Bioinformatics Analysis of RNA-seq Data Reveals Genes Related to Cancer Stem Cells in Colorectal Cancerogenesis
Abstract
:1. Introduction
2. Results
2.1. Identification of DEGs
2.1.1. Identification of DEGs from Gene Expression Omnibus (GEO) Database
2.1.2. Comparing Results between Projects from GEO Database—CRC and Corresponding Normal Mucosa
2.1.3. Identification of DEGs through Joint Projects—CRC and Adenomas
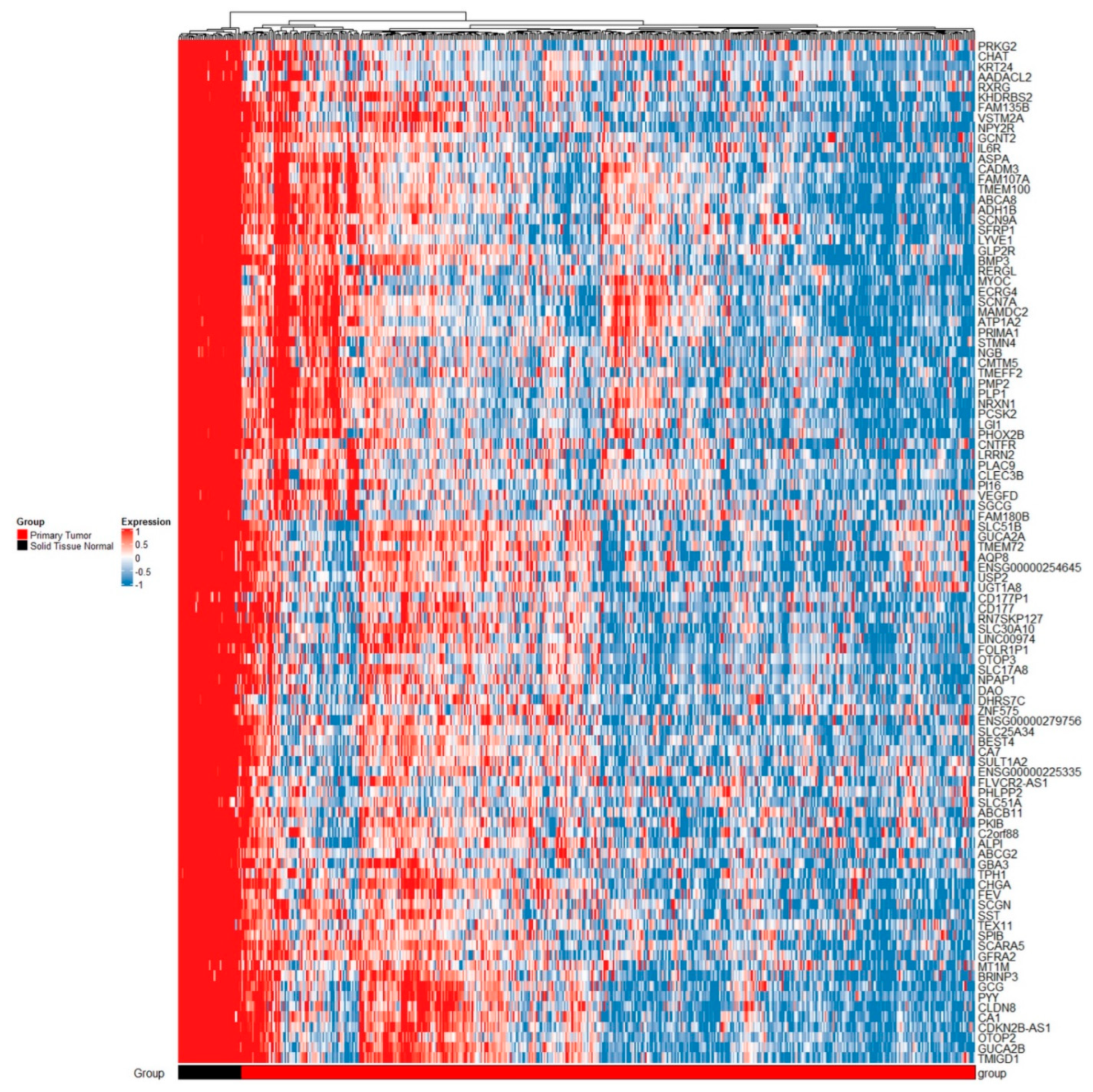
2.1.4. TCGA Analysis
2.1.5. Comparing Results from GEO Projects and TCGA Results
2.2. Identification of Candidate Genes for Further Validation
2.3. Validation of Identified Candidate Genes on Samples of Normal Mucosa, Adenoma, Adenoma with Early Carcinoma, and Carcinoma without and with Lymph Node Invasion
2.3.1. Differential Gene Expression in Adenoma and Adenoma with Early Carcinoma
2.3.2. Gene Expression in Carcinoma Compared to Corresponding Normal Mucosa
2.3.3. Association with Level of Malignancy
3. Discussion
3.1. Identified DEGs, PEI and PPI Related to CSC
3.2. Expression of Investigated Genes in Association with Malignant Transformation, Progression, and Mestastases of CRC
3.3. The Most Common Predicted Functional Partners in the PPI Analysis
3.4. Limitations of the Study
4. Materials and Methods
4.1. Bioinformatics Analysis of Publicly Available Data
4.1.1. Project Selection
4.1.2. Data Processing
4.1.3. Analysis of Projects Obtained from GEO/TCGA Database
4.2. Pathway Enrichment Analysis of Publicly Available Data
4.3. Protein–Protein Interaction Analysis
4.4. Validation of Selected Potential Candidate Genes
4.4.1. Patient and Tissue Selection
4.4.2. RNA Isolation and Quality Assessment
4.4.3. Reverse Transcription (RT) and Pre-Amplification
4.4.4. Selection of Primers and Probes
4.4.5. Quantitative Real-Time PCR (qPCR)
4.4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blank, A.; Roberts, D.E., 2nd; Dawson, H.; Zlobec, I.; Lugli, A. Tumor heterogeneity in primary colorectal cancer and corresponding metastases. Does the apple fall far from the tree? Front. Med. 2018, 5, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saiki, Y.; Ishimaru, S.; Mimori, K.; Takatsuno, Y.; Nagahara, M.; Ishii, H.; Yamada, K.; Mori, M. Comprehensive analysis of the clinical significance of inducing pluripotent stemness-related gene expression in colorectal cancer cells. Ann. Surg. Oncol. 2009, 16, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Dylla, S.J.; Beviglia, L.; Park, I.K.; Chartier, C.; Raval, J.; Ngan, L.; Pickell, K.; Aguilar, J.; Lazetic, S.; Smith-Berdan, S.; et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS ONE 2008, 3, e2428. [Google Scholar] [CrossRef]
- Urh, K.; Žlajpah, M.; Zidar, N.; Boštjančič, E. Identification and validation of new cancer stem cell-related genes and their regulatory microRNAs in colorectal cancerogenesis. Biomedicines 2021, 9, 179. [Google Scholar] [CrossRef]
- Urh, K.; Zidar, N.; Tomažič, A.; Boštjančič, E. Intra-tumor heterogeneity of cancer stem cell-related genes and their potential regulatory microRNAs in metastasizing colorectal carcinoma. Oncol. Rep. 2022, 48, 193. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, A.V.; Lipatova, A.V.; Zaretsky, A.R.; Moskalev, A.A.; Fedorova, M.S.; Rasskazova, A.S.; Shibukhova, G.A.; Snezhkina, A.V.; Kaprin, A.D.; Alekseev, B.Y.; et al. Important molecular genetic markers of colorectal cancer. Oncotarget 2016, 7, 53959–53983. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Xu, E.; Liu, H.; Wan, L.; Lai, M. Epithelial-mesenchymal transition in colorectal cancer metastasis: A system review. Pathol. Res. Pract. 2015, 211, 557–569. [Google Scholar] [CrossRef]
- Balch, C.; Ramapuram, J.B.; Tiwari, A.K. The epigenomics of embryonic pathway signaling in colorectal cancer. Front. Pharmacol. 2017, 8, 267. [Google Scholar] [CrossRef] [Green Version]
