Cellular Stress Assay in Peripheral Blood Mononuclear Cells: Factors Influencing Its Results
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Participants According to the Potential Factors Considered
2.2. Yields of PBMCs in RobosepTM-S and OptiPrep® Isolation Methods
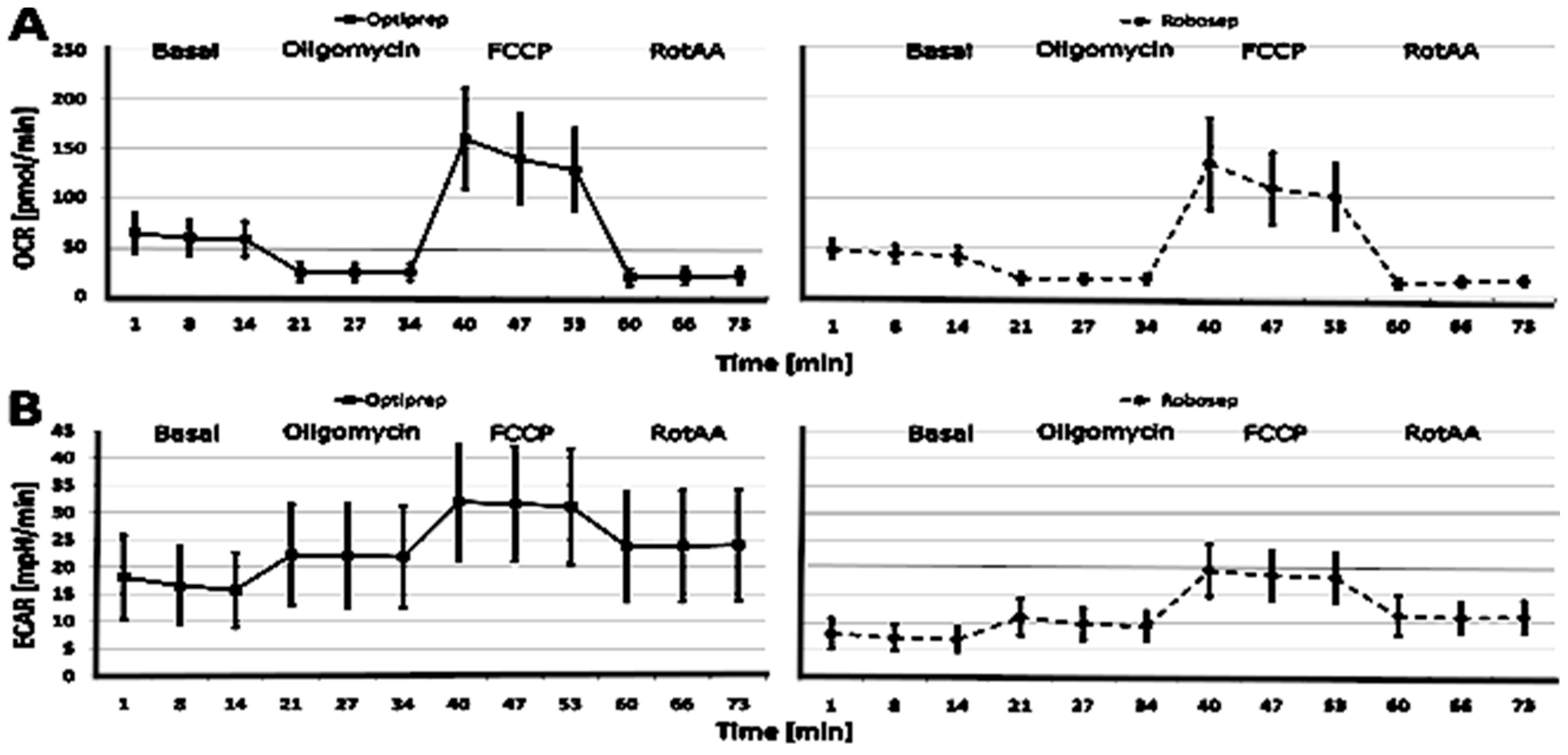
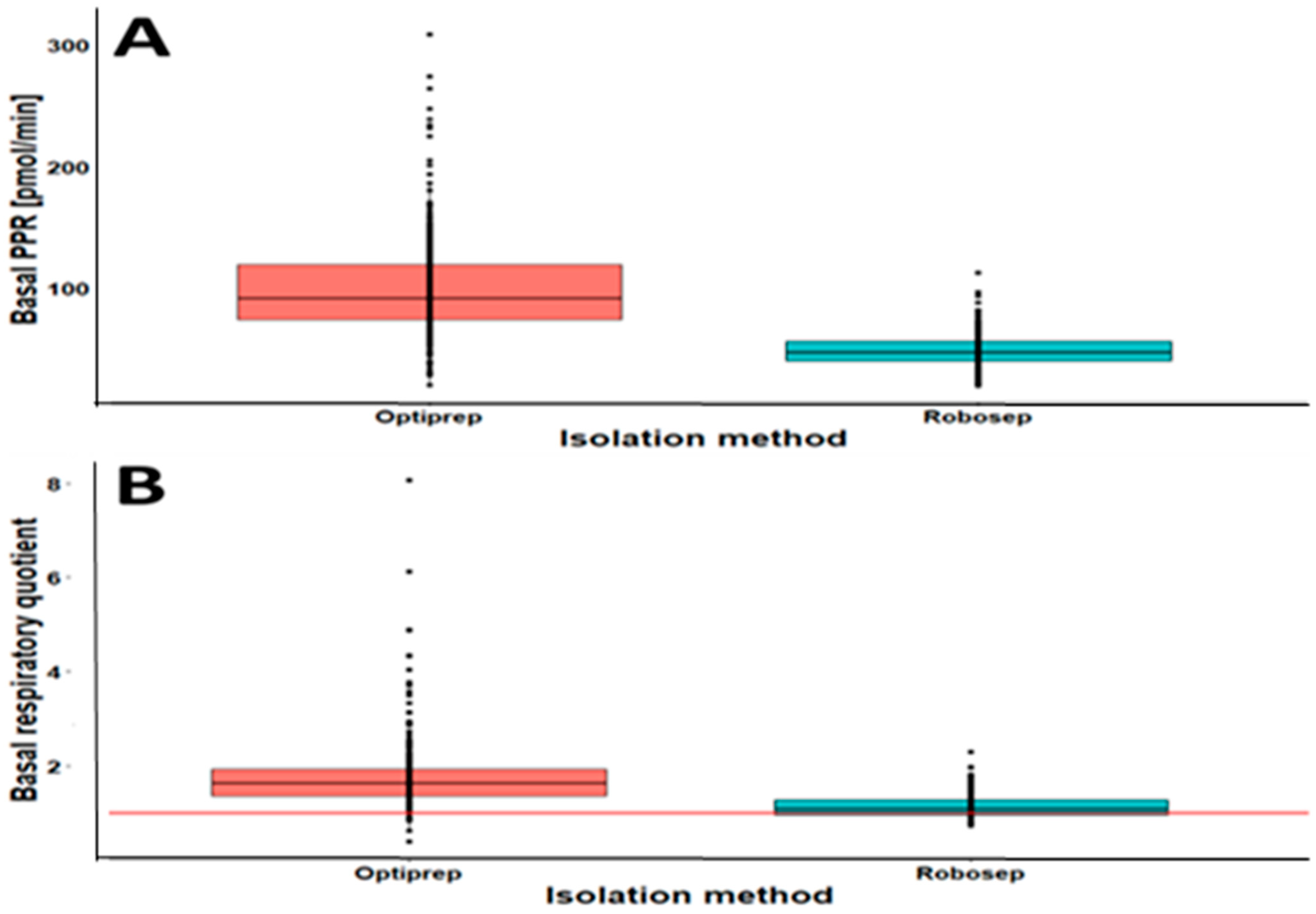
2.3. The Results of CSA Parameters in RobosepTM-S- and OptiPrep®-Isolated PBMCs
2.4. Younger Individuals Show Lower Oxygen Consumption and Lower Cellular Acidification
2.5. Oxygen Consumption Rate and Extracellular Acidification Rate Show Seasonal Variation
2.6. Males Show Higher Oxygen Consumption and Extracellular Acidification Rates Than Females
3. Discussion
4. Materials and Methods
4.1. Study Design, Period and Settings
4.2. Study Participants
4.3. Blood Sample Collection
4.4. PBMC Isolation by OptiPrep® Method
4.5. PBMC Isolation by RobosepTM-S Method
4.6. Cellular Stress Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blake, R.; Trounce, I.A. Mitochondrial Dysfunction and Complications Associated with Diabetes. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Ilkun, O.; Boudina, S. Cardiac Dysfunction and Oxidative Stress in the Metabolic Syndrome: An Update on Antioxidant Therapies. Curr. Pharm. Des. 2013, 19, 4806–4817. [Google Scholar] [CrossRef]
- Sundström, J.; Risérus, U.; Byberg, L.; Zethelius, B.; Lithell, H.; Lind, L. Clinical Value of the Metabolic Syndrome for Long Term Prediction of Total and Cardiovascular Mortality: Prospective, Population Based Cohort Study. BMJ 2006, 332, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G.; Benavides, G.A.; Lancaster, J.R.; Ballinger, S.; Dell’Italia, L.; Zhang, J.; Darley-Usmar, V.M. Integration of Cellular Bioenergetics with Mitochondrial Quality Control and Autophagy. Biol. Chem. 2012, 393, 1485–1512. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Darley-Usmar, V.; Zhang, J. Autophagy as an Essential Cellular Antioxidant Pathway in Neurodegenerative Disease. Redox Biol. 2014, 2, 82–90. [Google Scholar] [CrossRef]
- Apostolova, N.; Blas-Garcia, A.; V. Esplugues, J. Mitochondria Sentencing about Cellular Life and Death: A Matter of Oxidative Stress. Curr. Pharm. Des. 2011, 17, 4047–4060. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative Stress, Insulin Signaling, and Diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Kramer, P.A.; Chacko, B.K.; Ravi, S.; Johnson, M.S.; Mitchell, T.; Barnes, S.; Arabshahi, A.; Dell’Italia, L.J.; George, D.J.; Steele, C.; et al. Hemoglobin-Associated Oxidative Stress in the Pericardial Compartment of Postoperative Cardiac Surgery Patients. Lab. Investig. 2015, 95, 132–141. [Google Scholar] [CrossRef]
