; Saha, P.K.; Del, S.M.; Zhu, B.; York, B.; et al. Asprosin, a fasting-induced glucogenic protein hormone. Cell 2016, 165, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Li, W.; Zhu, Y.; Yu, B.; Wu, J. Asprosin: A Novel Player in Metabolic Diseases. Front. Endocrinol. 2020, 11, 64. [Google Scholar] [CrossRef]
- Hekim, M.G.; Kelestemur, M.M.; Bulmus, F.G.; Bilgin, B.; Bulut, F.; Gokdere, E.; Ozdede, M.R.; Kelestimur, H.; Canpolat, S.; Ozcan, M. Asprosin, a novel glucogenic adipokine: A potential therapeutic implication in diabetes mellitus. Arch. Physiol. Biochem. 2021, 1–7, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Kang, Y.R.; Xiao, Y.F. Increased asprosin is associated with non-alcoholic fatty liver disease in children with obesity. World J. Pediatr. 2021, 17, 394–399. [Google Scholar] [CrossRef]
- Maylem, E.R.S.; Spicer, L.J.; Batalha, I.; Schutz, L.F. Discovery of a possible role of asprosin in ovarian follicular function. J. Mol. Endocrinol. 2021, 66, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Guven, C.; Kafadar, H. Evaluation of Plasma Asprosin Concentration in Patients with Coronary Artery Disease. Braz. J. Cardiovasc. Surg. 2022, 37, 493–500. [Google Scholar] [CrossRef]
- Duerrschmid, C.; He, Y.; Wang, C.; Li, C.; Bournat, J.C.; Romere, C.; Saha, P.K.; Lee, M.E.; Phillips, K.J.; Jain, M.; et al. Asprosin is a centrally acting orexigenic hormone. Nat. Med. 2017, 23, 1444–1453. [Google Scholar] [CrossRef]
- Kocaman, N.; Kuloglu, T. Expression of asprosin in rat hepatic, renal, heart, gastric, testicular and brain tissues and its changes in a streptozotocin-induced diabetes mellitus model. Tissue Cell 2020, 66, 101397. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, H.J.; Li, P.; Gao, Q.; Zhou, Y.B.; Zhang, F.; Gao, X.Y.; Zhu, G.Q. Angiotensin-(1-7) in paraventricular nucleus modulates sympathetic activity and cardiac sympathetic afferent reflex in renovascular hypertensive rats. PLoS ONE 2012, 7, e48966. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cui, B.P.; Zhang, L.L.; Sun, H.J.; Liu, T.Y.; Zhu, G.Q. Melanocortin 3/4 receptors in paraventricular nucleus modulates sympathetic outflow and blood pressure. Exp. Physiol. 2013, 98, 435–443. [Google Scholar] [CrossRef]
- Shen, Y.H.; Chen, X.R.; Yang, C.X.; Liu, B.X.; Li, P. Alamandine injected into the paraventricular nucleus increases blood pressure and sympathetic activation in spontaneously hypertensive rats. Peptides 2018, 103, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, H.; Shen, J.; Wang, N.; Zhang, Y. Different roles of b-arrestin and the PKA pathway in mitochondrial ROS production induced by acute b-adrenergic receptor stimulation in neonatal mouse cardiomyocytes. Biochem. Biophys. Res. Commun. 2017, 489, 393–398. [Google Scholar] [CrossRef]
- Andersson, D.C.; Fauconnier, J.; Yamada, T.; Lacampagne, A.; Zhang, S.J.; Katz, A.; Westerblad, H. Mitochondrial production of reactive oxygen species contributes to the b-adrenergic stimulation of mouse cardiomycytes. J. Physiol. 2011, 589, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Yu, X.J.; Bai, J.; Su, Q.; Wang, M.L.; Huo, C.J.; Xia, W.J.; Yi, Q.Y.; Liu, K.L.; Fu, L.Y.; et al. Silencing salusin beta ameliorates heart failure in aged spontaneously hypertensive rats by ROS-relative MAPK/NF-kappaB pathways in the paraventricular nucleus. Int. J. Cardiol. 2019, 280, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.C.; Flor, A.F.; Franca-Silva, M.S.; Balarini, C.M.; Braga, V.A. Reactive oxygen species in the paraventricular nucleus of the hypothalamus alter sympathetic activity during metabolic syndrome. Front. Physiol. 2015, 6, 384. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, Y.; Wang, H.J.; Gao, X.Y.; Wang, W.; Zhu, G.Q. Reactive oxygen species in paraventricular nucleus modulates cardiac sympathetic afferent reflex in rats. Brain Res. 2005, 1058, 82–90. [Google Scholar] [CrossRef]
