Systematic Review of the Diagnostic and Clinical Utility of Salivary microRNAs in Traumatic Brain Injury (TBI)
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection and Exclusion Criteria
2.3. Data Extraction and Analysis
3. Results
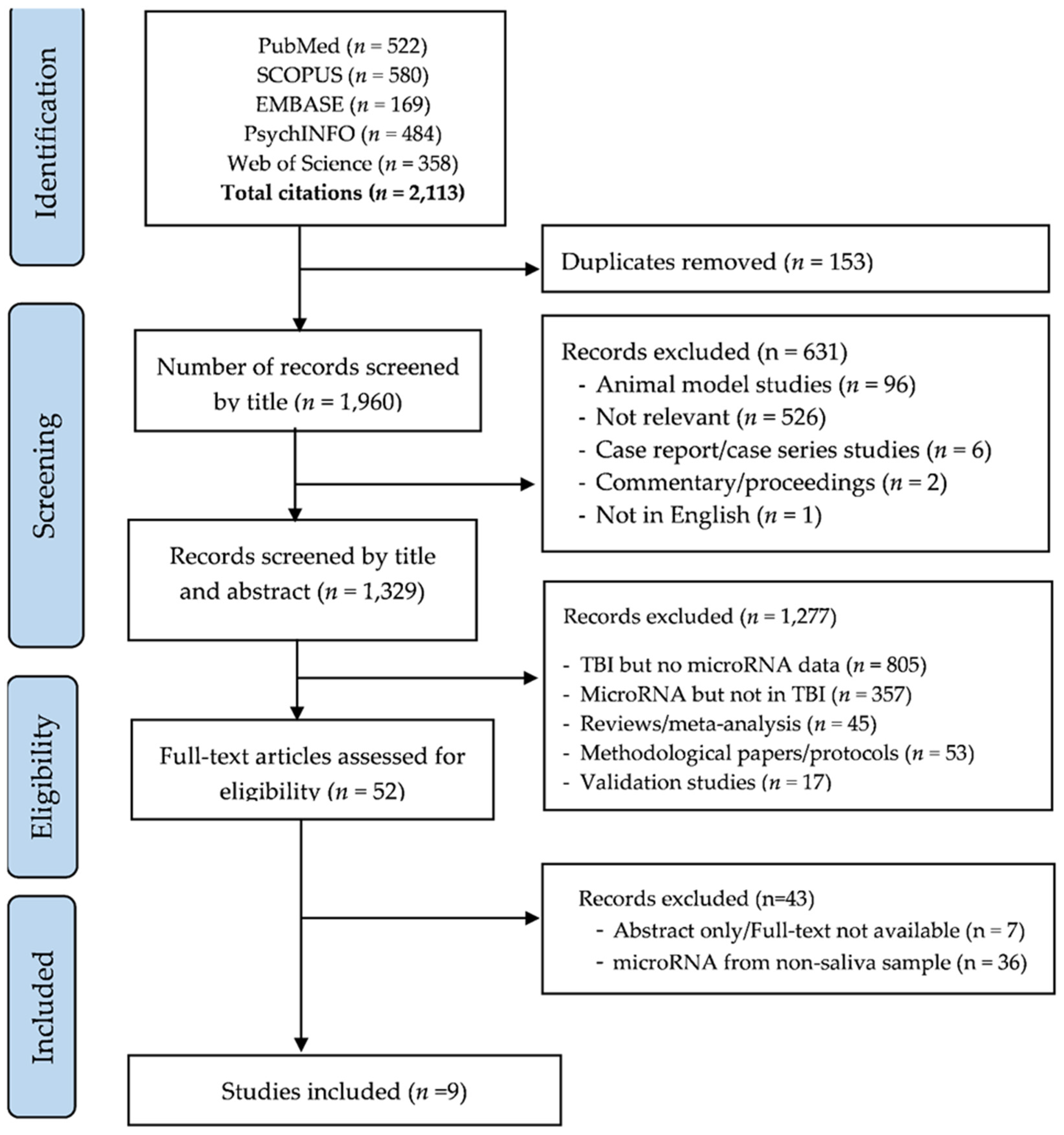
3.1. Study Selection and Characteristics
3.2. Circulating miRNAs and Their Relative Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khellaf, A.; Khan, D.Z.; Helmy, A. Recent advances in traumatic brain injury. J. Neurol. 2019, 266, 2878–2889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.B.; Sun, Z.L.; Feng, D.F. The Role of MicroRNA in Traumatic Brain Injury. Neuroscience 2017, 367, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 13, 1080–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef]
- Miller, G.F.; Daugherty, J.; Waltzman, D.; Sarmiento, K. Predictors of traumatic brain injury morbidity and mortality: Examination of data from the national trauma data bank: Predictors of TBI morbidity & mortality. Injury 2021, 52, 1138–1144. [Google Scholar] [PubMed]
- Rondina, C.; Videtta, W.; Petroni, G.; Lujan, S.; Schoon, P.; Mori, L.B.; Matkovich, J.; Carney, N.; Chesnut, R. Mortality and morbidity from moderate to severe traumatic brain injury in Argentina. J. Head Trauma Rehabil. 2005, 20, 368–376. [Google Scholar] [CrossRef]
- Harvey, L.A.; Close, J.C. Traumatic brain injury in older adults: Characteristics, causes and consequences. Injury 2012, 43, 1821–1826. [Google Scholar] [CrossRef]
- Hiskens, M.; Vella, R.; Schneiders, A.; Fenning, A. Celecoxib in a preclinical model of repetitive mild traumatic brain injury: Hippocampal learning deficits persist with inflammatory and excitotoxic neuroprotection. Trauma Care 2021, 1, 23–37. [Google Scholar] [CrossRef]
- Hiskens, M.I. Targets of Neuroprotection and Review of Pharmacological Interventions in Traumatic Brain Injury. J. Pharmacol. Exp. Ther. 2022, 382, 149–166. [Google Scholar] [CrossRef]
- McCrory, P.; Meeuwisse, W.; Dvorak, J.; Aubry, M.; Bailes, J.; Broglio, S.; Cantu, R.C.; Cassidy, D.; Echemendia, R.J.; Castellani, R.J.; et al. Consensus statement on concussion in sport—The 5th international conference on concussion in sport held in Berlin, October 2016. Br. J. Sport. Med. 2017, 51, 838–847. [Google Scholar]
- Cota, M.R.; Moses, A.D.; Jikaria, N.R.; Bittner, K.C.; Diaz-Arrastia, R.R.; Latour, L.L.; Turtzo, L.C. Discordance between Documented Criteria and Documented Diagnosis of Traumatic Brain Injury in the Emergency Department. J. Neurotrauma 2019, 36, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Pin, E.; Petricoin, E.F., III; Cortes, N.; Bowman, T.G.; Andersson, E.; Uhlen, M.; Nilsson, P.; Caswell, S.V. Immunoglobulin A Autoreactivity toward Brain Enriched and Apoptosis-Regulating Proteins in Saliva of Athletes after Acute Concussion and Subconcussive Impacts. J. Neurotrauma 2021, 38, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- Bowman, K.; Matney, C.; Berwick, D.M. Improving Traumatic Brain Injury Care and Research: A Report From the National Academies of Sciences, Engineering, and Medicine. JAMA 2022, 327, 419–420. [Google Scholar] [CrossRef]