- Srinivasan, S.; Yeh, M.; Danziger, E.C.; Hatley, M.E.; Riggan, A.E.; Leitinger, N.; Berliner, J.A.; Hedrick, C.C. Glucose Regulates Monocyte Adhesion through Endothelial Production of Interleukin-8. Circ. Res. 2003, 92, 371–377. [Google Scholar] [CrossRef]
- Yamagishi, S.; Edelstein, D.; Du, X.; Brownlee, M. Hyperglycemia Potentiates Collagen-Induced Platelet Activation through Mitochondrial Superoxide Overproduction. Diabetes 2001, 50, 1491–1494. [Google Scholar] [CrossRef]
- Widlansky, M.E.; Wang, J.; Shenouda, S.M.; Hagen, T.M.; Smith, A.R.; Kizhakekuttu, T.J.; Kluge, M.A.; Weihrauch, D.; Gutterman, D.D.; Vita, J.A. Altered Mitochondrial Membrane Potential, Mass, and Morphology in the Mononuclear Cells of Humans with Type 2 Diabetes. Transl. Res. 2010, 156, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.; Hanczko, R.; Doherty, E. Assessment of Mitochondrial Dysfunction in Lymphocytes of Patients with Systemic Lupus Erythematosus. In Autoimmunity; Humana Press: Totowa, NJ, USA, 2012; pp. 61–89. [Google Scholar]
- Onyango, I.; Khan, S.; Miller, B.; Swerdlow, R.; Trimmer, P.; Bennett, J. Mitochondrial Genomic Contribution to Mitochondrial Dysfunction in Alzheimer’s Disease. J. Alzheimers Dis. 2006, 9, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; de Leon, M.; Murray, J.; E, L.; Lu, J.; Javier, E.; McHugh, P.; Swerdlow, R.H. Reduced Mitochondria Cytochrome Oxidase Activity in Adult Children of Mothers with Alzheimer’s Disease. J. Alzheimers Dis. 2011, 27, 483–490. [Google Scholar] [CrossRef]
- Caimari, A.; Oliver, P.; Keijer, J.; Palou, A. Peripheral Blood Mononuclear Cells as a Model to Study the Response of Energy Homeostasis-Related Genes to Acute Changes in Feeding Conditions. OMICS A J. Integr. Biol. 2010, 14, 129–141. [Google Scholar] [CrossRef]
- Shikuma, C.M.; Gerschenson, M.; Chow, D.; Libutti, D.E.; Willis, J.H.; Murray, J.; Capaldi, R.A.; Marusich, M. Mitochondrial Oxidative Phosphorylation Protein Levels in Peripheral Blood Mononuclear Cells Correlate with Levels in Subcutaneous Adipose Tissue within Samples Differing by HIV and Lipoatrophy Status. AIDS Res. Hum. Retroviruses 2008, 24, 1255–1262. [Google Scholar] [CrossRef]
- Sternfeld, T.; Tischleder, A.; Schuster, M.; Bogner, J. Mitochondrial Membrane Potential and Apoptosis of Blood Mononuclear Cells in Untreated HIV-1 Infected Patients. HIV Med. 2009, 10, 512–519. [Google Scholar] [CrossRef]