- Sepulveda, A.R.; Del Portillo, A.J. Molecular basis of diseases of the gastrointestinal tract. In Molecular Pathology, 2nd ed.; Coleman, W.B., Tsongalis, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 387–415. [Google Scholar] [CrossRef]
- Jarvinen, H.J.; Mecklin, J.P.; Sistonen, P. Screening reduces colorectal cancer rate in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 1995, 108, 1405–1411. [Google Scholar] [CrossRef]
- Brenner, H.; Jansen, L.; Ulrich, A.; Chang-Claude, J.; Hoffmeister, M. Survival of patients with symptom- and screening-detected colorectal cancer. Oncotarget 2016, 7, 44695–44704. [Google Scholar] [CrossRef] [Green Version]
- Fischer, J.; Walker, L.C.; Robinson, B.A.; Frizelle, F.A.; Church, J.M.; Eglinton, T.W. Clinical implications of the genetics of sporadic colorectal cancer. ANZ J. Surg. 2019, 89, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, R.; Scartozzi, M.; Loretelli, C.; Piva, F.; Mandolesi, A.; Lezoche, G.; Del Prete, M.; Bittoni, A.; Faloppi, L.; Bianconi, M.; et al. Cancer stem cell gene profile as predictor of relapse in high risk stage II and stage III, radically resected colon cancer patients. PLoS ONE 2013, 8, e72843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlos-Suarez, A.; Barriga, F.M.; Jung, P.; Iglesias, M.; Cespedes, M.V.; Rossell, D.; Sevillano, M.; Hernando-Momblona, X.; da Silva-Diz, V.; Munoz, P.; et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 2011, 8, 511–524. [Google Scholar] [CrossRef] [Green Version]
- Colak, S.; Zimberlin, C.D.; Fessler, E.; Hogdal, L.; Prasetyanti, P.R.; Grandela, C.M.; Letai, A.; Medema, J.P. Decreased mitochondrial priming determines chemoresistance of colon cancer stem cells. Cell Death Differ. 2014, 21, 1170–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardo, Y.; Scopelliti, A.; Cammareri, P.; Todaro, M.; Iovino, F.; Ricci-Vitiani, L.; Gulotta, G.; Dieli, F.; de Maria, R.; Stassi, G. Bone morphogenetic protein 4 induces differentiation of colorectal cancer stem cells and increases their response to chemotherapy in mice. Gastroenterology 2011, 140, 297–309. [Google Scholar] [CrossRef]
- Lotti, F.; Jarrar, A.M.; Pai, R.K.; Hitomi, M.; Lathia, J.; Mace, A.; Gantt, G.A., Jr.; Sukhdeo, K.; DeVecchio, J.; Vasanji, A.; et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J. Exp. Med. 2013, 210, 2851–2872. [Google Scholar] [CrossRef] [Green Version]
- Zeuner, A.; Todaro, M.; Stassi, G.; De Maria, R. Colorectal cancer stem cells: From the crypt to the clinic. Cell Stem Cell 2014, 15, 692–705. [Google Scholar] [CrossRef] [Green Version]
- Safiyeva, A.; Bayramov, N.; Avunduk, M. The role of “Prominin-1” marker in adenomatous colorectal polyps. Pol. Przegl. Chir. 2021, 94, 19–26. [Google Scholar] [CrossRef]
- Sahin, I.; Gundogdu, B.; Ceylan, A.C.; Erdem, H.B.; Tatar, A. High expression of stem cell-related genes in polyps with villous features and high-grade dysplasia support malignant phenotype and colorectal carcinogenesis. Asian Pac. J. Cancer Prev. 2021, 22, 2429–2435. [Google Scholar] [CrossRef]
- Cui, G.; Li, G.; Pang, Z.; Florholmen, J.; Goll, R. The presentation and regulation of the IL-8 network in the epithelial cancer stem-like cell niche in patients with colorectal cancer. Biomed. Pharmacother. 2022, 152, 113252. [Google Scholar] [CrossRef]
- Becker, W.R.; Nevins, S.A.; Chen, D.C.; Chiu, R.; Horning, A.M.; Guha, T.K.; Laquindanum, R.; Mills, M.; Chaib, H.; Ladabaum, U.; et al. Single-cell analyses define a continuum of cell state and composition changes in the malignant transformation of polyps to colorectal cancer. Nat. Genet. 2022, 54, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Scurrah, C.R.; McKinley, E.T.; Simmons, A.J.; Ramirez-Solano, M.A.; Zhu, X.; Markham, N.O.; Heiser, C.N.; Vega, P.N.; Rolong, A.; et al. Differential pre-malignant programs and microenvironment chart distinct paths to malignancy in human colorectal polyps. Cell 2021, 184, 6262–6280.e26. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.; Bataille, F.; Schmid, C.; Ruemmele, P.; Waldeck, A.; Blaszyk, H.; Hartmann, A.; Hofstaedter, F.; Dietmaier, W. Gene expression profiling of colorectal cancer and metastases divides tumours according to their clinicopathological stage. J. Pathol. 2004, 204, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Kwon, C.H.; Choi, Y.; Park, H.J.; Kim, H.S.; Jo, H.J.; Oh, N.; Park, D.Y. Transcriptome analysis of paired primary colorectal carcinoma and liver metastases reveals fusion transcripts and similar gene expression profiles in primary carcinoma and liver metastases. BMC Cancer 2016, 16, 539. [Google Scholar] [CrossRef] [Green Version]