- Huff, J.S.; Jahar, S. Differences in interpretation of cranial computed tomography in ED traumatic brain injury patients by expert neuroradiologists. Am. J. Emerg. Med. 2014, 32, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Wilde, E.A.; Wanner, I.-B.; Kenney, K.; Gill, J.; Stone, J.R.; Disner, S.; Schnakers, C.; Meyer, R.; Prager, E.M.; Haas, M.; et al. A Framework to Advance Biomarker Development in the Diagnosis, Outcome Prediction, and Treatment of Traumatic Brain Injury. J. Neurotrauma 2022, 39, 436–457. [Google Scholar] [CrossRef]
- Thelin, E.P.; Nelson, D.W.; Bellander, B.M. A review of the clinical utility of serum S100B protein levels in the assessment of traumatic brain injury. Acta Neurochir. 2017, 159, 209–225. [Google Scholar] [CrossRef] [Green Version]
- Hiskens, M.I.; Schneiders, A.G.; Angoa-Perez, M.; Vella, R.K.; Fenning, A.S. Blood biomarkers for assessment of mild traumatic brain injury and chronic traumatic encephalopathy. Biomarkers 2020, 25, 213–227. [Google Scholar] [CrossRef]
- Zetterberg, H.; Blennow, K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat. Rev. Neurol. 2016, 12, 563–574. [Google Scholar] [CrossRef]
- Nguyen, R.; Fiest, K.M.; McChesney, J.; Kwon, C.-S.; Jette, N.; Frolkis, A.D.; Atta, C.; Mah, S.; Dhaliwal, H.; Reid, A.; et al. The International Incidence of Traumatic Brain Injury: A Systematic Review and Meta-Analysis. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. Can. J. Neurol. Sci. 2016, 43, 774–785. [Google Scholar] [CrossRef] [Green Version]
- Kellermann, I.; Kleindienst, A.; Hore, N.; Buchfelder, M.; Brandner, S. Early CSF and Serum S100B Concentrations for Outcome Prediction in Traumatic Brain Injury and Subarachnoid Hemorrhage. Clin. Neurol. Neurosurg. 2016, 145, 79–83. [Google Scholar] [CrossRef]
- Hawkins, T.; Greenslade, J.H.; Suna, J.; Williams, J.; Rickard, C.M.; Jensen, M.; Donohue, M.; Cho, E.; Van Hise, C.; Egerton-Warburton, D.; et al. Peripheral Intravenous Cannula Insertion and Use in the Emergency Department: An Intervention Study. Acad. Emerg. Med. 2018, 25, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-W.; Rissland, O.S.; Koppstein, D.; Abreu-Goodger, C.; Jan, C.H.; Agarwal, V.; Yildirim, M.A.; Rodriguez, A.; Bartel, D.P. Global analyses of the effect of different cellular contexts on microRNA targeting. Mol. Cell 2014, 53, 1031–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redell, J.B.; Moore, A.N.; Ward, N.H.; Hergenroeder, G.W., 3rd; Dash, P.K. Human traumatic brain injury alters plasma microRNA levels. J. Neurotrauma 2010, 27, 2147–2156. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Di Pietro, V.; Ragusa, M.; Davies, D.; Su, Z.; Hazeldine, J.; Lazzarino, G.; Hill, L.G.; Crombie, N.; Foster, M.; Purrello, M.; et al. MicroRNAs as novel biomarkers for the diagnosis and prognosis of mild and severe traumatic brain injury. J. Neurotrauma 2017, 34, 1948–1956. [Google Scholar] [CrossRef]
- Das Gupta, S.; Ciszek, R.; Heiskanen, M.; Lapinlampi, N.; Kukkonen, J.; Leinonen, V.; Puhakka, N.; Pitkänen, A. Plasma miR-9-3p and miR-136-3p as Potential Novel Diagnostic Biomarkers for Experimental and Human Mild Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 1563. [Google Scholar] [CrossRef]
- Gilad, S.; Meiri, E.; Yogev, Y.; Benjamin, S.; Lebanony, D.; Yerushalmi, N.; Benjamin, H.; Kushnir, M.; Cholakh, H.; Melamed, N.; et al. Serum microRNAs are promising novel biomarkers. PLoS ONE. 2008, 3, e3148. [Google Scholar] [CrossRef] [Green Version]
