Microbiota Alterations in Patients with Autoimmune Thyroid Diseases: A Systematic Review
Abstract
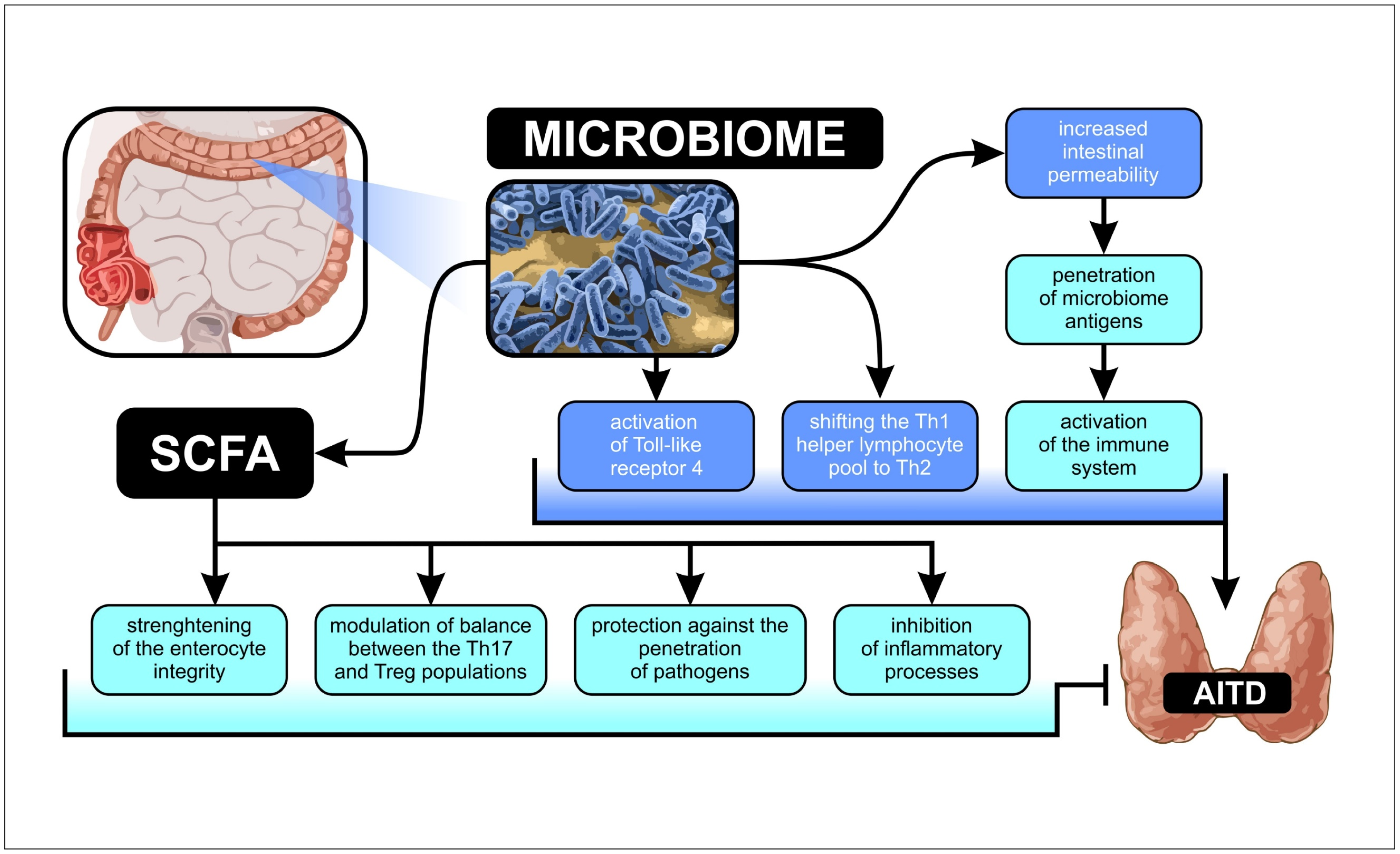
1. Introduction
2. Results
3. Discussion
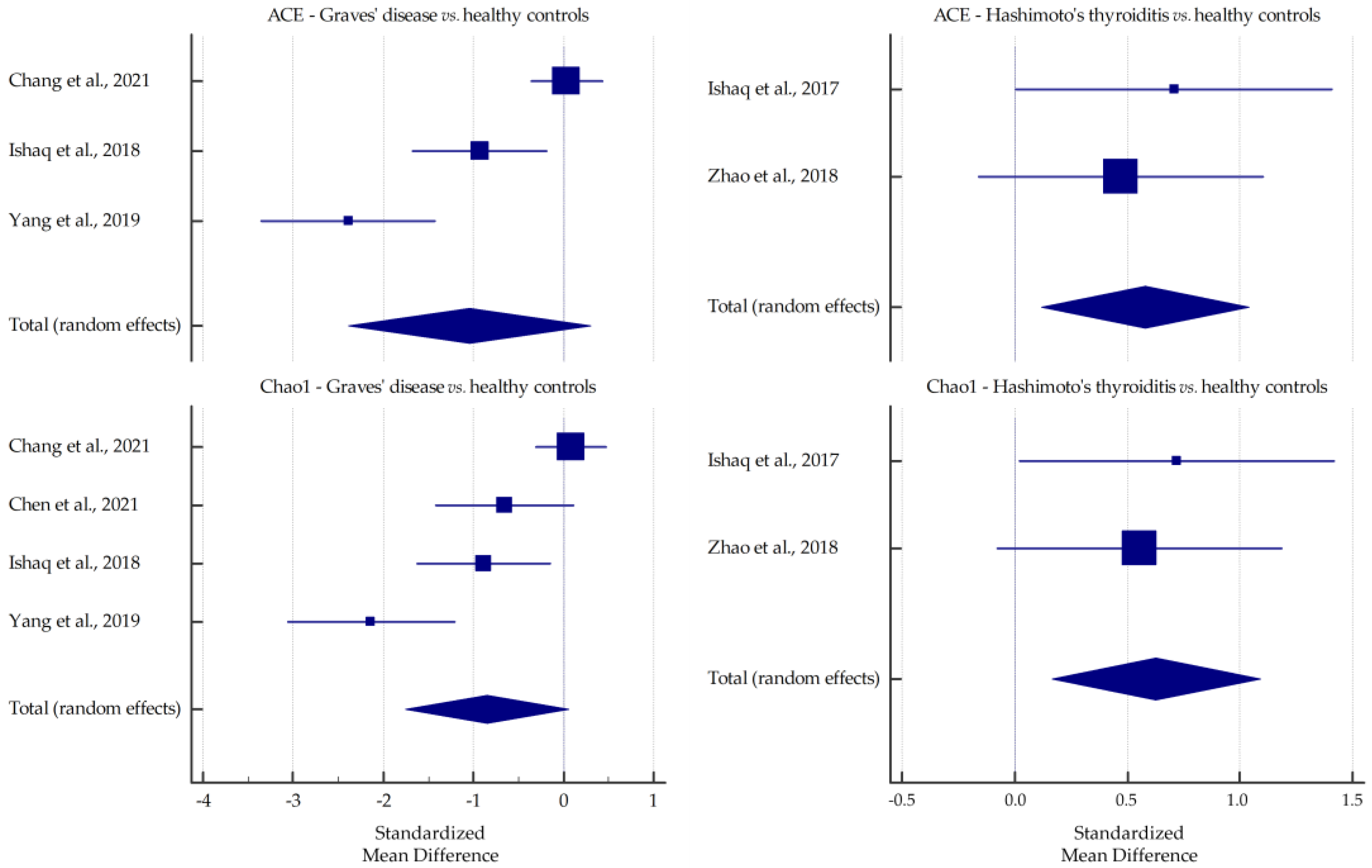
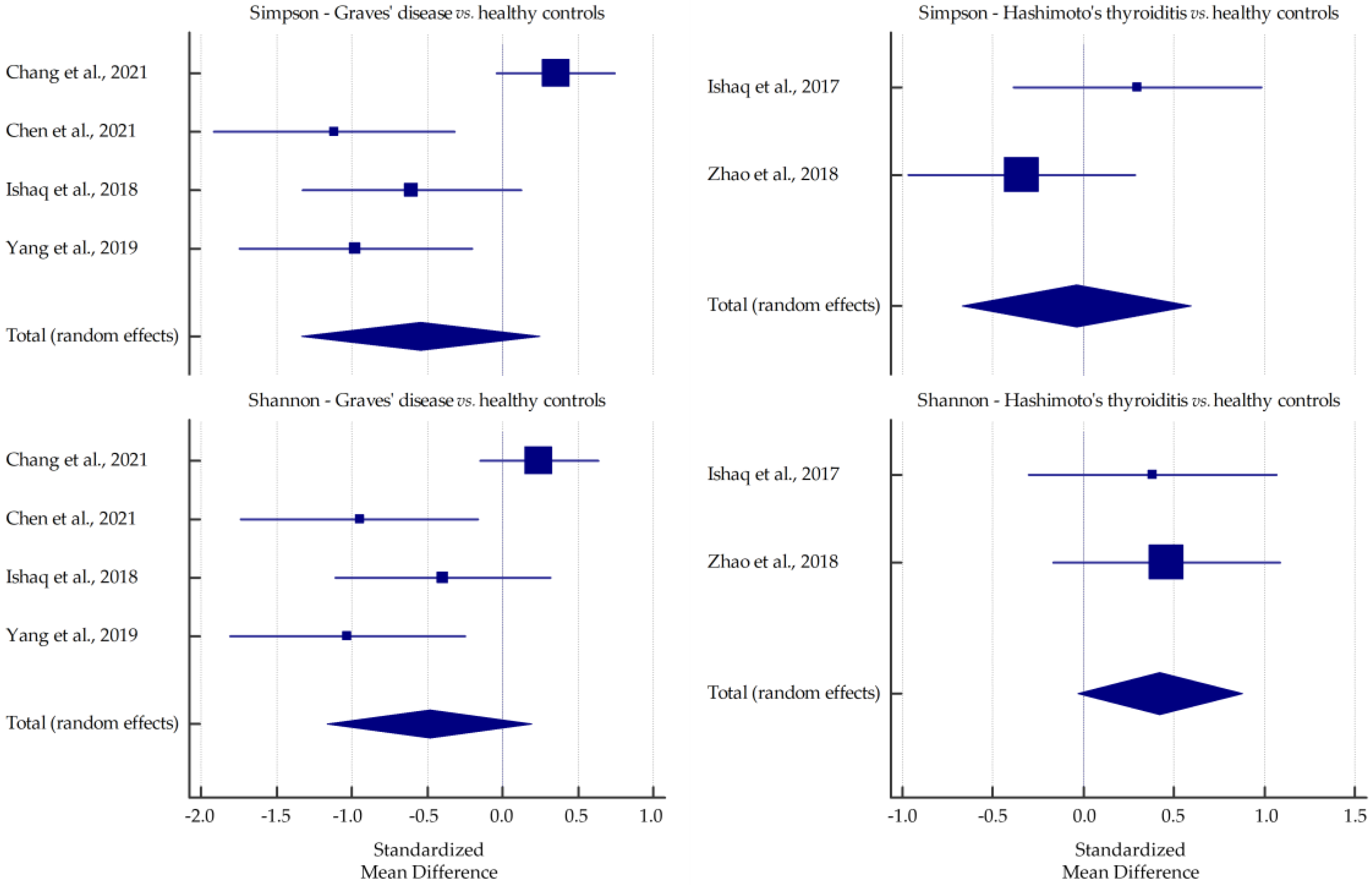
3.1. Microbiota Alpha-Diversity in AITD Patients
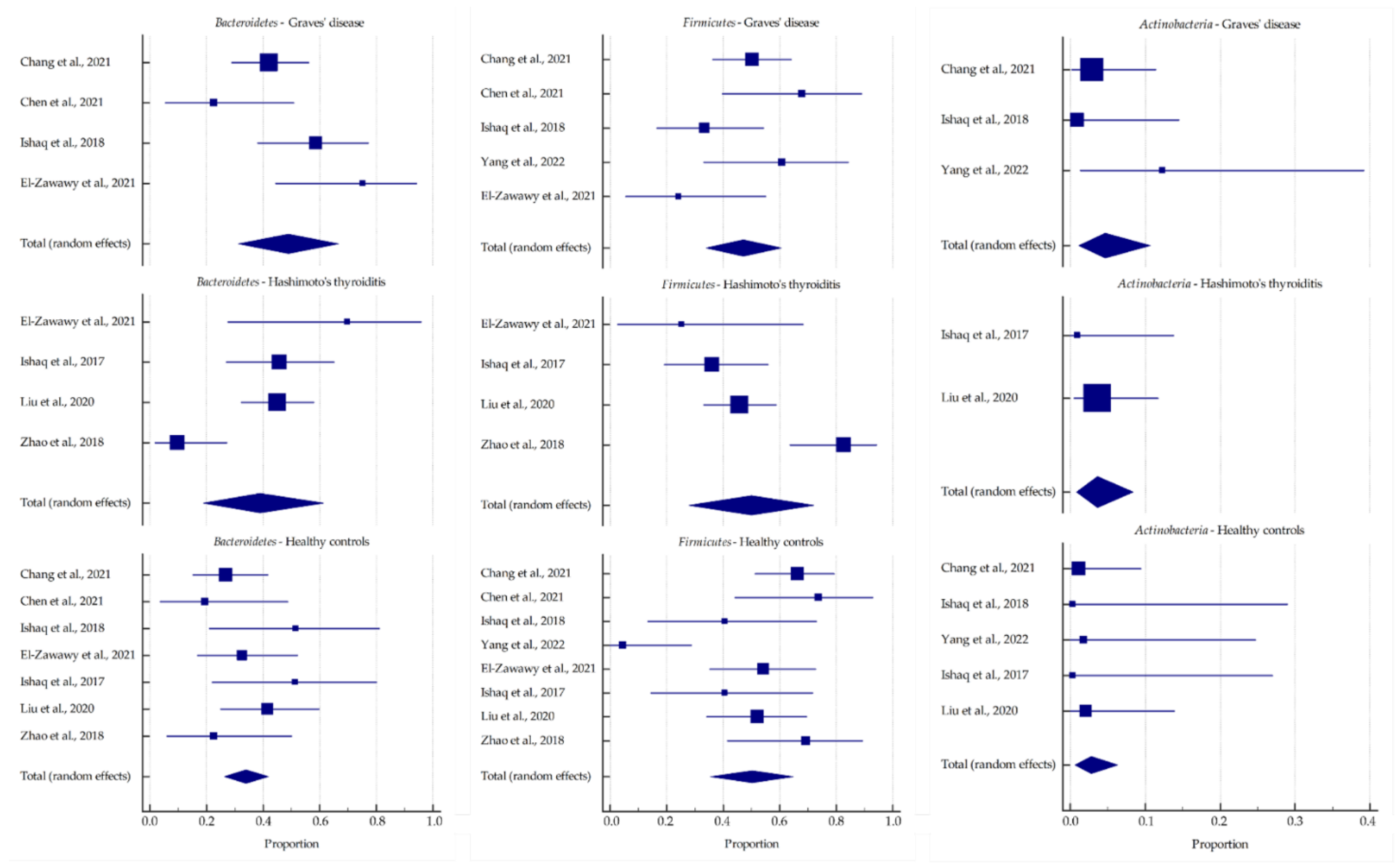
3.2. Microbiota Relative Abundance in GD and HT Patients
3.3. Correlations between Microbiota Alterations and Thyroid Functional Parameters
3.4. Study Limitations
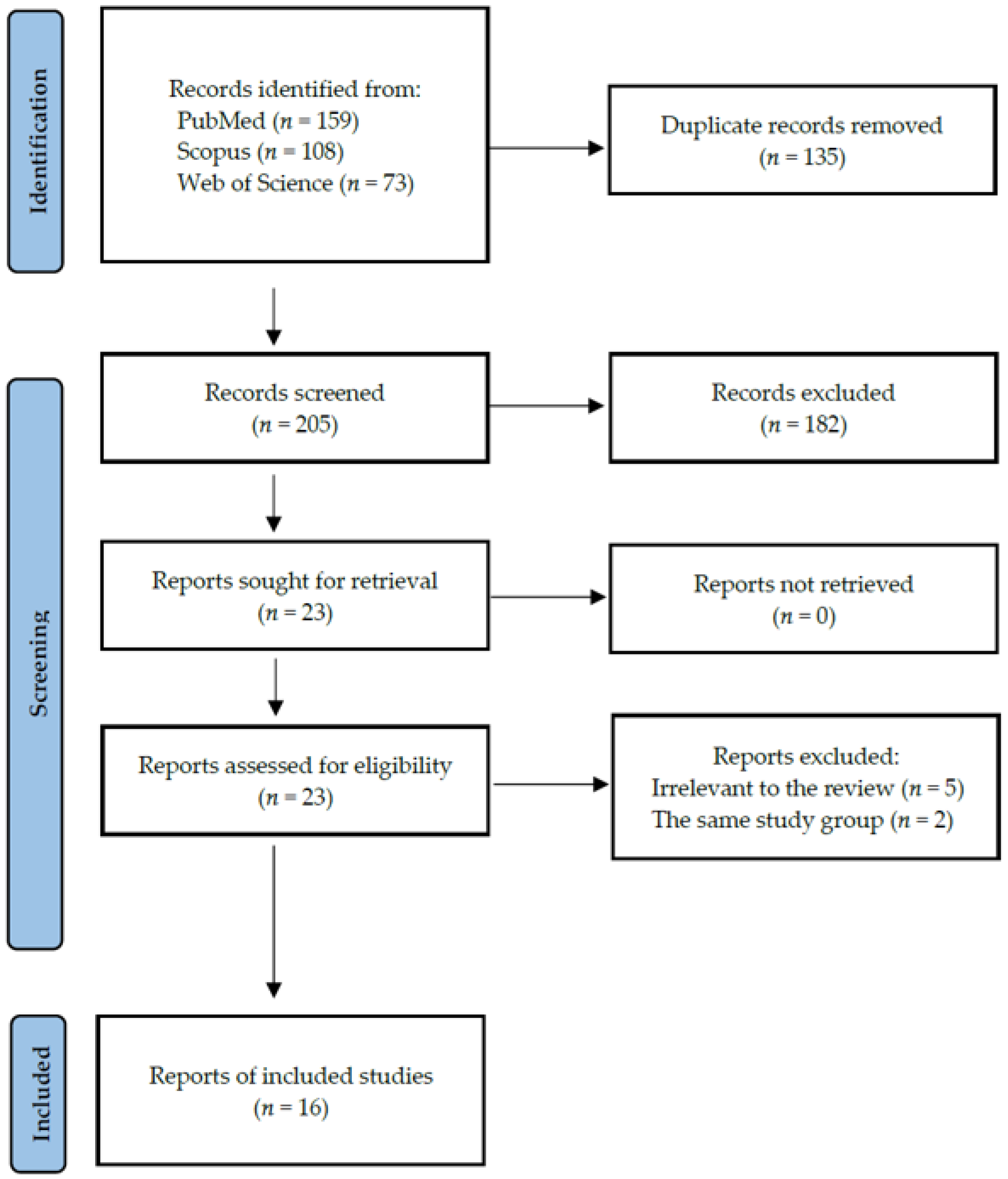
4. Materials and Methods
4.1. Search Strategy and Data Extraction
4.2. Quality Assessment and Critical Appraisal for the Systematic Review of the Included Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bogusławska, J.; Godlewska, M.; Gajda, E.; Piekiełko-Witkowska, A. Cellular and Molecular Basis of Thyroid Autoimmunity. Eur. Thyroid J. 2022, 11, e210024. [Google Scholar] [CrossRef]
- Luty, J.; Ruckemann-Dziurdzińska, K.; Witkowski, J.M.; Bryl, E. Immunological Aspects of Autoimmune Thyroid Disease—Complex Interplay between Cells and Cytokines. Cytokine 2019, 116, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Mikoś, H.; Mikoś, M.; Obara-Moszyńska, M.; Niedziela, M. The Role of