Leukocyte Telomere Length as Potential Biomarker of HD Progression: A Follow-Up Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample
4.2. Laboratory Methods
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Futter, M.; Diekmann, H.; Schoenmakers, E.; Sadiq, O.; Chatterjee, K.; Rubinsztein, D.C. Wild-Type but Not Mutant Huntingtin Modulates the Transcriptional Activity of Liver X Receptors. J. Med. Genet. 2009, 46, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Schulte, J.; Littleton, J.T. The Biological Function of the Huntingtin Protein and Its Relevance to Huntington’s Disease Pathology. Curr. Trends Neurol. 2011, 5, 65–78. [Google Scholar] [PubMed]
- Bates, G.P.; Dorsey, R.; Gusella, J.F.; Hayden, M.R.; Kay, C.; Leavitt, B.R.; Nance, M.; Ross, C.A.; Scahill, R.I.; Wetzel, R.; et al. Huntington Disease. Nat. Rev. Dis. Primer 2015, 1, 15005. [Google Scholar] [CrossRef]
- Myers, R.H. Huntington’s Disease Genetics. NeuroRx, J. Am. Soc. Exp. Neurother. 2004, 1, 255–262. [Google Scholar] [CrossRef]
- Wexler, N.S.; Lorimer, J.; Porter, J.; Gomez, F.; Moskowitz, C.; Shackell, E.; Marder, K.; Penchaszadeh, G.; Roberts, S.A.; Gayán, J.; et al. Venezuelan Kindreds Reveal That Genetic and Environmental Factors Modulate Huntington’s Disease Age of Onset. Proc. Natl. Acad. Sci. USA 2004, 101, 3498–3503. [Google Scholar] [CrossRef] [PubMed]
- Gusella, J.F.; MacDonald, M.E.; Lee, J.-M. Genetic Modifiers of Huntington’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 1359–1365. [Google Scholar] [CrossRef]
- Wright, G.E.B.; Collins, J.A.; Kay, C.; McDonald, C.; Dolzhenko, E.; Xia, Q.; Bečanović, K.; Drögemöller, B.I.; Semaka, A.; Nguyen, C.M.; et al. Length of Uninterrupted CAG, Independent of Polyglutamine Size, Results in Increased Somatic Instability, Hastening Onset of Huntington Disease. Am. J. Hum. Genet. 2019, 104, 1116–1126. [Google Scholar] [CrossRef]