- Hauptman, N.; Boštjančič, E.; Žlajpah, M.; Ranković, B.; Zidar, N. Bioinformatics analysis reveals most prominent gene candidates to distinguish colorectal adenoma from adenocarcinoma. Biomed. Res. Int. 2018, 2018, 9416515. [Google Scholar] [CrossRef] [Green Version]
- Uhan, S.; Zidar, N.; Tomazič, A.; Hauptman, N. Hypermethylated promoters of genes UNC5D and KCNA1 as potential novel diagnostic biomarkers in colorectal cancer. Epigenomics 2020, 12, 1677–1688. [Google Scholar] [CrossRef]
- Hrašovec, S.; Hauptman, N.; Glavač, D.; Jelenc, F.; Ravnik-Glavač, M. TMEM25 is a candidate biomarker methylated and down-regulated in colorectal cancer. Dis. Markers 2013, 34, 93–104. [Google Scholar] [CrossRef]
- Szigeti, K.A.; Kalmár, A.; Galamb, O.; Valcz, G.; Barták, B.K.; Nagy, Z.B.; Zsigrai, S.; Felletár, I.; VPatai, Á.; Micsik, T.; et al. Global DNA hypomethylation of colorectal tumours detected in tissue and liquid biopsies may be related to decreased methyl-donor content. BMC Cancer 2022, 22, 605. [Google Scholar] [CrossRef]
- Muller, D.; Gyorffy, B. DNA methylation-based diagnostic, prognostic, and predictive biomarkers in colorectal cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188722. [Google Scholar] [CrossRef]
- Mehrgou, A.; Teimourian, S. Update of gene expression/methylation and miRNA profiling in colorectal cancer; application in diagnosis, prognosis, and targeted therapy. PLoS ONE 2022, 17, e0265527. [Google Scholar] [CrossRef]
- Caruso, F.P.; D’Andrea, M.R.; Coppola, L.; Landriscina, M.; Condelli, V.; Cerulo, L.; Giordano, G.; Porras, A.; Pancione, M. Lymphocyte antigen 6G6D-mediated modulation through p38alpha MAPK and DNA methylation in colorectal cancer. Cancer Cell Int. 2022, 22, 253. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, K.; Zheng, J.; Sun, X.; Zhao, W. Establishment of a novel prognostic signature based on an identified expression profile of integrin superfamily to predict overall survival of patients with colorectal adenocarcinoma. Gene 2022, 808, 145990. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Villanueva, M.; Duran-Sanchon, S.; Martin, A.C.; Perez-Palacios, R.; Vila-Navarro, E.; Marcuello, M.; Diaz-Centeno, M.; Cubiella, J.; Diez, M.S.; Bujanda, L.; et al. Plasma microRNA signature validation for early detection of colorectal cancer. Clin. Transl. Gastroenterol. 2019, 10, e00003. [Google Scholar] [CrossRef] [PubMed]
- Islam Khan, M.Z.; Tam, S.Y.; Azam, Z.; Law, H.K.W. Proteomic profiling of metabolic proteins as potential biomarkers of radioresponsiveness for colorectal cancer. J. Proteom. 2022, 262, 104600. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zheng, H.C.; Zhang, Z.G. Roles of Fascin mRNA expression in colorectal cancer: Meta-analysis and bioinformatics analysis. Mol. Clin. Oncol. 2020, 13, 119–128. [Google Scholar] [CrossRef]
- Zhou, J.; Xie, Z.; Cui, P.; Su, Q.; Zhang, Y.; Luo, L.; Li, Z.; Ye, L.; Liang, H.; Huang, J. SLC1A1, SLC16A9, and CNTN3 are potential biomarkers for the occurrence of colorectal cancer. Biomed. Res. Int. 2020, 2020, 1204605. [Google Scholar] [CrossRef] [PubMed]
- Komor, M.A.; de Wit, M.; van den Berg, J.; Martens de Kemp, S.R.; Delis-van Diemen, P.M.; Bolijn, A.S.; Tijssen, M.; Schelfhorst, T.; Piersma, S.R.; Chiasserini, D.; et al. Molecular characterization of colorectal adenomas reveals POFUT1 as a candidate driver of tumor progression. Int. J. Cancer 2020, 146, 1979–1992. [Google Scholar] [CrossRef]