- Bhomia, M.; Balakathiresan, N.S.; Wang, K.K.; Papa, L.; Maheshwari, R.K. A Panel of Serum MiRNA Biomarkers for the Diagnosis of Severe to Mild Traumatic Brain Injury in Humans. Sci. Rep. 2016, 6, 28148. [Google Scholar] [CrossRef]
- Mitra, B.; Rau, T.F.; Surendran, N.; Brennan, J.H.; Thaveenthiran, P.; Sorich, E.; Fitzgerald, M.C.; Rosenfeld, J.V.; Patel, S.A. Plasma micro-RNA biomarkers for diagnosis and prognosis after traumatic brain injury: A pilot study. J. Clin. Neurosci. 2017, 38, 37–42. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, V.; O’Halloran, P.; Watson, C.N.; Begum, G.; Acharjee, A.; Yakoub, K.M.; Bentley, C.; Davies, D.J.; Iliceto, P.; Candilera, G.; et al. Unique diagnostic signatures of concussion in the saliva of male athletes: The Study of Concussion in Rugby Union through MicroRNAs (SCRUM). BJSM 2021, 55, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, V.; Porto, E.; Ragusa, M.; Barbagallo, C.; Davies, D.; Forcione, M.; Logan, A.; Pietro, C.D.; Purrello, M.; Grey, M.; et al. Salivary MicroRNAs: Diagnostic Markers of Mild Traumatic Brain Injury in Contact-Sport. Front. Mol. Neurosci. 2018, 11, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedorchak, G.; Rangnekar, A.; Onks, C.; Loeffert, A.C.; Loeffert, J.; Olympia, R.P.; DeVita, S.; Leddy, J.; Haider, M.N.; Roberts, A.; et al. Saliva RNA biomarkers predict concussion duration and detect symptom recovery: A comparison with balance and cognitive testing. J. Neurol. 2021, 268, 4349–4361. [Google Scholar] [CrossRef]
- Hicks, S.D.; Johnson, J.; Carney, M.C.; Bramley, H.; Olympia, R.P.; Loeffert, A.C.; Thomas, N.J. Overlapping MicroRNA Expression in Saliva and Cerebrospinal Fluid Accurately Identifies Pediatric Traumatic Brain Injury. J. Neurotrauma 2018, 35, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.D.; Olympia, R.P.; Onks, C.; Kim, R.Y.; Zhen, K.J.; Fedorchak, G.; DeVita, S.; Rangnekar, A.; Heller, M.; Zwibel, H.; et al. Saliva microRNA Biomarkers of Cumulative Concussion. Int. J. Mol. Sci. 2020, 21, 7758. [Google Scholar] [CrossRef]
- Hicks, S.D.; Onks, C.; Kim, R.Y.; Zhen, K.J.; Loeffert, J.; Loeffert, A.C.; Olympia, R.P.; Fedorchak, G.; DeVita, S.; Gagnon, Z.; et al. Refinement of saliva microRNA biomarkers for sports-related concussion. J. Sport Health Sci. 2021. [Google Scholar] [CrossRef]
- Hicks, S.D.; Onks, C.; Kim, R.Y.; Zhen, K.J.; Loeffert, J.; Loeffert, A.C.; Olympia, R.P.; Fedorchak, G.; DeVita, S.; Rangnekar, A.; et al. Diagnosing mild traumatic brain injury using saliva RNA compared to cognitive and balance testing. Clin. Transl. Med. 2020, 10, e197. [Google Scholar] [CrossRef]
- Johnson, J.J.; Loeffert, A.C.; Stokes, J.; Olympia, R.P.; Bramley, H.; Hicks, S.D. Association of Salivary MicroRNA Changes With Prolonged Concussion Symptoms. JAMA Pediatr. 2018, 172, 65–73. [Google Scholar] [CrossRef] [Green Version]