- Chen, W.W.; Sun, H.J.; Zhang, F.; Zhou, Y.B.; Xiong, X.Q.; Wang, J.J.; Zhu, G.Q. Salusin-b in paraventricular nucleus increases blood pressure and sympathetic outflow via vasopressin in hypertensive rats. Cardiovasc. Res. 2013, 98, 344–351. [Google Scholar] [CrossRef]
- Zheng, F.; Ye, C.; Wan, G.W.; Zhou, B.; Tong, Y.; Lei, J.Z.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. Interleukin-1b in hypothalamic paraventricular nucleus mediates excitatory renal reflex. Pflugers. Arch. 2020, 472, 1577–1586. [Google Scholar] [CrossRef]
- Li, P.; Jie, Y.; YuGen, S.; Yu, W.; Yan, S. High mobility group box-1 in hypothalamic paraventricular nuclei attenuates sympathetic tone in rats at post-myocardial infarction. Cardiol. J. 2019, 26, 555–563. [Google Scholar] [CrossRef]
- Tai, P.; Ascoli, M. Reactive oxygen species (ROS) play a critical role in the cAMP-induced activation of Ras and the phosphorylation of ERK1/2 in Leydig cells. Mol. Endocrinol. 2011, 25, 885–893. [Google Scholar] [CrossRef]
- Badoer, E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin. Exp. Pharmacol. Physiol. 2001, 28, 95–99. [Google Scholar] [CrossRef]
- Palasz, A.; Della, V.A.; Saganiak, K.; Worthington, J.J. Neuropeptides of the human magnocellular hypothalamus. J. Chem. Neuroanat. 2021, 117, 102003. [Google Scholar] [CrossRef]
- Ye, C.; Zheng, F.; Xu, T.; Wu, N.; Tong, Y.; Xiong, X.Q.; Zhou, Y.B.; Wang, J.J.; Chen, Q.; Li, Y.H.; et al. Norepinephrine acting on adventitial fibroblasts stimulates vascular smooth muscle cell proliferation via promoting small extracellular vesicle release. Theranostics 2022, 12, 4718–4733. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.S.; Tong, Y.; Qiu, Y.; Ye, C.; Wu, N.; Xiong, X.Q.; Wang, J.J.; Han, Y.; Zhou, Y.B.; Zhang, F.; et al. MiR155-5p in adventitial fibroblasts-derived extracellular vesicles inhibits vascular smooth muscle cell proliferation via suppressing angiotensin-converting enzyme expression. J. Extracell. Vesicles. 2020, 9, 1698795. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Zheng, F.; Nan, W.; Zhu, G.Q.; Li, X.Z. Extracellular vesicles in vascular remodeling. Acta. Pharmacol. Sin. 2022, 43, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zheng, F.; Ye, C.; Chen, A.D.; Wang, J.J.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. Angiotensin type 1 receptors and superoxide anion production in hypothalamic paraventricular nucleus contribute to capsaicin-induced excitatory renal reflex and sympathetic activation. Neurosci. Bull. 2020, 36, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Zhang, F.; Zhang, L.L.; Gao, J.; Zhou, Y.B.; Han, Y.; Zhu, G.Q. SOD1 gene transfer into paraventricular nucleus attenuates hypertension and sympathetic activity in spontaneously hypertensive rats. Pflugers. Arch. 2013, 465, 261–270. [Google Scholar] [CrossRef]
- Han, Y.; Shi, Z.; Zhang, F.; Yu, Y.; Zhong, M.K.; Gao, X.Y.; Wang, W.; Zhu, G.Q. Reactive oxygen species in the paraventricular nucleus mediate the cardiac sympathetic afferent reflex in chronic heart failure rats. Eur. J. Heart Fail. 2007, 9, 967–973. [Google Scholar] [CrossRef]