- Fan, Y.; Guo, L.; Zheng, H.; Ji, C.; Wang, W.; Sun, H. BMP-9 is a novel marker for colorectal tumorigenesis undergoing the normal mucosa-adenoma-adenocarcinoma sequence and is associated with colorectal cancer prognosis. Oncol. Lett. 2020, 19, 271–282. [Google Scholar] [CrossRef] [Green Version]
- An, N.; Zhao, C.; Yu, Z.; Yang, X. Identification of prognostic genes in colorectal cancer through transcription profiling of multi-stage carcinogenesis. Oncol. Lett. 2019, 17, 432–441. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Shen, X.; Cui, X.; Guo, Y. Identification of novel biomarkers and small molecule drugs in human colorectal cancer by microarray and bioinformatics analysis. Mol. Genet Genomic. Med. 2019, 7, e00713. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wu, W.; Qu, H. Integrated analysis of the gene expression changes during colorectal cancer progression by bioinformatic methods. J. Comput. Biol. 2019, 26, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Zeng, Q.; Jiang, W.; Xie, J.; Cheng, J.; Yan, H.; He, J.; Xu, Y.; Guan, G.; Guo, Z.; et al. A qualitative transcriptional signature for the risk assessment of precancerous colorectal lesions. Front. Genet. 2020, 11, 573787. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Pan, Z.; Shi, Q.; Zhang, X.; Shen, W.; Huo, L.; Guo, H.; Tang, C.; Ling, Y. Bioinformatics profiling and experimental validation of 4 differentially-expressed LIM genes in the course of colorectal-adenoma-carcinoma. Med. Sci. Monit. 2022, 28, e937081. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Xu, X.Y. PI3K/Akt/mTOR signaling pathway in cancer stem cells: From basic research to clinical application. Am. J. Cancer Res. 2015, 5, 1602–1609. [Google Scholar]
- Yoon, C.; Lu, J.; Yi, B.C.; Chang, K.K.; Simon, M.C.; Ryeom, S.; Yoon, S.S. PI3K/Akt pathway and Nanog maintain cancer stem cells in sarcomas. Oncogenesis 2021, 10, 12. [Google Scholar] [CrossRef]
- Karami Fath, M.; Ebrahimi, M.; Nourbakhsh, E.; Zia Hazara, A.; Mirzaei, A.; Shafieyari, S.; Salehi, A.; Hoseinzadeh, M.; Payandeh, Z.; Barati, G. PI3K/Akt/mTOR signaling pathway in cancer stem cells. Pathol. Res. Pract. 2022, 237, 154010. [Google Scholar] [CrossRef]
- Choi, J.; Pacheco, C.M.; Mosbergen, R.; Korn, O.; Chen, T.; Nagpal, I.; Englart, S.; Angel, P.W.; Wells, C.A. Stemformatics: Visualize and download curated stem cell data. Nucleic Acids Res. 2019, 47, D841–D846. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.S.; Huang, T.; Zhong, X.M.; Zhang, H.W.; Cong, X.L.; Xu, H.; Lu, G.X.; Yu, F.; Xue, S.B.; Lv, Z.W.; et al. Proteogenomic characterization and comprehensive integrative genomic analysis of human colorectal cancer liver metastasis. Mol. Cancer 2018, 17, 139. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhao, L.M.; Li, S.L.; Li, J.; Gao, B.; Wang, F.F.; Wang, S.P.; Hu, X.H.; Cao, J.; Wang, G.Y. Differentially expressed lncRNAs and mRNAs identified by NGS analysis in colorectal cancer patients. Cancer Med. 2018, 7, 4650–4664. [Google Scholar] [CrossRef] [Green Version]
- Kanth, P.; Bronner, M.P.; Boucher, K.M.; Burt, R.W.; Neklason, D.W.; Hagedorn, C.H.; Delker, D.A. Gene Signature in sessile serrated polyps identifies colon cancer subtype. Cancer Prev. Res. 2016, 9, 456–465. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Z.; Ge, W.; Shou, J.; You, L.; Pan, H.; Han, W. Analysis of potential genes and pathways associated with the colorectal normal mucosa-adenoma-carcinoma sequence. Cancer Med. 2018, 7, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Druliner, B.R.; Wang, P.; Bae, T.; Baheti, S.; Slettedahl, S.; Mahoney, D.; Vasmatzis, N.; Xu, H.; Kim, M.; Bockol, M.; et al. Molecular characterization of colorectal adenomas with and without malignancy reveals distinguishing genome, transcriptome and methylome alterations. Sci. Rep. 2018, 8, 3161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Žlajpah, M.; Hauptman, N.; Boštjančič, E.; Zidar, N. Differential expression of extracellular matrixrelated genes DCN, EPHA4, FN1, SPARC, SPON2 and SPP1 in colorectal carcinogenesis. Oncol. Rep. 2019, 42, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Kesh, K.; Gupta, V.K.; Durden, B.; Garrido, V.; Mateo-Victoriano, B.; Lavania, S.P.; Banerjee, S. Therapy resistance, cancer stem cells and ECM in cancer: The matrix reloaded. Cancers 2020, 12, 3067. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Wang, J.; Shen, Y.; Tang, X.; Yu, F.; Wang, R. Expression and function of PPARs in cancer stem cells. Curr. Stem. Cell Res. Ther. 2016, 11, 226–234. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, C.; Feng, Y.; Qi, W.; Cui, Y.; Xuan, Y. Tenascin-C is involved in promotion of cancer stemness via the Akt/HIF1a axis in esophageal squamous cell carcinoma. Exp. Mol. Pathol. 2019, 109, 104239. [Google Scholar] [CrossRef]
- Efremov, Y.R.; Proskurina, A.S.; Potter, E.A.; Dolgova, E.V.; Efremova, O.V.; Taranov, O.S.; Ostanin, A.A.; Chernykh, E.R.; Kolchanov, N.A.; Bogachev, S.S. Cancer Stem Cells: Emergent Nature of Tumor Emergency. Front. Genet. 2018, 9, 544. [Google Scholar] [CrossRef]
- Liot, S.; Aubert, A.; Hervieu, V.; Kholti, N.E.; Schalkwijk, J.; Verrier, B.; Valcourt, U.; Lambert, E. Loss of Tenascin-X expression during tumor progression: A new pan-cancer marker. Matrix Biol. Plus 2020, 6-7, 100021. [Google Scholar] [CrossRef]
- Devarajan, E.; Song, Y.H.; Krishnappa, S.; Alt, E. Epithelial-mesenchymal transition in breast cancer lines is mediated through PDGF-D released by tissue-resident stem cells. Int. J. Cancer 2012, 131, 1023–1031. [Google Scholar] [CrossRef]
- Huang, F.; Wang, M.; Yang, T.; Cai, J.; Zhang, Q.; Sun, Z.; Wu, X.; Zhang, X.; Zhu, W.; Qian, H.; et al. Gastric cancer-derived MSC-secreted PDGF-DD promotes gastric cancer progression. J. Cancer Res. Clin. Oncol. 2014, 140, 1835–1848. [Google Scholar] [CrossRef]
- Huang, Z.; Shen, G.; Gao, J. CDK1 promotes the stemness of lung cancer cells through interacting with Sox2. Clin. Transl. Oncol. 2021, 23, 1743–1751. [Google Scholar] [CrossRef]
- Miller, T.J.; McCoy, M.J.; Hemmings, C.; Iacopetta, B.; Platell, C.F. Expression of PD-L1 and SOX2 during rectal tumourigenesis: Potential mechanisms for immune escape and tumour cell invasion. Oncol. Lett. 2018, 16, 5761–5768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talebi, A.; Kianersi, K.; Beiraghdar, M. Comparison of gene expression of SOX2 and OCT4 in normal tissue, polyps, and colon adenocarcinoma using immunohistochemical staining. Adv. Biomed. Res. 2015, 4, 234. [Google Scholar] [CrossRef]
- Neganova, I.; Tilgner, K.; Buskin, A.; Paraskevopoulou, I.; Atkinson, S.P.; Peberdy, D.; Passos, J.F.; Lako, M. CDK1 plays an important role in the maintenance of pluripotency and genomic stability in human pluripotent stem cells. Cell Death Dis. 2014, 5, e1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Naydenov, N.G.; Dozmorov, M.G.; Koblinski, J.E.; Ivanov, A.I. Anillin regulates breast cancer cell migration, growth, and metastasis by non-canonical mechanisms involving control of cell stemness and differentiation. Breast Cancer Res. 2020, 22, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maryam, A.; Chin, Y.R. ANLN enhances triple-negative breast cancer stemness through TWIST1 and BMP2 and promotes its spheroid growth. Front Mol. Biosci. 2021, 8, 700973. [Google Scholar] [CrossRef]
- Gong, D.Y.; Chen, X.; Yang, T.L.; Wang, Y.; Guo, Y.; Zeng, J.H.; Chen, S.Z. Upregulation of ECT2 is associated with transcriptional program of cancer stem cells and predicts poor clinical outcome in gastric cancer. Oncol. Lett. 2020, 20, 54. [Google Scholar] [CrossRef]
- Egilmez, R.; Elagoz, S.; Kanik, E.A. CDK1/P34CDC2 and P21WAF expression in colorectal adenomas and carcinomas. J. Exp. Clin. Cancer Res. 2001, 20, 549–552. [Google Scholar]