- LaRocca, D.; Barns, S.; Hicks, S.D.; Brindle, A.; Williams, J.; Uhlig, R.; Johnson, P.; Neville, C.; Middleton, F.A. Comparison of serum and saliva miRNAs for identification and characterization of mTBI in adult mixed martial arts fighters. PLoS ONE 2019, 14, e0207785. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Yin, J.; Wang, Y.; Zhuang, X.; He, Z.; Chen, Z.; Yang, X. MicroRNAs as potential biomarkers for the diagnosis of Traumatic Brain Injury: A systematic review and meta-analysis. Int. J. Med. Sci. 2021, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Hsueh, H.; Delongchamp, R.R.; Lin, C.; Tsai, C. Reproducibility of microarray data: A further analysis of microarray quality control (MAQC) data. BMC Bioinform. 2007, 8, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novianti, P.W.; Roes, K.C.; Eijkemans, M.J. Evaluation of gene expression classification studies: Factors associated with classification performance. PLoS ONE 2014, 9, e96063. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, D.; Haberberger, A.; Kirchner, B.; Spornraft, M.; Riedmaier, I.; Schelling, G.; Pfaffl, M.W. Toward reliable biomarker signatures in the age of liquid biopsies-how to standardize the small RNA-Seq workflow. Nucleic Acids Res. 2016, 44, 5995–6018. [Google Scholar] [CrossRef]
- Goutnik, M.; Lucke-Wold, B. Commentary: Evaluating potential glioma serum biomarkers, with future applications. World J. Clin. Oncol. 2022, 13, 412–416. [Google Scholar] [CrossRef]
- Schulte, L.N.; Eulalio, A.; Mollenkopf, H.J.; Reinhardt, R.; Vogel, J. Analysis of the host microRNA response to Salmonella uncovers the control of major cytokines by the let-7 family. EMBO J. 2011, 30, 1977–1989. [Google Scholar] [CrossRef] [Green Version]
- Balakathiresan, N.; Bhomia, M.; Chandran, R.; Chavko, M.; McCarron, R.M.; Maheshwari, R.K. MicroRNA let-7i is a promising serum biomarker for blast-induced traumatic brain injury. J. Neurotrauma 2012, 29, 1379–1387. [Google Scholar] [CrossRef] [Green Version]
- Sabirzhanov, B.; Zhao, Z.; Stoica, B.A.; Loane, D.; Wu, J.; Borroto, C.; Dorsey, S.G.; Faden, A.I. Downregulation of miR-23a and miR-27a following experimental traumatic brain injury induces neuronal cell death through activation of proapoptotic Bcl-2 proteins. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 10055–10071. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Xu, J.; Li, L.; Li, H.; Mao, S.; Zhang, F.; Zen, K.; Zhang, C.-Y.; Zhang, Q. MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell Death Dis. 2014, 5, e1132. [Google Scholar] [CrossRef] [Green Version]
- Xi, T.; Jin, F.; Zhu, Y.; Wang, J.; Tang, L.; Wang, Y.; Liebeskind, D.S.; Scalzo, F.; He, Z. miR-27a-3p protects against blood–brain barrier disruption and brain injury after intracerebral hemorrhage by targeting endothelial aquaporin-11. J. Biol. Chem. 2018, 293, 20041–20050. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Q.; Lv, H.W.; Wang, Z.L.; Tan, W.F.; Fang, B.; Ma, H. MiR-27a ameliorates inflammatory damage to the blood-spinal cord barrier after spinal cord ischemia: Reperfusion injury in rats by downregulating TICAM-2 of the TLR4 signaling pathway. J. Neuroinflamm. 2015, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Shultz, S.R.; Taylor, C.J.; Aggio-Bruce, R.; O’Brien, W.T.; Sun, M.; Cioanca, A.V.; Neocleous, G.; Symons, G.F.; Brady, R.D.; Hardikar, A.A.; et al. Decrease in Plasma miR-27a and miR-221 After Concussion in Australian Football Players. Biomark. Insights 2022, 17, 11772719221081318. [Google Scholar] [CrossRef] [PubMed]