- Japiassú, A.M.; Santiago, A.P.S.A.; D’Avila, J.d.C.P.; Garcia-Souza, L.F.; Galina, A.; Castro Faria-Neto, H.C.; Bozza, F.A.; Oliveira, M.F. Bioenergetic Failure of Human Peripheral Blood Monocytes in Patients with Septic Shock Is Mediated by Reduced F1Fo Adenosine-5’-Triphosphate Synthase Activity*. Crit. Care Med. 2011, 39, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Chacko, B.K.; Kramer, P.A.; Ravi, S.; Johnson, M.S.; Hardy, R.W.; Ballinger, S.W.; Darley-Usmar, V.M. Methods for Defining Distinct Bioenergetic Profiles in Platelets, Lymphocytes, Monocytes, and Neutrophils, and the Oxidative Burst from Human Blood. Lab. Investig. 2013, 93, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Hedges, C.P.; Woodhead, J.S.T.; Wang, H.W.; Mitchell, C.J.; Cameron-Smith, D.; Hickey, A.J.R.; Merry, T.L. Peripheral Blood Mononuclear Cells Do Not Reflect Skeletal Muscle Mitochondrial Function or Adaptation to High-Intensity Interval Training in Healthy Young Men. J. Appl. Physiol. 2019, 126, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, S.A.; Brand, M.D. Measurement and Analysis of Extracellular Acid Production to Determine Glycolytic Rate. J. Vis. Exp. 2015, 12, e53464. [Google Scholar] [CrossRef]
- Dranka, B.P.; Benavides, G.A.; Diers, A.R.; Giordano, S.; Zelickson, B.R.; Reily, C.; Zou, L.; Chatham, J.C.; Hill, B.G.; Zhang, J.; et al. Assessing Bioenergetic Function in Response to Oxidative Stress by Metabolic Profiling. Free Radic. Biol. Med. 2011, 51, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Sansbury, B.E.; Jones, S.P.; Riggs, D.W.; Darley-Usmar, V.M.; Hill, B.G. Bioenergetic Function in Cardiovascular Cells: The Importance of the Reserve Capacity and Its Biological Regulation. Chem. Biol. Interact. 2011, 191, 288–295. [Google Scholar] [CrossRef]
- Suomalainen, A.; Battersby, B.J. Mitochondrial Diseases: The Contribution of Organelle Stress Responses to Pathology. Nat. Rev. Mol. Cell Biol. 2018, 19, 77–92. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondrial DNA Mutations in Disease and Aging. Environ. Mol. Mutagen. 2010, 51, 440–450. [Google Scholar] [CrossRef]
- Mookerjee, S.A.; Gerencser, A.A.; Nicholls, D.G.; Brand, M.D. Quantifying Intracellular Rates of Glycolytic and Oxidative ATP Production and Consumption Using Extracellular Flux Measurements. J. Biol. Chem. 2018, 293, 12649–12652. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Bharadwaj, M.S.; Van Horn, C.G.; Marsh, A.P.; Nicklas, B.J.; Molina, A.J.A. Blood-Cell Bioenergetics Are Associated with Physical Function and Inflammation in Overweight/Obese Older Adults. Exp. Gerontol. 2015, 70, 84–91. [Google Scholar] [CrossRef]
- Ravi, S.; Mitchell, T.; Kramer, P.A.; Chacko, B.; Darley-Usmar, V.M. Mitochondria in Monocytes and Macrophages-Implications for Translational and Basic Research. Int. J. Biochem. Cell Biol. 2014, 53, 202–207. [Google Scholar] [CrossRef]