- Langbehn, D.R.; Brinkman, R.R.; Falush, D.; Paulsen, J.S.; Hayden, M.R.; International Huntington’s Disease Collaborative Group. A New Model for Prediction of the Age of Onset and Penetrance for Huntington’s Disease Based on CAG Length. Clin. Genet. 2004, 65, 267–277. [Google Scholar] [CrossRef]
- Langbehn, D.R.; Hayden, M.R.; Paulsen, J.S.; the PREDICT-HD Investigators of the Huntington Study Group. CAG-Repeat Length and the Age of Onset in Huntington Disease (HD): A Review and Validation Study of Statistical Approaches. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2010, 153B, 397–408. [Google Scholar] [CrossRef]
- Silajdžić, E.; Björkqvist, M. A Critical Evaluation of Wet Biomarkers for Huntington’s Disease: Current Status and Ways Forward. J. Huntingt. Dis. 2018, 7, 109–135. [Google Scholar] [CrossRef]
- Tang, H.; van Eimeren, T.; Sampaio, C.; Mestre, T.A. Validation of Biomarkers in Huntington Disease to Support the Development of Disease-Modifying Therapies: A Systematic Review and Critical Appraisal Scheme. Parkinsonism Relat. Disord. 2021, 93, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.Y.; Collins, K. Telomere Maintenance and Disease. Lancet Lond. Engl. 2003, 362, 983–988. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Greider, C.W.; Szostak, J.W. Telomeres and Telomerase: The Path from Maize, Tetrahymena and Yeast to Human Cancer and Aging. Nat. Med. 2006, 12, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human Telomere Biology: A Contributory and Interactive Factor in Aging, Disease Risks, and Protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Daniali, L.; Benetos, A.; Susser, E.; Kark, J.D.; Labat, C.; Kimura, M.; Desai, K.; Granick, M.; Aviv, A. Telomeres Shorten at Equivalent Rates in Somatic Tissues of Adults. Nat. Commun. 2013, 4, 1597. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Epel, E. Stress and Telomere Shortening: Insights from Cellular Mechanisms. Ageing Res. Rev. 2022, 73, 101507. [Google Scholar] [CrossRef]