- Cook, D.R.; Kang, M.; Martin, T.D.; Galanko, J.A.; Loeza, G.H.; Trembath, D.G.; Justilien, V.; Pickering, K.A.; Vincent, D.F.; Jarosch, A.; et al. Aberrant expression and subcellular localization of ECT2 drives colorectal cancer progression and growth. Cancer Res. 2022, 82, 90–104. [Google Scholar] [CrossRef]
- Hanamura, N.; Yoshida, T.; Matsumoto, E.; Kawarada, Y.; Sakakura, T. Expression of fibronectin and tenascin-C mRNA by myofibroblasts, vascular cells and epithelial cells in human colon adenomas and carcinomas. Int. J. Cancer 1997, 73, 10–15. [Google Scholar] [CrossRef]
- Sis, B.; Sagol, O.; Kupelioglu, A.; Sokmen, S.; Terzi, C.; Fuzun, M.; Ozer, E.; Bishop, P. Prognostic significance of matrix metalloproteinase-2, cathepsin D, and tenascin-C expression in colorectal carcinoma. Pathol. Res. Pract. 2004, 200, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Satow, R.; Inagaki, S.; Kato, C.; Shimozawa, M.; Fukami, K. Identification of zinc finger protein of the cerebellum 5 as a survival factor of prostate and colorectal cancer cells. Cancer Sci. 2017, 108, 2405–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Chen, J.; Yuan, W.; Ji, J.; Liu, Z.; Wu, L.; Tang, Q.; Shu, X. Platelet-derived growth factor-D promotes colorectal cancer cell migration, invasion and proliferation by regulating Notch1 and matrix metalloproteinase-9. Oncol. Lett. 2018, 15, 1573–1579. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Shen, W.; Cui, L.; Chen, W.; Hu, X.; Fu, J. Overexpression of Anillin (ANLN) is correlated with colorectal cancer progression and poor prognosis. Cancer Biomark. 2016, 16, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, P.; Cao, F.; Wang, S.; He, Y.; Xu, Y.; Wang, Y. ANLN, Regulated by SP2, promotes colorectal carcinoma cell proliferation via PI3K/AKT and MAPK Signaling Pathway. J. Invest. Surg. 2022, 35, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.W.; Lin, Y.M.; Wu, P.R.; Yen, H.H.; Lai, H.W.; Su, T.C.; Huang, R.H.; Wen, C.K.; Chen, C.Y.; Chen, C.J.; et al. High nuclear/cytoplasmic ratio of CDK1 expression predicts poor prognosis in colorectal cancer patients. BMC Cancer 2014, 14, 951. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Kawakami, H.; Liu, W.; Zeng, X.; Strebhardt, K.; Tao, K.; Huang, S.; Sinicrope, F.A. Targeting CDK1 and MEK/ERK overcomes apoptotic resistance in BRAF-mutant human colorectal cancer. Mol. Cancer Res. 2018, 16, 378–389. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cai, X.; Chen, B.; Gu, H.; Liu, C. Overexpression of epithelial cell transforming 2 protein in colorectal carcinoma predicts a poor prognosis. Exp. Ther. Med. 2017, 14, 4862–4868. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Qin, S.L.; Mu, Y.F.; Wang, Z.S.; Zhong, M.; Bian, Z.Q. Elevated expression of ECT2 predicts unfavorable prognosis in patients with colorectal cancer. Biomed. Pharmacother. 2015, 73, 135–139. [Google Scholar] [CrossRef]
- Koh, H.M.; Jang, B.G.; Hyun, C.L.; Kim, Y.S.; Hyun, J.W.; Chang, W.Y.; Maeng, Y.H. Aurora kinase a is a prognostic marker in colorectal adenocarcinoma. J. Pathol. Transl. Med. 2017, 51, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Shan, B.; Zhao, R.; Zhou, J.; Zhang, M.; Qi, X.; Wang, T.; Gong, J.; Wu, Y.; Zhu, Y.; Yang, W.; et al. AURKA increase the chemosensitivity of colon cancer cells to oxaliplatin by inhibiting the TP53-mediated dna damage response genes. Biomed. Res. Int. 2020, 2020, 8916729. [Google Scholar] [CrossRef] [PubMed]