- Chacko, B.K.; Kramer, P.A.; Ravi, S.; Benavides, G.A.; Mitchell, T.; Dranka, B.P.; Ferrick, D.; Singal, A.K.; Ballinger, S.W.; Bailey, S.M.; et al. The Bioenergetic Health Index: A New Concept in Mitochondrial Translational Research. Clin. Sci. 2014, 127, 367–373. [Google Scholar] [CrossRef]
- Betsou, F.; Gaignaux, A.; Ammerlaan, W.; Norris, P.J.; Stone, M. Biospecimen Science of Blood for Peripheral Blood Mononuclear Cell (PBMC) Functional Applications. Curr. Pathobiol. Rep. 2019, 7, 17–27. [Google Scholar] [CrossRef]
- Payne, B.A.I.; Chinnery, P.F. Mitochondrial Dysfunction in Aging: Much Progress but Many Unresolved Questions. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 1347–1353. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Fares, A. Factors Influencing the Seasonal Patterns of Infectious Diseases. Int. J. Prev. Med. 2013, 4, 128–132. [Google Scholar] [PubMed]
- Dhahbi, W.; Sellami, M.; Chaouachi, A.; Padulo, J.; Milic, M.; Mekki, I.; Chamari, K. Seasonal Weather Conditions Affect Training Program Efficiency and Physical Performance among Special Forces Trainees: A Long-Term Follow-up Study. PLoS ONE 2018, 13, e0206088. [Google Scholar] [CrossRef]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender Difference in Oxidative Stress: A New Look at the Mechanisms for Cardiovascular Diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, E.M.; Shim, H.; Zhang, Y.S.; Kim, J.K. Differences between Men and Women in Mortality and the Health Dimensions of the Morbidity Process. Clin. Chem. 2019, 65, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Widmaier, E.P.; Raff, H.; Strang, K.T. Vander’s Human Physiology: The Mechanisms of Body Function, 14th ed.; McGraw Hill: New York, NY, USA, 2016; ISBN 9781259294099. [Google Scholar]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial Proton and Electron Leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Haber, E.P.; Hirabara, S.M.; Rebelato, E.L.O.; Procopio, J.; Morgan, D.; Oliveira-Emilio, H.C.; Carpinelli, A.R.; Curi, R. Diabetes Associated Cell Stress and Dysfunction: Role of Mitochondrial and Non-Mitochondrial ROS Production and Activity. J. Physiol. 2007, 583, 9–24. [Google Scholar] [CrossRef]
- Maynard, S.; Keijzers, G.; Gram, M.; Desler, C.; Bendix, L.; Budtz-Jørgensen, E.; Molbo, D.; Croteau, D.L.; Osler, M.; Stevnsner, T.; et al. Relationships between Human Vitality and Mitochondrial Respiratory Parameters, Reactive Oxygen Species Production and DNTP Levels in Peripheral Blood Mononuclear Cells. Aging 2013, 5, 850–864. [Google Scholar] [CrossRef]