- Yeh, J.-K.; Wang, C.-Y. Telomeres and Telomerase in Cardiovascular Diseases. Genes 2016, 7, 58. [Google Scholar] [CrossRef]
- Cheng, F.; Carroll, L.; Joglekar, M.V.; Januszewski, A.S.; Wong, K.K.; Hardikar, A.A.; Jenkins, A.J.; Ma, R.C.W. Diabetes, Metabolic Disease, and Telomere Length. Lancet Diabetes Endocrinol. 2021, 9, 117–126. [Google Scholar] [CrossRef]
- Gavia-García, G.; Rosado-Pérez, J.; Arista-Ugalde, T.L.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Telomere Length and Oxidative Stress and Its Relation with Metabolic Syndrome Components in the Aging. Biology 2021, 10, 253. [Google Scholar] [CrossRef]
- Epel, E.S.; Prather, A.A. Stress, Telomeres, and Psychopathology: Toward a Deeper Understanding of a Triad of Early Aging. Annu. Rev. Clin. Psychol. 2018, 14, 371–397. [Google Scholar] [CrossRef]
- Habib, R.; Ocklenburg, S.; Hoffjan, S.; Haghikia, A.; Epplen, J.T.; Arning, L. Association between Shorter Leukocyte Telomeres and Multiple Sclerosis. J. Neuroimmunol. 2020, 341, 577187. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Pelini, L.; Ercolani, S.; Ruggiero, C.; Mecocci, P. From Cellular Senescence to Alzheimer’s Disease: The Role of Telomere Shortening. Ageing Res. Rev. 2015, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huo, Y.R.; Wang, J.; Wang, C.; Liu, S.; Liu, S.; Wang, J.; Ji, Y. Telomere Shortening in Alzheimer’s Disease Patients. Ann. Clin. Lab. Sci. 2016, 46, 260–265. [Google Scholar] [PubMed]
- Scarabino, D.; Broggio, E.; Gambina, G.; Corbo, R.M. Leukocyte Telomere Length in Mild Cognitive Impairment and Alzheimer’s Disease Patients. Exp. Gerontol. 2017, 98, 143–147. [Google Scholar] [CrossRef]
- Scarabino, D.; Peconi, M.; Broggio, E.; Gambina, G.; Maggi, E.; Armeli, F.; Mantuano, E.; Morello, M.; Corbo, R.M.; Businaro, R. Relationship between Proinflammatory Cytokines (Il-1beta, Il-18) and Leukocyte Telomere Length in Mild Cognitive Impairment and Alzheimer’s Disease. Exp. Gerontol. 2020, 136, 110945. [Google Scholar] [CrossRef]