the Immune System and Cytokines Involved in the Pathogenesis of Autoimmune Thyroid Disease (AITD). Endokrynol. Pol. 2014, 65, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, A.A.; Karaźniewicz-Łada, M.; Filipowicz, D.; Ruchała, M.; Główka, F.K. Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management—An Overview. Int. J. Mol. Sci. 2022, 23, 6580. [Google Scholar] [CrossRef] [PubMed]
- Szczepanek-Parulska, E.; Adamska, M.; Korda, O.; Kosicka, W.; Skowrońska, D.; Świejkowska, A.; Tuzimek, D.; Dadej, D.; Krygier, A.; Ruchała, M. Changes in Complete Blood Count Parameters Influenced by Endocrine Disorders. Endokrynol. Pol. 2021, 72, 261–270. [Google Scholar] [CrossRef]
- Mohammad, M.Y.H.; Bushulaybi, N.A.; AlHumam, A.S.; AlGhamdi, A.Y.; Aldakhil, H.A.; Alumair, N.A.; Shafey, M.M. Prevalence of Depression among Hypothyroid Patients Attending the Primary Healthcare and Endocrine Clinics of King Fahad Hospital of the University (KFHU). J. Fam. Med. Prim. Care 2019, 8, 2708–2713. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Ragusa, F.; Elia, G.; Paparo, S.R.; Ruffilli, I.; Patrizio, A.; Giusti, C.; Gonnella, D.; Cristaudo, A.; et al. Graves’ Disease: Epidemiology, Genetic and Environmental Risk Factors and Viruses. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101387. [Google Scholar] [CrossRef]