- Goos, J.A.; Coupe, V.M.; Diosdado, B.; Delis-Van Diemen, P.M.; Karga, C.; Belien, J.A.; Carvalho, B.; van den Tol, M.P.; Verheul, H.M.; Geldof, A.A.; et al. Aurora kinase A (AURKA) expression in colorectal cancer liver metastasis is associated with poor prognosis. Br. J. Cancer 2013, 109, 2445–2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Song, G.; Xiang, J.; Zhang, H.; Zhao, S.; Zhan, Y. AURKA promotes cancer metastasis by regulating epithelial-mesenchymal transition and cancer stem cell properties in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2017, 486, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Yue, C.; Li, G.; He, B.; Cheng, W.; Wang, X.; Yan, M.; Long, Z.; Qiu, W.; Yuan, Z.; et al. Nuclear AURKA acquires kinase-independent transactivating function to enhance breast cancer stem cell phenotype. Nat. Commun. 2016, 7, 10180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.K.; Wang, Q.Q.; Huang, J.L.; Zhang, L.B.; Zhou, X.; Liu, J.Q.; Chen, Z.J.; Liao, X.W.; Huang, R.; Yang, C.K.; et al. Novel candidate biomarkers of origin recognition complex 1, 5 and 6 for survival surveillance in patients with hepatocellular carcinoma. J. Cancer 2020, 11, 1869–1882. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Ho, J.N.; Jin, H.; Lee, S.C.; Lee, S.E.; Hong, S.K.; Lee, J.W.; Lee, E.S.; Byun, S.S. Upregulated expression of BCL2, MCM7, and CCNE1 indicate cisplatin-resistance in the set of two human bladder cancer cell lines: T24 cisplatin sensitive and T24R2 cisplatin resistant bladder cancer cell lines. Investig. Clin. Urol. 2016, 57, 63–72. [Google Scholar] [CrossRef]
- Shibata, E.; Dutta, A. A human cancer cell line initiates DNA replication normally in the absence of ORC5 and ORC2 proteins. J. Biol. Chem. 2020, 295, 16949–16959. [Google Scholar] [CrossRef]
- Song, S.; Wang, Y.; Liu, P. DNA replication licensing factors: Novel targets for cancer therapy via inhibiting the stemness of cancer cells. Int. J. Biol. Sci. 2022, 18, 1211–1219. [Google Scholar] [CrossRef]
- Shi, Y.; Ge, C.; Fang, D.; Wei, W.; Li, L.; Wei, Q.; Yu, H. NCAPG facilitates colorectal cancer cell proliferation, migration, invasion and epithelial-mesenchymal transition by activating the Wnt/beta-catenin signaling pathway. Cancer Cell Int. 2022, 22, 119. [Google Scholar] [CrossRef]
- Li, P.; Wen, J.; Ren, X.; Zhou, Y.; Xue, Y.; Yan, Z.; Li, S.; Tian, H.; Tang, X.G.; Zhang, G.J. MicroRNA-23b-3p targets non-SMC condensing I complex subunit G to promote proliferation and inhibit apoptosis of colorectal cancer cells via regulation of the PI3K/AKT signaling pathway. Oncol. Lett. 2021, 22, 812. [Google Scholar] [CrossRef]
- Sanz-Pamplona, R.; Berenguer, A.; Cordero, D.; Mollevi, D.G.; Crous-Bou, M.; Sole, X.; Pare-Brunet, L.; Guino, E.; Salazar, R.; Santos, C.; et al. Aberrant gene expression in mucosa adjacent to tumor reveals a molecular crosstalk in colon cancer. Mol. Cancer 2014, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Polley, A.C.; Mulholland, F.; Pin, C.; Williams, E.A.; Bradburn, D.M.; Mills, S.J.; Mathers, J.C.; Johnson, I.T. Proteomic analysis reveals field-wide changes in protein expression in the morphologically normal mucosa of patients with colorectal neoplasia. Cancer Res. 2006, 66, 6553–6562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Smyth, G.K.; Shi, W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019, 47, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Shi, W. Read trimming is not required for mapping and quantification of RNA-seq reads at the gene level. NAR Genom. Bioinform. 2020, 2, lqaa068. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.; Carey, V.J.; Gentleman, R.; Anders, S.; Carlson, M.; Carvalho, B.S.; Bravo, H.C.; Davis, S.; Gatto, L.; Girke, T.; et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 2015, 12, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Meltzer, P.S. GEOquery: A bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 2007, 23, 1846–1847. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, D.J.; Chen, Y.; Smyth, G.K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012, 40, 4288–4297. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [Green Version]
- Latham, G.J. Normalization of microRNA quantitative RT-PCR data in reduced scale experimental designs. Methods Mol. Biol. 2010, 667, 19–31. [Google Scholar] [CrossRef] [PubMed]
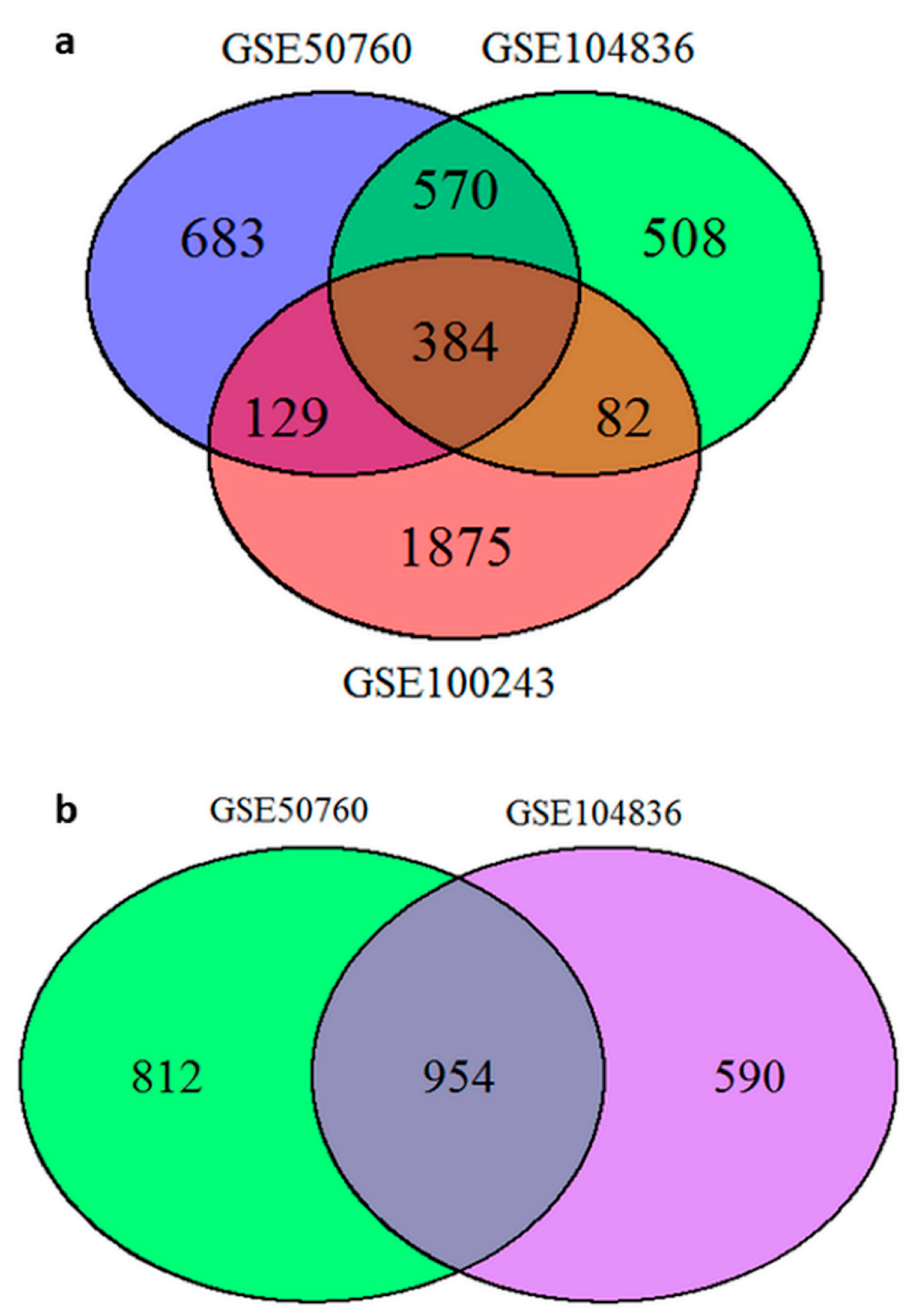





| Project Number | Comparison | Number of DEGs | Additional Data |