- Savaskan, N.E.; Brauer, A.U.; Nitsch, R. Molecular cloning and expression regulation of PRG-3, a new member of the plasticity-related gene family. Eur. J. Neurosci. 2004, 19, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.P.; Fiori, L.M.; Gross, J.A.; Labonte, B.; Yerko, V.; Mechawar, N.; Turecki, G. Regulatory role of miRNAs in polyamine gene expression in the prefrontal cortex of depressed suicide completers. Int. J. Neuropsychopharmacol. 2014, 17, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.T.; Tsai, P.C.; Liao, Y.C.; Hsu, C.Y.; Juo, S.H.H. Circulating microRNAs have a sex-specific association with metabolic syndrome. J. Biomed. Sci. 2013, 20, 72. [Google Scholar] [CrossRef] [Green Version]
- Morgan, C.P.; Bale, T.L. Sex differences in microRNA regulation of gene expression: No smoke, just miRs. Biol. Sex Differ. 2012, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Rekker, K.; Saare, M.; Roost, A.M.; Salumets, A.; Peters, M. Circulating microRNA Profile throughout the menstrual cycle. PLoS ONE 2013, 8, e81166. [Google Scholar]
- Luizon, M.R.; Conceição, I.M.C.A.; Viana-Mattioli, S.; Caldeira-Dias, M.; Cavalli, R.C.; Sandrim, V.C. Circulating MicroRNAs in the Second Trimester From Pregnant Women Who Subsequently Developed Preeclampsia: Potential Candidates as Predictive Biomarkers and Pathway Analysis for Target Genes of miR-204-5p. Front. Physiol. 2021, 1536. [Google Scholar] [CrossRef]
- Witwer, K.W.; Hirschi, K.D. Transfer and functional consequences of dietary microRNAs in vertebrates: Concepts in search of corroboration: Negative results challenge the hypothesis that dietary xenomiRs cross the gut and regulate genes in ingesting vertebrates, but important questions persist. Bioessays 2014, 36, 394–406. [Google Scholar]
- Gomes, C.P.; Oliveira, G.P.; Jr Madrid, B.; Almeida, J.A.; Franco, O.L.; Pereira, R.W. Circulating miR-1, miR-133a, and miR-206 levels are increased after a half-marathon run. Biomarkers 2014, 19, 585–589. [Google Scholar] [CrossRef]
- Nielsen, S.; Åkerström, T.; Rinnov, A.R.; Yfanti, C.; Scheele, C.; Pedersen, B.K.; Laye, M.J. The miRNA plasma signature in response to acute aerobic exercise and endurance training. PLoS ONE 2014, 9, e87308. [Google Scholar] [CrossRef] [PubMed]
- Horak, M.; Zlamal, F.; Iliev, R.; Kučera, J.; Cacek, J.; Svobodova, L.; Hlavoňová, Z.; Kalina, T.; Slaby, O.; Bienertova-Vasku, J. Exercise-induced circulating microRNA changes in athletes in various training scenarios. PLoS ONE 2018, 13, e0191060. [Google Scholar] [CrossRef] [PubMed]
- Eyileten, C.; Wicik, Z.; Fitas, A.; Marszalek, M.; Simon, J.E.; De Rosa, S.; Wiecha, S.; Palatini, J.; Postula, M.; Malek, L.A. Altered Circulating MicroRNA Profiles After Endurance Training: A Cohort Study of Ultramarathon Runners. Front. Physiol. 2021, 12, 792931. [Google Scholar] [CrossRef] [PubMed]