- Hintzpeter, B.; Mensink, G.B.M.; Thierfelder, W.; Müller, M.J.; Scheidt-Nave, C. Vitamin D Status and Health Correlates among German Adults. Eur. J. Clin. Nutr. 2008, 62, 1079–1089. [Google Scholar] [CrossRef]
- Gangwisch, J.E. Seasonal Variation in Metabolism: Evidence for the Role of Circannual Rhythms in Metabolism? Hypertens. Res. 2013, 36, 392–393. [Google Scholar] [CrossRef]
- Tucker, P.; Gilliland, J. The Effect of Season and Weather on Physical Activity: A Systematic Review. Public Health 2007, 121, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Calculating and Reporting Effect Sizes to Facilitate Cumulative Science: A Practical Primer for t-Tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]





| CSA Parameters | Opti-Isolated PBMCs | Robo-Isolated PBMCs | Cohen’s d * | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Shapiro–Wilk Test | Mean (SD) | 95% CI | Shapiro–Wilk Test | ||
| Basal respiration (pmol/min) | 39.84 (13.69) | 38.30–41.38 | 0.95 | 29.37 (6.76) | 28.50–30.30 | 0.99 | 0.92 |
| Basal OCR (pmol/min) | 57.56 (17.92) | 55.54–59.58 | 0.96 | 42.88 (8.54) | 41.70–44.00 | 0.99 | 0.99 |
| Non-mitochondrial respiration (pmol/min) | 17.70 (6.10) | 17.01–18.39 | 0.98 | 13.66 (4.33) | 13.10–14.20 | 0.99 | 0.74 |
| Non-mitochondrial respiration (%) | 31.16 (7.53) | 30.31–32.01 | 0.95 | 31.74 (8.56) | 30.60–32.90 | 0.97 | 0.07 |
| Coupling efficiency (%) | 87.74 (9.00) | 86.72–88.76 | 0.87 | 83.66 (10.18) | 82.30–85.00 | 0.96 | 0.43 |
| Proton leak (pmol/min) | 5.11 (3.10) | 4.76–5.46 | 0.92 | 5.14 (3.48) | 4.68–5.60 | 0.89 | 0.01 |
| Proton leak (%) | 12.99 (7.21) | 12.18–13.80 | 0.92 | 16.89 (9.47) | 15.60–18.20 | 0.95 | 0.47 |
| Maximal respiration (pmol/min) | 141.83 (47.93) | 136.42–147.24 | 0.98 | 120.75 (44.00) | 115.00–127.00 | 0.96 | 0.46 |
| Spare capacity (pmol/min) | 102.00 (38.15) | 97.70–106.30 | 0.98 | 81.22 (40.24) | 85.90–96.60 | 0.97 | 0.28 |
| Spare capacity (%) | 263.45 (79.82) | 254.45–272.45 | 0.98 | 312.60 (114.46) | 257.00–328.00 | 0.98 | 0.51 |
| Bioenergetic Health Index (BHI) | 1.64 (0.31) | 1.61–1.67 | 0.97 | 1.56 (0.36) | 1.51–1.61 | 0.96 | 0.27 |
| Basal ECAR (mpH/min) | 15.76 (6.86) | 14.99–16.53 | 0.89 | 7.01 (2.27) | 6.71–7.31 | 0.94 | 1.61 |
| ECAR oligomycin (mpH/min) | 22.61 (9.52) | 21.54–23.68 | 0.90 | 11.05 (3.31) | 10.60–11.50 | 0.98 | 1.52 |
| Maximal ECAR (FCCP) (mpH/min) | 32.25 (10.74) | 31.04–33.46 | 0.95 | 19.58 (4.85) | 18.90–20.20 | 0.98 | 1.44 |
| CSA Parameter | Opti-Isolated PBMCs | Robo-Isolated PBMCs | Cohen’s d * | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Shapiro–Wilk Test | Mean (SD) | 95% CI | Shapiro–Wilk Test | ||