- Sawicka-Gutaj, N.; Ziółkowska, P.; Wojciechowska, K.; Shawkat, S.; Czarnywojtek, A.; Warchoł, W.; Sowiński, J.; Szczepanek-Parulska, E.; Ruchała, M. Eye Symptoms in Patients with Benign Thyroid Diseases. Sci. Rep. 2021, 11, 18706. [Google Scholar] [CrossRef]
- Gontarz-Nowak, K.; Szychlińska, M.; Matuszewski, W.; Stefanowicz-Rutkowska, M.; Bandurska-Stankiewicz, E. Current Knowledge on Graves’ Orbitopathy. J. Clin. Med. 2021, 10, 16. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Racioppo, A.; Altieri, C.; Bevilacqua, A. How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients 2022, 14, 2456. [Google Scholar] [CrossRef] [PubMed]
- Docimo, G.; Cangiano, A.; Romano, R.M.; Pignatelli, M.F.; Offi, C.; Paglionico, V.A.; Galdiero, M.; Donnarumma, G.; Nigro, V.; Esposito, D.; et al. The Human Microbiota in Endocrinology: Implications for Pathophysiology, Treatment, and Prognosis in Thyroid Diseases. Front. Endocrinol. 2020, 11, 586529. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef]
- Zhang, L.; Masetti, G.; Colucci, G.; Salvi, M.; Covelli, D.; Eckstein, A.; Kaiser, U.; Draman, M.S.; Muller, I.; Ludgate, M.; et al. Combining Micro-RNA and Protein Sequencing to Detect Robust Biomarkers for Graves’ Disease and Orbitopathy. Sci. Rep. 2018, 8, 8386. [Google Scholar] [CrossRef]