- Uhlemann, M.; Möbius-Winkler, S.; Fikenzer, S.; Adam, J.; Redlich, M.; Möhlenkamp, S.; Hilberg, T.; Schuler, G.C.; Adams, V. Circulating microRNA-126 increases after different forms of endurance exercise in healthy adults. Eur. J. Prev. Cardiol. 2014, 21, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Kroh, E.; Wood, B.; Arroyo, J.D.; Dougherty, K.J.; Miyaji, M.M.; Tait, J.F.; Tewari, M. Blood Cell Origin of Circulating MicroRNAs: A Cautionary Note for Cancer Biomarker StudiesCirculating MicroRNA Biomarkers and Blood Cells. Cancer Prev. Res. 2012, 5, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Majem, B.; Rigau, M.; Reventós, J.; Wong, D.T. Non-coding RNAs in saliva: Emerging biomarkers for molecular diagnostics. Int. J. Mol. Sci. 2015, 16, 8676–8698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullan, M.J.; Asken, B.M.; Jaffee, M.S.; DeKosky, S.T.; Bauer, R.M. Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy. Neurosci. Biobehav. Rev. 2018, 84, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Liu, D.Z.; Jickling, G.C.; Sharp, F.R.; Yin, K.J. MicroRNA-based therapeutics in central nervous system injuries. J. Cereb. Blood Flow Metab. 2018, 38, 1125–1148. [Google Scholar] [CrossRef]
- Madathil, S.K.; Nelson, P.T.; Saatman, K.E.; Wilfred, B.R. MicroRNAs in CNS injury: Potential roles and therapeutic implications. Bioessays 2011, 33, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Hiskens, M.I.; Vella, R.K.; Schneiders, A.G.; Fenning, A.S. Minocycline improves cognition and molecular measures of inflammation and neurodegeneration following repetitive mTBI. Brain Inj. 2021, 35, 831–841. [Google Scholar] [CrossRef]
| Author (Year) | Study Type/Design | Study Participants | Study Sample (n) | Sex | Age Range or Mean (SD) | Saliva Sample Collection Time | microRNA Measurement | microRNA Expression (Type) | Summary of Key Findings | Reported AUC or ROC (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Cases | Controls | ||||||||||
| Di Pietro, V. et al. (2018) [33] | Case-control | Rugby players | 12 (for discovery group) | 6 Concussed (For discovery group) | 6 non-concussed (For discovery group) | Male | 16–65 years | 48–72 hrs. from concussion | Nano-string Profiling | let-7i-5p, miR-142-3p, miR-107, miR-27b-3p, miR-135b-5p |
| AUCs for:
|
| 32 (validation group) | 22 concussed (validation group) | 10 non-concussed (validation group) | ||||||||||
| Di Pietro, V. et al. (2021) [32] | Prospective cohort | Rugby players | 324 | 106 HIA+ | HIA- non-concussed (n = 50); Uninjured (n = 102); musculoskeletal injury (n = 66) | Male |
| 3 sample collection time points for 2 seasons
| NGS | 32 differentially expressed from HIA+ and HIA- compared at T1, T2, T3 in season 1: let-7a-5p, miR-1246, let-7f-5p, let-7i-5p, miR-107, miR-148a-3p, miR-135b-5p, miR-126-3p, miR-21-5p, miR-465, miR-34b-3p, miR-92a-3p, miR-6, miR-476, miR-144-3p, miR-103a-3p, RNU6-6, RNU6-4, RNU6-45, RNU6-7, RNU6-73, tRNA27-MetCAT, tRNA18-ArgCCT, tRNA2-LeuTAA, Y_RNA.255, SNORD3B-2, tRNA120-AlaAGC, U2.3, snoU13.120, tRNA73-ArgCCG, U6.375, U6.601 14 with the highest accuracy compared with HIA- and controls combined as a panel in season 2: let-7a5p, miR-143-3p, miR-103a-3p, miR-34b-3p, RNU6-7, RNU6-45, Snora57, snoU13.120, tRNA18Arg-CCT, U6-168, U6-428, U6-1249, Uco22cjg1, YRNA_255 |
| Season 1 T1 AUC HIA+ Vs. HIA- =1.00 (1.00–1.00) T2 AUC HIA+ Vs. All combined: 0.91 (0.81–1.00) Vs. HIA-: 0.88 (0.74–1.00) Vs. Uninjured: 0.93 (0.84–1.00) Vs. MSK: 0.90 (0.78–1.00) Vs. Baseline: 0.95 (0.90–1.00) T3 AUC HIA+ Vs. All combined: 0.94 (0.86–1.00) Vs. HIA-: 0.96 (0.89–1.00) Vs. Uninjured: 0.96 (0.88–1.00) Vs. MSK: 0.90 (0.69–1.00) Vs. Baseline: 0.91 (0.84–0.98) Season 2 T2 AUC HIA+ Vs. All combined: 0.96 (0.92–1.00) Vs. HIA−: 0.94 (0.85–1.00) Vs. Uninjured: 0.94 (0.87–1.00) Vs. MSK: 1.00 (1.00–1.00) T3 AUC HIA+ Vs. All combined: 0.93 (0.86–1.00) Vs. HIA−: 0.86 (0.73–1.00) Vs. Uninjured: 0.95 (0.89–1.00) Vs. MSK: 0.95 (0.88–1.00) |