| Basal PPR (pmol/min) | 100.07 (41.98) | 95.26–104.88 | 0.90 | 49.11 (1.155) | 47.01–51.21 | 0.94 | 1.72 |
| PPR Oligomycin (mpH/rnin) | 140.29 (58.08) | 131.64–146.94 | 0.91 | 72.21 (20.29) | 69.29–75.13 | 0.98 | 1.56 |
| Maximal PPR (FCCP) (mpH/min) | 157.18 (66.41) | 189.69–204.67 | 0.96 | 121.66 (29.57) | 117.40–125.92 | 0.97 | 0.69 |
| Basal RQ | 1.77 (1.76) | 1.68–1.86 | 0.71 | 1.14 (0.27) | 1.10–1.18 | 0.92 | 0.50 |
| Maximal RQ | 1.27 (0.44) | 1.22–1.32 | 0.76 | 0.92 (0.25) | 0.88–0.96 | 0.82 | 0.98 |
| PBMC Isolation Method | CSA Parameters | Age Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Young (n = 40) | Midlife (n = 72) | Mature Adulthood (n = 77) | Late Adulthood (n = 64) | Old (n = 49) | Overall (n = 302) | Cohen’s d * (Young vs. Old) | ||
| Opti-isolated PBMCs | Spare capacity (pmol/min) | 0.6 | ||||||
| Mean (SD) | 86.7 (32.0) | 96.0 (33.8) | 105 (38.0) | 111 (46.2) | 106 (33.4) | 102 (38.1) | ||
| Median (Min, Max) | 78.1 (25.5, 160) | 95.8 (4.73, 191) | 104 (25.7, 2819) | 111 (16.7, 218) | 105 (36.2, 211) | 100 (4.73, 281) | ||
| Spare capacity (%) | 0.16 | |||||||
| Mean (SD) | 251 (66.3) | 255 (76.6) | 265 (77.0) | 280 (94.2) | 263 (77.8) | 263 (79.8) | ||
| Median (Min, Max) | 248 (117, 390) | 254 (17.8, 476) | 254 (102, 449) | 268 (47.5, 529) | 260 (18.5, 411) | 255 (17.8, 529) | ||
| Maximal respiration (pmol/min) | 0.65 | |||||||
| Mean (SD) | 122 (392) | 134 (41.5) | 147 (50.3) | 152 (57.2) | 148 (403) | 142 (47.9) | ||
| Median (Min, Max) | 116 (38.4, 207) | 133 (29.9, 251) | 141 (4.10, 352) | 151 (35.4, 279) | 146 (71.7, 278) | 138 (29.9, 3529) | ||
| Robo-isolated PBMCs | CSA Parameters | Young (n = 21) | Midlife (n = 56) | Mature adulthood (n = 66) | Late adulthood (n = 48) | Old (n = 25) | Overall (n = 216) | |
| Spare capacity (pmol/min) | 1.01 | |||||||
| Mean (SD) | 70.8 (31.1) | 95.3 (39.5) | 87.0 (42.1) | 90.6 (34.4) | 111 (46.2) | 91.2 (40.2) | ||
| Median (Min, Max) | 59.2 (34.4, 160) | 87.6 (12.7.211) | 87.9 (5.66, 219) | 86.1 (37.9, 198) | 102 (27.3, 218) | 87.7 (5.66, 219) | ||
| Spare capacity (%) | 0.71 | |||||||
| Mean (SD) | 271 (72.1) | 313 (113) | 300 (116) | 327 (111) | 352 (140) | 313 (114) | ||
| Median (Min, Max) | 278 (144, 397) | 313 (37.0, 784) | 315 (35.8, 500) | 314 (141, 710) | 347 (95.1, 653) | 311 (35.8, 784) | ||
| Maximal respiration (pmol/min) | 1.01 | |||||||
| Mean (SD) | 97.0 (36.4) | 126 (43.2) | 117 (45.4) | 119 (38.0) | 143 (49.0) | 121 (44.0) | ||
| Median (Min, Max) | 87.5 (47.1, 210) | 119 (471, 252) | 114 (25.2, 263) | 117 (55.4, 237) | 142 (55.8, 266) | 118 (25.2, 266) | ||