- Benvenga, S.; Guarneri, F. Molecular Mimicry and Autoimmune Thyroid Disease. Rev. Endocr. Metab. Disord. 2016, 17, 485–498. [Google Scholar] [CrossRef]
- Hou, J.; Tang, Y.; Chen, Y.; Chen, D. The Role of the Microbiota in Graves’ Disease and Graves’ Orbitopathy. Front. Cell. Infect. Microbiol. 2021, 11, 739707. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Microbiota and Thyroid Interaction in Health and Disease. Trends Endocrinol. Metab. TEM 2019, 30, 479–490. [Google Scholar] [CrossRef]
- Mohammad Hosseini, A.; Majidi, J.; Baradaran, B.; Yousefi, M. Toll-Like Receptors in the Pathogenesis of Autoimmune Diseases. Adv. Pharm. Bull. 2015, 5, 605–614. [Google Scholar] [CrossRef]
- Bargiel, P.; Szczuko, M.; Stachowska, L.; Prowans, P.; Czapla, N.; Markowska, M.; Petriczko, J.; Kledzik, J.; Jędrzejczyk-Kledzik, A.; Palma, J.; et al. Microbiome Metabolites and Thyroid Dysfunction. J. Clin. Med. 2021, 10, 3609. [Google Scholar] [CrossRef]