- Scarabino, D.; Veneziano, L.; Peconi, M.; Frontali, M.; Mantuano, E.; Corbo, R.M. Leukocyte Telomere Shortening in Huntington’s Disease. J. Neurol. Sci. 2019, 396, 25–29. [Google Scholar] [CrossRef]
- Scarabino, D.; Veneziano, L.; Fiore, A.; Nethisinghe, S.; Mantuano, E.; Garcia-Moreno, H.; Bellucci, G.; Solanky, N.; Morello, M.; Zanni, G.; et al. Leukocyte Telomere Length Variability as a Potential Biomarker in Patients with PolyQ Diseases. Antioxid. Basel Switz. 2022, 11, 1436. [Google Scholar] [CrossRef]
- Forero, D.A.; González-Giraldo, Y.; López-Quintero, C.; Castro-Vega, L.J.; Barreto, G.E.; Perry, G. Telomere Length in Parkinson’s Disease: A Meta-Analysis. Exp. Gerontol. 2016, 75, 53–55. [Google Scholar] [CrossRef]
- Kota, L.N.; Bharath, S.; Purushottam, M.; Moily, N.S.; Sivakumar, P.T.; Varghese, M.; Pal, P.K.; Jain, S. Reduced Telomere Length in Neurodegenerative Disorders May Suggest Shared Biology. J. Neuropsychiatry Clin. Neurosci. 2015, 27, e92–e96. [Google Scholar] [CrossRef]
- Castaldo, I.; De Rosa, M.; Romano, A.; Zuchegna, C.; Squitieri, F.; Mechelli, R.; Peluso, S.; Borrelli, C.; Del Mondo, A.; Salvatore, E.; et al. DNA Damage Signatures in Peripheral Blood Cells as Biomarkers in Prodromal Huntington Disease. Ann. Neurol. 2019, 85, 296–301. [Google Scholar] [CrossRef]
- PerezGrovas-Saltijeral, A.; Ochoa-Morales, A.; Miranda-Duarte, A.; Martínez-Ruano, L.; Jara-Prado, A.; Camacho-Molina, A.; Hidalgo-Bravo, A. Telomere Length Analysis on Leukocytes Derived from Patients with Huntington Disease. Mech. Ageing Dev. 2020, 185, 111189. [Google Scholar] [CrossRef] [PubMed]
- Andre, R.; Carty, L.; Tabrizi, S.J. Disruption of Immune Cell Function by Mutant Huntingtin in Huntington’s Disease Pathogenesis. Curr. Opin. Pharmacol. 2016, 26. [Google Scholar] [CrossRef] [PubMed]