| PBMC Isolation Method | CSA Parameters | Age Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Young (n = 40) | Midlife (n = 72) | Mature Adulthood (n = 77) | Late Adulthood (n = 64) | Old (n = 49) | Overall (n = 302) | Cohen’s d * (Young vs. Old) | ||
| Opti-isolated PBMCs | Basal ECAR (mph/min) | 0.15 | ||||||
| Mean (SD) | 14.7 (8.69) | 14.3 (4.94) | 16.4 (7.29) | 17.2 (6.99) | 15.8 (6.42) | 15.8 (6.86) | ||
| Median (Min, Max) | 13.0 (3.97, 50.3) | 13.8 (5.67, 27.6) | 14.6 (3.98, 45.5) | 15.9 (5.64, 43.21) | 14.5 (5.16, 36.2) | 14.3 (3.97, 50.3) | ||
| ECAR oligomycin (mph/min) | 0.27 | |||||||
| Mean (SD) | 20.2 (10.0) | 20.6 (7.23) | 24.5 (11.9) | 24.0 (8.45) | 22.7 (8.61) | 22.6 (9.52) | ||
| Median (Min, Max) | 16.8 (5.26, 63.2) | 19.6 (0.29, 40.6) | 22.3 (8.17, 75.1) | 22.9 (7.00, 50.6) | 20.2 (5.64, 47.2) | 20.7 (5.26, 75.1) | ||
| Maximal ECAR (FCCP) (mph/min) | 0.27 | |||||||
| Mean (SD) | 29.4 (11.2) | 29.7 (8.55) | 34.5 (12.7) | 34.3 (10.2) | 32 1 (9.68) | 32.3 (10.7) | ||
| Median (Min, Max) | 25.7 (8.68, 588) | 27.6 (11.3, 53.7) | 30.8 (132, 80.8) | 33.7 (10.5, 60.1) | 30.8 (02.7, 56.1) | 30.2 (8.68, 80.8) | ||
| Robo-isolated PBMCs | Basal ECAR (mph/min) | 0.95 | ||||||
| Mean (SD) | 5.88 (1.52) | 7.15 (2.27) | 6.84 (2.27) | 7.14 (82.29) | 7.87 (2.48) | 7.01 (2.27) | ||
| Median (Min, Max) | 6.11 (3.19. 8.89) | 6.66 (3.13,17.7) | 6.50 (2.50, 13.1) | 6.75 (3.19, 14.5) | 7.24 (4.91, 14.8) | 6.63 (2.50, 17.7) | ||
| ECAR oligomycin (mph/min) | 1.24 | |||||||
| Mean (SD) | 8.75 (2.36) | 11.0 (3.38) | 11.1 (3.21) | 11.5 (3.44) | 12.2 (3.17) | 11.0 (3.31) | ||
| Median (Min, Max) | 8.61 (4.90, 14.0) | 10.2 (5.32. 21.9) | 11.5 (3.42, 19.49) | 11.4 (4.44, 20.7) | 11.8 (6.88, 20.5) | 10.8 (3.42, 21.9) | ||
| Maximal ECAR (FCCP) (mph/min) | 1.13 | |||||||
| Mean (SD) | 16.4 (3.65) | 20.1 (4–98) | 19.6 (4.85) | 19.4 (4.68) | 21.3 (4.82) | 19.6 (4.85) | ||
| Median (Min, Max) | 16.6 (8.90, 24.5) | 19.5 (10.4, 31.81) | 19.4 (5.91, 34.0) | 18.4 (9.82. 31.719) | 20.8 (11.8, 31.7) | 19.2 (5.91, 34.0) | ||
| CSA Parameters | Opti-Isolated PBMCs | Robo-Isolated PBMCs | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Cohen’s d | Mean (SD) | Cohen’s d | |||
| Warm (n = 55) | Cold (n = 138) | Warm (n = 78) | Cold (n = 44) | |||
| Basal respiration (pmol/min) | 33.64 (9.6) | 40.99 (15.15) | 0.53 | 30.72 (6.56) | 26.55 (6.76) | 0.63 |
| Basal OCR (pmol/min) | 48.43 (13.11) | 59.44 (19.09) | 0.63 | 43.92 (8.5) | 40.64 (9.47) | 0.37 |
| Non-mitochondrial respiration (pmol/min) | 14.79 (4.7) | 18.5 (5.99) | 0.66 | 13.35 (4.42) | 14.09 (5.53) | 0.15 |