- Yamamoto, E.A.; Jørgensen, T.N. Relationships Between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front. Immunol. 2020, 10, 3141. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, H.; Liu, C.; Shang, M.; Wei, T.; Yin, P. Gut Microbiome and the Role of Metabolites in the Study of Graves’ Disease. Front. Mol. Biosci. 2022, 9, 841223. [Google Scholar] [CrossRef] [PubMed]
- Virili, C.; Centanni, M. Does Microbiota Composition Affect Thyroid Homeostasis? Endocrine 2015, 49, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Virili, C.; Fallahi, P.; Antonelli, A.; Benvenga, S.; Centanni, M. Gut Microbiota and Hashimoto’s Thyroiditis. Rev. Endocr. Metab. Disord. 2018, 19, 293–300. [Google Scholar] [CrossRef]
- Fernández-García, V.; González-Ramos, S.; Martín-Sanz, P.; Laparra, J.M.; Boscá, L. Beyond Classic Concepts in Thyroid Homeostasis: Immune System and Microbiota. Mol. Cell. Endocrinol. 2021, 533, 111333. [Google Scholar] [CrossRef]
- Virili, C.; Centanni, M. “With a Little Help from My Friends”—The Role of Microbiota in Thyroid Hormone Metabolism and Enterohepatic Recycling. Mol. Cell. Endocrinol. 2017, 458, 39–43. [Google Scholar] [CrossRef]
- Vought, R.L.; Brown, F.A.; Sibinovic, K.H.; Daniel, E.G.M. Effect of Changing Intestinal Bacterial Flora on Thyroid Function in the Rat. Horm. Metab. Res. 1972, 4, 43–47. [Google Scholar] [CrossRef]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship with Diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef]