| Fedorchak, G. et al. (2021) [34] | Case-control | Patients with clinical diagnosis of mTBI |
|
|
|
| 8–24 y | 2 time point sample collection
| NGS | wiRNA_48, miR-1246, miR-486-59, wiRNA_147, wiRNA_1590, wiRNA9924, miR-92b-3p, wiRNA_7971, miR-203a-5p, SNORD81, wiRNA_9447, miR-148a-5p, wiRNA_1385, wiRNA_7876, miR-100-5p, miR-148-3p |
| Performance of 16 ncRNAs to predict PPCS AUC 0.86 (0.84–0.88) |
| Hicks, S. D. et al. (2018) [35] | Case–cohort | Children with sTBI and mTBI (salivary group and CSF group) | 78 Salivary group * | 60 mTBI | 18 controls | 29 F 31 M | 5–21 y | Within 14 days after injury | NGS | miR-182-5p, miR-221-3p, miR-26b-5p, miR-320c, miR-29c-3p, miR-30e5p |
| The performance of 6 miRNAs to diagnose mTBI was: AUC = 0.852 (0.69–0.98) with similar validation accuracy (AUC 0.8) |
| Hicks, S. D. et al. (2020) [36] | Case- control | Former football athletes with diagnosed or recurrent concussion | 31 | 13 participants | 18 (age and sex matched) | 31 M | 46–89 y | 8–18 h to collect form all participants | NGS | miR-101-3p, miR-582-3p, miR-424-5p, miR-340-5p, miR-181c-5p, miR-155-5p, miR-28-3p, miR-26b-5p, miR-30a-3p, miR-3184-3p, miR-423-5p, miR-4776-5p, miR-339-3p, miR-576-5p, miR-361-5p, miR-3074-5p, miR-24-3p, miR-574-5p, miR23a-3p, miR-23b-3p |
| NA |
| Cross sectional | NA | 310 | 230 without history of concussion | 80 with single or recurrent concussion | 102 F 208 M | 7–39 y | 7–19 h to collect from all participants | |||||
| Hicks, S. D. et al. (2020) [38] | Case-control | Patients with mTBI and controls | 538 participants | 251 mTBI (201 mTBI cases used for testing) | 287 controls (no mTBI in the last 12 weeks) (229 controls used for testing) | 207 F 331 M | 5–66 Mean (SD) 18 (±6) y | ≤14 days postinjury Saliva sample collected 5 timepoints post injury | NGS | miR-4510, miR-34a-5p, miR-744-5p, miR-192-5p, miR-25-3p, miR-30e-3p, miR-30a-3p, miR-3074-5p, miR-3614-5p, miR-378a-5p, miR-27a-5p, miR-181c-5p, miR-708-5p, miR-1246, let-7e-5p, miR-944, miR-1290, miR-181a-5p, miR-582-3p, miR-183-5p, miR-1180-3p, miR-12136 |
| Training group: AUC 0.857; (0.836-0.918) |
| 108 (Testing group) | 50 mTBI for training | 58 controls using for training | Testing group: AUC 0.823 | |||||||||
| Hicks, S. D. et al. (2021) [37] | Case-control | Athletes with SRC-related concussion and non-concussed athletes as comparator | 172 participants with miRNAs not affected by exercise | 75 concussed | 97 non-concussed | 198 M 116 F | 8–58 y | ≤24 h post-injury (for cases) | NGS | 15 out of 40 miRNAs were unaffected by acute exercise and listed below: miR-27a-5p, miR-1246, miR-30e-3p, miR-30a-3p, miR-151a-3p, miR-192-5p, miR-7-1-3p, miR-181c-5p, miR-30e-5p, miR-1307-5p, miR-182-5p, miR-3074-5p, miR-629-5p, miR-944, miR-27b-3p |
|
|
| Johnson, J. J. et al. (2018) [39] | Prospective cohort | mTBI patients with acute or prolonged symptoms | 52 participants | 30 with prolonged symptom (PCS) group | 22 acute symptom (ACS) group | 22 F 30 M | 7–21 y Mean (SD) 14 (±3) y | 14 days of injury | NGS | miR-769-5p, miR-4792, miR-629-5p, let-7a-5p, miR-320c-1, miR-140-3p, miR-133a-5p, let-7b-5p, miR-192-5p, miR-30e, miR-4508, miR-1307-3p, miR-200b-3p, miR-145-5p, miR-629 |