| Non-mitochondrial respiration (%) | 30.71 (6.02) | 31.83 (8.33) | 0.14 | 30.12 (8.5) | 34.31 (10.51) | 0.45 |
| Coupling efficiency (%) | 85.95 (9.95) | 88.54 (9.25) | 0.27 | 80.58 (10.01) | 87.51 (10.67) | 0.68 |
| Proton leak (pmol/min) | 5.14 (2.68) | 5.05 (3.52) | 0.03 | 6.12 (3.78) | 3.78 (2.79) | 0.68 |
| Proton leak (%) | 15.06 (6.77) | 12.26 (7.48) | 0.38 | 19.56 (10.12) | 13.81 (8.94) | 0.59 |
| Maximal respiration (pmol/min) | 140.26 (44.14) | 135.09 (44.34) | 0.12 | 129.42 (40.88) | 86.72 (29.56) | 1.15 |
| Spare capacity (pmol/min) | 106.62 (36.81) | 94.1 (32.82) | 0.37 | 98.7 (36.96) | 60.16 (29.23) | 1.12 |
| Spare capacity (%) | 317.89 (71.56) | 239.13 (68.34) | 1.14 | 321.28 (102.81) | 249.25 (135.07) | 0.62 |
| Bioenergetic Health Index (BHI) | 1.65 (0.29) | 1.62 (0.31) | 0.10 | 1.52 (0.36) | 1.49 (0.4) | 0.08 |
| Basal ECAR (mpH/min) | 11.98 (4.22) | 17.58 (8.13) | 0.77 | 7.24 (2.67) | 7.14 (2.05) | 0.04 |
| ECAR oligomycin (mpH/min) | 16.18 (5.39) | 25.11 (11.12) | 0.91 | 10.82 (3.49) | 11.57 (3.11) | 0.22 |
| Maximal ECAR (FCCP) (mpH/min) | 25.77 (6.92) | 34.25 (11.9) | 0.79 | 19.58 (4.85) | 20.02 (4.3) | 0.20 |
| Basal PPR (pmol/min) | 76.27 (26.46) | 111.39 (48.55) | 0.81 | 49.81 (16.41) | 50.17 (14.35) | 0.08 |
| PPR oligomycin (mpH/rnin) | 100.58 (33.83) | 155.48 (66.09) | 0.93 | 70.78 (21.29) | 76.75 (20.05) | 0.28 |
| Maximal PPR (FCCP) (mpH/min) | 155.75 (43.77) | 209.13 (70.87) | 0.83 | 119.36 (29.97) | 125.9 (26.83) | 0.23 |
| Basal RO | 1.58 (0.37) | 1.95 (0.94) | 0.45 | 1.14 (0.28) | 1.25 (0.26) | 0.42 |
| Maximal RQ | 1.02 (0.2) | 1.42 (0.52) | 0.89 | 0.88 (0.22) | 1.32 (0.35) | 1.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessema, B.; Riemer, J.; Sack, U.; König, B. Cellular Stress Assay in Peripheral Blood Mononuclear Cells: Factors Influencing Its Results. Int. J. Mol. Sci. 2022, 23, 13118. https://doi.org/10.3390/ijms232113118
Tessema B, Riemer J, Sack U, König B. Cellular Stress Assay in Peripheral Blood Mononuclear Cells: Factors Influencing Its Results. International Journal of Molecular Sciences. 2022; 23(21):13118. https://doi.org/10.3390/ijms232113118
Chicago/Turabian StyleTessema, Belay, Janine Riemer, Ulrich Sack, and Brigitte König. 2022. "Cellular Stress Assay in Peripheral Blood Mononuclear Cells: Factors Influencing Its Results" International Journal of Molecular Sciences 23, no. 21: 13118. https://doi.org/10.3390/ijms232113118
APA StyleTessema, B., Riemer, J., Sack, U., & König, B. (2022). Cellular Stress Assay in Peripheral Blood Mononuclear Cells: Factors Influencing Its Results. International Journal of Molecular Sciences, 23(21), 13118. https://doi.org/10.3390/ijms232113118