- Mina, E.; van Roon-Mom, W.; Hettne, K.; van Zwet, E.; Goeman, J.; Neri, C.; A.C.’t Hoen, P.; Mons, B.; Roos, M. Common Disease Signatures from Gene Expression Analysis in Huntington’s Disease Human Blood and Brain. Orphanet J. Rare Dis. 2016, 11, 97. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Scahill, R.I.; Owen, G.; Durr, A.; Leavitt, B.R.; Roos, R.A.; Borowsky, B.; Landwehrmeyer, B.; Frost, C.; Johnson, H.; et al. Predictors of Phenotypic Progression and Disease Onset in Premanifest and Early-Stage Huntington’s Disease in the TRACK-HD Study: Analysis of 36-Month Observational Data. Lancet Neurol. 2013, 12, 637–649. [Google Scholar] [CrossRef]
- Paulsen, J.S.; Long, J.D.; Ross, C.A.; Harrington, D.L.; Erwin, C.J.; Williams, J.K.; Westervelt, H.J.; Johnson, H.J.; Aylward, E.H.; Zhang, Y.; et al. Prediction of Manifest Huntington’s Disease with Clinical and Imaging Measures: A Prospective Observational Study. Lancet Neurol. 2014, 13, 1193–1201. [Google Scholar] [CrossRef]
- Müezzinler, A.; Zaineddin, A.K.; Brenner, H. A Systematic Review of Leukocyte Telomere Length and Age in Adults. Ageing Res. Rev. 2013, 12, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Byrne, L.M.; Rodrigues, F.B.; Johnson, E.B.; Wijeratne, P.A.; De Vita, E.; Alexander, D.C.; Palermo, G.; Czech, C.; Schobel, S.; Scahill, R.I.; et al. Evaluation of Mutant Huntingtin and Neurofilament Proteins as Potential Markers in Huntington’s Disease. Sci. Transl. Med. 2018, 10, eaat7108. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere Measurement by Quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
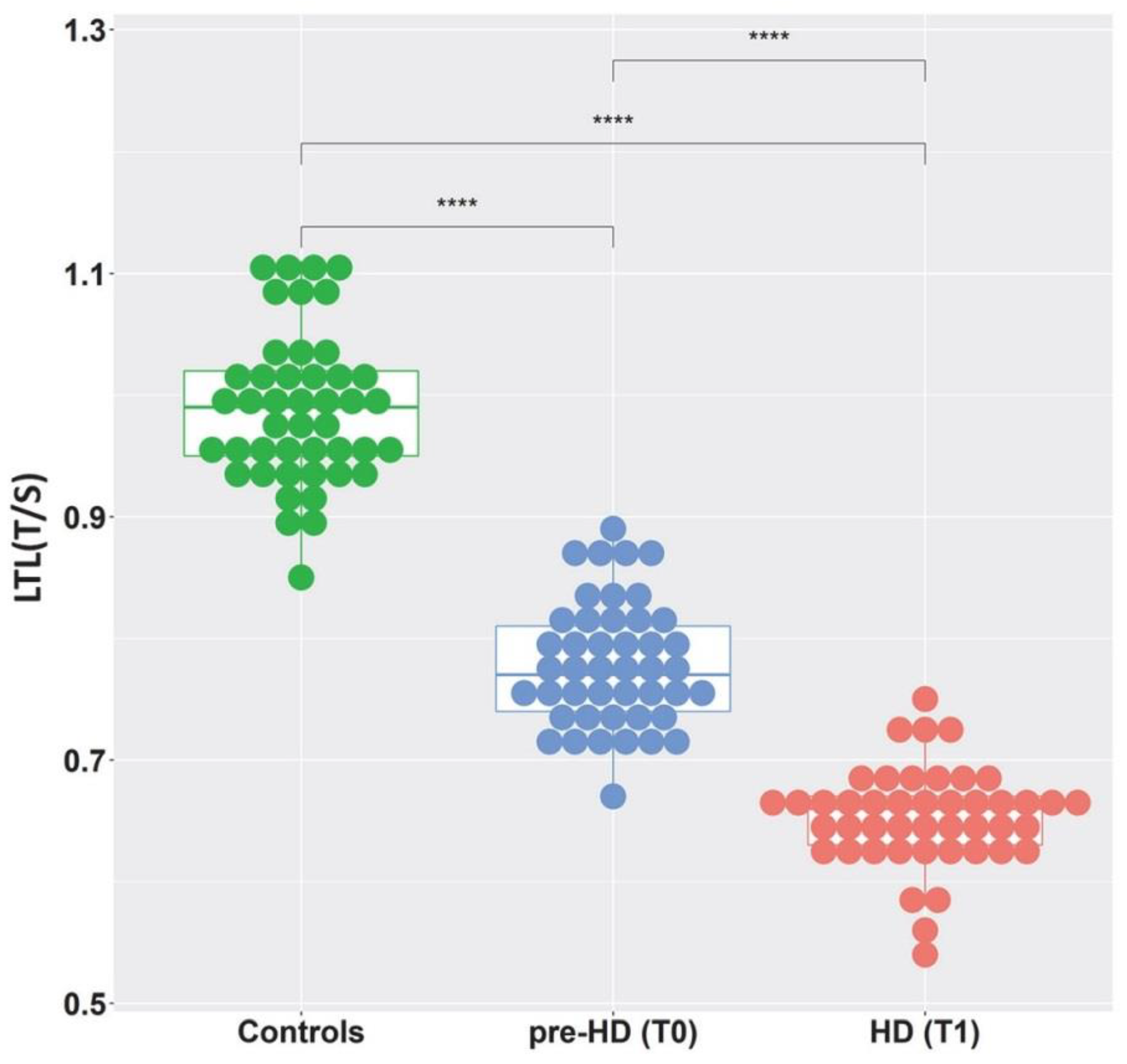
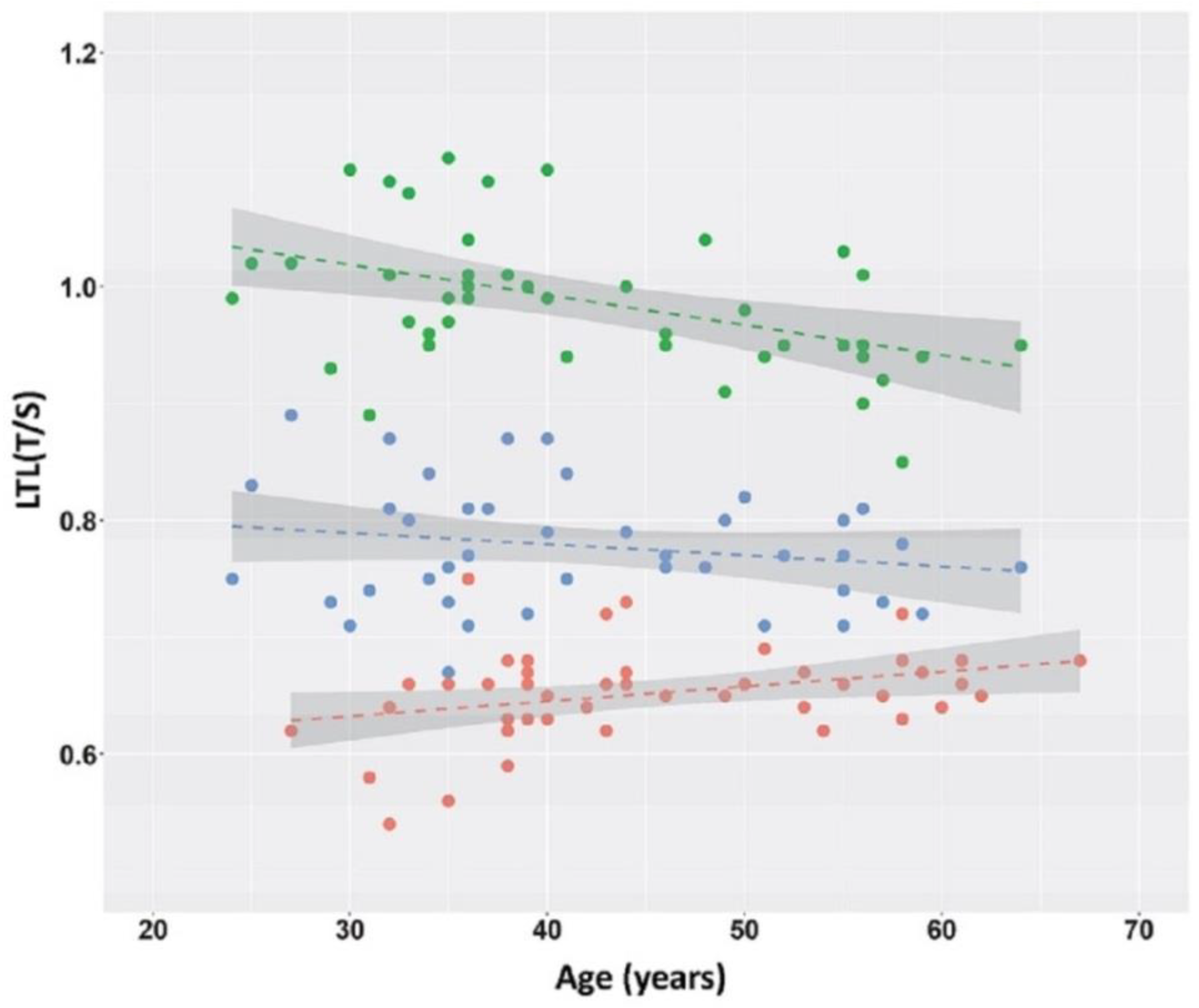



| Controls N = 45 | Pre-HD (T0) N = 45 | HD (T1) N = 45 | |
|---|---|---|---|
| Age at blood sampling (years) | 41.9 ± 10.5 | 41.9 ± 10.4 | 45.3 ± 10.2 |
| Sex (males, %) | 32.4 | 42.1 | 42.1 |
| Median CAG repeat (range) | NA | 43 (39–51) | 43 (39–51) |
| TMS | NA | 3.3 ± 2.4 | 13.2 ± 5.2 |
| TFC | NA | 12.5± 1.2 | 11.8 ± 2.1 |
| Age at onset (years) | NA | NA | 44.4 ± 10.7 |
| CAG Repeat (n.) | Age at T0 (years) | LTL at T0 pre-HD | LTL at T1 HD | LTL Reduction (T/S) |