|
|
| ||||||||||||
| ||||||||||||
| LaRocca, D. et al. (2019) [40] | Cohort study | Adult mixed martial arts fighters | 42 MMA fighters | MMAs with HTH | Those who
| 2 F 40 M | Mean (SD) 26.5 (±5.8) y |
| NGS | miR-7-1-3p, miR-10a-5p, miR-10b-5p, miR-20a-5p, miR-30b-5p, miR-92a-3p, miR-122-5p, miR-128-3p, miR-155-5p, miR-455-5p, miR-1307-3p, miR-3146, miR-3678-3p miR-376a-5p, miR-4637, miR-4649-3p miR-4693-5p, miR-4766-5p, miR-5694, miR-6770-5p, miR-6809-3p | 21 microRNAs from saliva and serum
| Salivary miRNAs combined with serum miRNAs provided AUC of 0.89 for predicting mTBI |
| miRNA | Di Pietro 2018 [33] | Di Pietro 2021 [32] | Fedorchak 2021 [34] | Hicks 2018 [35] | Hicks 2020 [36] | Hicks 2020 [38] | Hicks 2021 [37] | Johnson 2018 [39] | Larocca 2019 [40] |
|---|---|---|---|---|---|---|---|---|---|
| miR-1246 | DOWN | DOWN | UP | DOWN | |||||
| miR-30a-3p | UP | DOWN | DOWN | ||||||
| miR-181c-5p | UP | DOWN | DOWN | ||||||
| miR-192-5p | UP | DOWN | UP | ||||||
| miR-3074-5p | DOWN | UP | UP | ||||||
| let-7a-5p | UP | DOWN | |||||||
| let-7i-5p * | UP | UP | |||||||
| miR-107 * | UP | UP | |||||||
| miR-135b-5p * | UP | UP | |||||||
| miR-148a-3p * | UP | UP | |||||||
| miR-92a-3p | UP | DOWN | |||||||
| miR-20a-5p * | UP | UP | |||||||
| miR-24-3p * | UP | UP | |||||||
| miR-27b-3p * | UP | UP | |||||||
| miR-29c-3p * | UP | UP | |||||||
| miR-181a-5p * | UP | UP | |||||||
| miR-221-3p | UP | DOWN | |||||||
| miR-424-5p | UP | DOWN | |||||||
| miR-182-5p * | DOWN | DOWN | |||||||
| miR-26b-5p * | DOWN | DOWN | |||||||
| miR-320c * | DOWN | DOWN | |||||||
| miR-30e-5p | UP | DOWN | |||||||
| miR-582-3p | UP | DOWN | |||||||
| miR-155-5p | DOWN | UP | |||||||
| miR-27a-5p * | DOWN | DOWN | |||||||
| miR-30e-3p | UP | DOWN | |||||||
| miR-7-1-3p * | DOWN | DOWN | |||||||
| miR-629-5p | UP | DOWN | |||||||
| miR-944 | UP | DOWN | |||||||
| miR-1307-3p | UP | DOWN |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiskens, M.I.; Mengistu, T.S.; Li, K.M.; Fenning, A.S. Systematic Review of the Diagnostic and Clinical Utility of Salivary microRNAs in Traumatic Brain Injury (TBI). Int. J. Mol. Sci. 2022, 23, 13160. https://doi.org/10.3390/ijms232113160
Hiskens MI, Mengistu TS, Li KM, Fenning AS. Systematic Review of the Diagnostic and Clinical Utility of Salivary microRNAs in Traumatic Brain Injury (TBI). International Journal of Molecular Sciences. 2022; 23(21):13160. https://doi.org/10.3390/ijms232113160
Chicago/Turabian StyleHiskens, Matthew I., Tesfaye S. Mengistu, Katy M. Li, and Andrew S. Fenning. 2022. "Systematic Review of the Diagnostic and Clinical Utility of Salivary microRNAs in Traumatic Brain Injury (TBI)" International Journal of Molecular Sciences 23, no. 21: 13160. https://doi.org/10.3390/ijms232113160
APA StyleHiskens, M. I., Mengistu, T. S., Li, K. M., & Fenning, A. S. (2022). Systematic Review of the Diagnostic and Clinical Utility of Salivary microRNAs in Traumatic Brain Injury (TBI). International Journal of Molecular Sciences, 23(21), 13160. https://doi.org/10.3390/ijms232113160