|---|---|---|---|
| GSE72820 | 7 adenomas and corresponding normal tissue | / | / |
| GSE76987 | 8 adenomas and corresponding normal tissue | 3 * | Supplementary File S1, table GSE76987 |
| GSE104836 | 10 carcinomas and corresponding normal tissue | 1544 | Supplementary File S1, table GSE104836 |
| GSE92914 | 3 carcinomas and corresponding normal tissue | / | / |
| GSE100243 | 6 carcinomas and corresponding normal tissue | 2470 | Supplementary File S1, table GSE100243 |
| GSE50760 | 18 carcinomas and corresponding normal tissue | 1766 | Supplementary File S1, table GSE50760 |
| TNC | Normal Mucosa | Adenoma | Adenoma with Early Carcinoma | CRC N0 | CRC N+ |
|---|---|---|---|---|---|
| Normal mucosa | 1 | 0.000 | / | 0.039 | / |
| Adenoma | 1 | 0.012 | 0.000 | 0.002 | |
| Adenoma with early carcinoma | 1 | 0.003 | / | ||
| CRC N0 | 1 | / | |||
| CRC N+ | 1 | ||||
| TNXB | Normal mucosa | Adenoma | Adenoma with early carcinoma | CRC N0 | CRC N+ |
| Normal mucosa | 1 | 0.000 | 0.000 | / | / |
| Adenoma | 1 | / | 0.000 | 0.001 | |
| Adenoma with early carcinoma | 1 | 0.000 | 0.004 | ||
| CRC N0 | 1 | / | |||
| CRC N+ | 1 | ||||
| PDGFD | Normal mucosa | Adenoma | Adenoma with early carcinoma | CRC N0 | CRC N+ |
| Normal mucosa | 1 | / | / | / | 0.008 |
| Adenoma | 1 | / | 0.018 | 0.003 | |
| Adenoma with early carcinoma | 1 | 0.029 | 0.000 | ||
| CRC N0 | 1 | 0.03 | |||
| CRC N+ | 1 | ||||
| CDK1 | Normal mucosa | Adenoma | Adenoma with early carcinoma | CRC N0 | CRC N+ |
| Normal mucosa | 1 | / | 0.006 | / | 0.042 |
| Adenoma | 1 | 0.027 | / | / | |
| Adenoma with early carcinoma | 1 | / | / | ||
| CRC N0 | 1 | / | |||
| CRC N+ | 1 | ||||
| ANLN | Normal mucosa | Adenoma | Adenoma with early carcinoma | CRC N0 | CRC N+ |
| Normal mucosa | 1 | / | 0.000 | / | 0.019 |
| Adenoma | 1 | 0.000 | / | / | |
| Adenoma with early carcinoma | 1 | / | / | ||
| CRC N0 | 1 | 0.046 | |||
| CRC N+ | 1 | ||||
| ECT2 | Normal mucosa | Adenoma | Adenoma with early carcinoma | CRC N0 | CRC N+ |
| Normal mucosa | 1 | 0.000 | 0.000 | / | / |
| Adenoma | 1 | 0.002 | / | 0.02 | |
| Adenoma with early carcinoma | 1 | 0.023 | 0.001 | ||
| CRC N0 | 1 | / | |||
| CRC N+ | 1 |
| Project | Platform | Tissue type | Normal | Adenoma | Carcinoma |
|---|---|---|---|---|---|
| GSE72820 | Illumina | No data | 7 | 7 | 0 |
| GSE104836 | Illumina | Fresh frozen | 10 | 0 | 10 |
| GSE50760 | Illumina | No data | 18 | 0 | 18 |
| GSE76987 | Illumina | RNA-later | 8 | 8 | 0 |
| GSE92914 | Illumina | Fresh frozen | 3 | 0 | 3 |
| GSE100243 | Ion Torrent | FFPE | 6 | 0 | 8 |
| Patients | Adenoma | Adenoma with Early Carcinoma | CRC without Lymph Node Metastases | CRC with Lymph Node Metastases |
|---|---|---|---|---|
| M:F | 10:1 | 9:4 | 4:6 | 9:4 |
| Age | 62.3 ± 10.7 | 64.9 ± 5.7 | 72.7 ± 11.6 | 73.2 ± 11.8 |
| Gene | Entrez Gene ID | Assay ID |
|---|---|---|
| B2M | 567 | Hs99999907_m1 |
| IPO8 | 10526 | Hs00183533_m1 |
| TNC | 3371 | Hs01115665_m1 |
| TNXB | 7148 | Hs00372889_g1 |
| PDGFD | 80310 | Hs00228671_m1 |
| CDK1 | 983 | Hs00364293_m1 |
| ANLN | 54443 | Hs01122612_m1 |
| ECT2 | 1894 | Hs00978168_m1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urh, K.; Zidar, N.; Boštjančič, E. Bioinformatics Analysis of RNA-seq Data Reveals Genes Related to Cancer Stem Cells in Colorectal Cancerogenesis. Int. J. Mol. Sci. 2022, 23, 13252. https://doi.org/10.3390/ijms232113252
Urh K, Zidar N, Boštjančič E. Bioinformatics Analysis of RNA-seq Data Reveals Genes Related to Cancer Stem Cells in Colorectal Cancerogenesis. International Journal of Molecular Sciences. 2022; 23(21):13252. https://doi.org/10.3390/ijms232113252
Chicago/Turabian StyleUrh, Kristian, Nina Zidar, and Emanuela Boštjančič. 2022. "Bioinformatics Analysis of RNA-seq Data Reveals Genes Related to Cancer Stem Cells in Colorectal Cancerogenesis" International Journal of Molecular Sciences 23, no. 21: 13252. https://doi.org/10.3390/ijms232113252