- Gorini, F.; Vassalle, C. Selenium and Selenoproteins at the Intersection of Type 2 Diabetes and Thyroid Pathophysiology. Antioxidants 2022, 11, 1188. [Google Scholar] [CrossRef]
- Chang, S.-C.; Lin, S.-F.; Chen, S.-T.; Chang, P.-Y.; Yeh, Y.-M.; Lo, F.-S.; Lu, J.-J. Alterations of Gut Microbiota in Patients with Graves’ Disease. Front. Cell. Infect. Microbiol. 2021, 11, 663131. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Guo, Z.; Huang, S.; Lei, H.; Zang, P.; Lu, B.; Shao, J.; Gu, P. Associations between Gut Microbiota and Thyroidal Function Status in Chinese Patients with Graves’ Disease. J. Endocrinol. Investig. 2021, 44, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, H.M.; Mohammad, I.S.; Shahzad, M.; Ma, C.; Raza, M.A.; Wu, X.; Guo, H.; Shi, P.; Xu, J. Molecular Alteration Analysis of Human Gut Microbial Composition in Graves’ Disease Patients. Int. J. Biol. Sci. 2018, 14, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yu, X.; Kosik, R.O.; Song, Y.; Qiao, T.; Tong, J.; Liu, S.; Fan, S.; Luo, Q.; Chai, L.; et al. Gut Microbiota May Play a Significant Role in the Pathogenesis of Graves’ Disease. Thyroid Off. J. Am. Thyroid Assoc. 2021, 31, 810–820. [Google Scholar] [CrossRef]