- Lu, Q.B.; Sun, J.; Kang, Y.; Sun, H.J.; Wang, H.S.; Wang, Y.; Zhu, G.Q.; Zhou, Y.B. Superoxide Anions and NO in the Paraventricular Nucleus Modulate the Cardiac Sympathetic Afferent Reflex in Obese Rats. Int. J. Mol. Sci. 2017, 19, 59. [Google Scholar] [CrossRef]
- Patel, K.P.; Mayhan, W.G.; Bidasee, K.R.; Zheng, H. Enhanced angiotensin II-mediated central sympathoexcitation in streptozotocin-induced diabetes: Role of superoxide anion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R311–R320. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I. Asprosin-a fasting-induced, glucogenic, and orexigenic adipokine as a new promising player. Will it be a new factor in the treatment of obesity, diabetes, or infertility? A review of the literature. Nutrients 2021, 13, 620. [Google Scholar] [CrossRef]
- Wang, R.; Lin, P.; Sun, H.; Hu, W. Increased serum asprosin is correlated with diabetic nephropathy. Diabetol. Metab. Syndr. 2021, 13, 51. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, Y.; Zhu, L.; Zhang, B.; Feng, P.; Gao, E.; Xu, C.; Wang, X.; Yi, W.; Sun, Y. Asprosin improves the survival of mesenchymal stromal cells in myocardial infarction by inhibiting apoptosis via the activated ERK1/2-SOD2 pathway. Life Sci. 2019, 231, 116554. [Google Scholar] [CrossRef]
- Li, E.; Shan, H.; Chen, L.; Long, A.; Zhang, Y.; Liu, Y.; Jia, L.; Wei, F.; Han, J.; Li, T.; et al. OLFR734 Mediates Glucose Metabolism as a Receptor of Asprosin. Cell Metab. 2019, 30, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Long, A.; Wang, Y. The Asprosin-OLFR734 hormonal signaling axis modulates male fertility. Cell Discov. 2019, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Yun, S.; Jeong, J.H.; Jung, T.W. Asprosin impairs insulin secretion in response to glucose and viability through TLR4/JNK-mediated inflammation. Mol. Cell Endocrinol. 2019, 486, 96–104. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-L.; Wang, J.-X.; Chen, J.-L.; Hao, W.-Y.; Xu, W.-Z.; Xu, Z.-Q.; Jiang, Y.-T.; Luo, P.-Q.; Chen, Q.; Li, Y.-H.; et al. Asprosin in the Paraventricular Nucleus Induces Sympathetic Activation and Pressor Responses via cAMP-Dependent ROS Production. Int. J. Mol. Sci. 2022, 23, 12595. https://doi.org/10.3390/ijms232012595
Wang X-L, Wang J-X, Chen J-L, Hao W-Y, Xu W-Z, Xu Z-Q, Jiang Y-T, Luo P-Q, Chen Q, Li Y-H, et al. Asprosin in the Paraventricular Nucleus Induces Sympathetic Activation and Pressor Responses via cAMP-Dependent ROS Production. International Journal of Molecular Sciences. 2022; 23(20):12595. https://doi.org/10.3390/ijms232012595
Chicago/Turabian StyleWang, Xiao-Li, Jing-Xiao Wang, Jun-Liu Chen, Wen-Yuan Hao, Wen-Zhou Xu, Zhi-Qin Xu, Yu-Tong Jiang, Pei-Qi Luo, Qi Chen, Yue-Hua Li, and et al. 2022. "Asprosin in the Paraventricular Nucleus Induces Sympathetic Activation and Pressor Responses via cAMP-Dependent ROS Production" International Journal of Molecular Sciences 23, no. 20: 12595. https://doi.org/10.3390/ijms232012595
APA StyleWang, X.-L., Wang, J.-X., Chen, J.-L., Hao, W.-Y., Xu, W.-Z., Xu, Z.-Q., Jiang, Y.-T., Luo, P.-Q., Chen, Q., Li, Y.-H., Zhu, G.-Q., & Li, X.-Z. (2022). Asprosin in the Paraventricular Nucleus Induces Sympathetic Activation and Pressor Responses via cAMP-Dependent ROS Production. International Journal of Molecular Sciences, 23(20), 12595. https://doi.org/10.3390/ijms232012595