|---|---|---|---|---|
| Total (45) | 40 (34–51.5) | 0.77 (0.74–0.81) | 0.66 (0.63–0.67) | 0.12(0.09–0.16) |
| ≤40 (6) | 57.5 (55–60.3) | 0.77 (0.73–0.79) | 0.68 (0.68–0.69) | 0.08 (0.07–0.11) |
| 41 (6) | 53.5 (46.8–55.3) | 0.79 (0.76–0.83) | 0.66 (0.64–0.69) | 0.13(0.08–0.16) |
| 42 (8) | 43.5 (40.3–49.0) | 0.80 (0.75–0.86) | 0.67 (0.64–0.72) | 0.12(0.10–0.14) |
| 43 (7) | 36.0 (33.0–44.0) | 0.79 (0.75–0.87) | 0.65 (0.63–0.66) | 0.12 (0.09–0.21) |
| 44 (6) | 35.0 (33.8–39.0) | 0.74 (0.70–0.77) | 0.65 (0.61–0.68) | 0.09(0.06–0.12) |
| 45 (6) | 32.5 (28.0–37.8) | 0.80 (0.74–0.83) | 0.61 (0.56–67) | 0.18 (0.15–0.21) |
| ≥46(6) | 33.5 (28.5–36.8) | 0.76 (0.72–0.81) | 0.65 (0.63–0.66) | 0.13(0.07–0.15) |
| p | p < 0.00001 | p = 0.26 | p = 0.15 | p = 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarabino, D.; Veneziano, L.; Mantuano, E.; Arisi, I.; Fiore, A.; Frontali, M.; Corbo, R.M. Leukocyte Telomere Length as Potential Biomarker of HD Progression: A Follow-Up Study. Int. J. Mol. Sci. 2022, 23, 13449. https://doi.org/10.3390/ijms232113449
Scarabino D, Veneziano L, Mantuano E, Arisi I, Fiore A, Frontali M, Corbo RM. Leukocyte Telomere Length as Potential Biomarker of HD Progression: A Follow-Up Study. International Journal of Molecular Sciences. 2022; 23(21):13449. https://doi.org/10.3390/ijms232113449
Chicago/Turabian StyleScarabino, Daniela, Liana Veneziano, Elide Mantuano, Ivan Arisi, Alessia Fiore, Marina Frontali, and Rosa Maria Corbo. 2022. "Leukocyte Telomere Length as Potential Biomarker of HD Progression: A Follow-Up Study" International Journal of Molecular Sciences 23, no. 21: 13449. https://doi.org/10.3390/ijms232113449
APA StyleScarabino, D., Veneziano, L., Mantuano, E., Arisi, I., Fiore, A., Frontali, M., & Corbo, R. M. (2022). Leukocyte Telomere Length as Potential Biomarker of HD Progression: A Follow-Up Study. International Journal of Molecular Sciences, 23(21), 13449. https://doi.org/10.3390/ijms232113449