- Shi, T.-T.; Xin, Z.; Hua, L.; Zhao, R.-X.; Yang, Y.-L.; Wang, H.; Zhang, S.; Liu, W.; Xie, R.-R. Alterations in the Intestinal Microbiota of Patients with Severe and Active Graves’ Orbitopathy: A Cross-Sectional Study. J. Endocrinol. Investig. 2019, 42, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.-T.; Xin, Z.; Hua, L.; Wang, H.; Zhao, R.-X.; Yang, Y.-L.; Xie, R.-R.; Liu, H.-Y.; Yang, J.-K. Comparative Assessment of Gut Microbial Composition and Function in Patients with Graves’ Disease and Graves’ Orbitopathy. J. Endocrinol. Investig. 2021, 44, 297–310. [Google Scholar] [CrossRef]
- Su, X.; Yin, X.; Liu, Y.; Yan, X.; Zhang, S.; Wang, X.; Lin, Z.; Zhou, X.; Gao, J.; Wang, Z.; et al. Gut Dysbiosis Contributes to the Imbalance of Treg and Th17 Cells in Graves’ Disease Patients by Propionic Acid. J. Clin. Endocrinol. Metab. 2020, 105, dgaa511. [Google Scholar] [CrossRef]
- Yan, H.-X.; An, W.-C.; Chen, F.; An, B.; Pan, Y.; Jin, J.; Xia, X.-P.; Cui, Z.-J.; Jiang, L.; Zhou, S.-J.; et al. Intestinal Microbiota Changes in Graves’ Disease: A Prospective Clinical Study. Biosci. Rep. 2020, 40, BSR20191242. [Google Scholar] [CrossRef]
- Yang, M.; Li, F.; Zhang, R.; Wu, Y.; Yang, Q.; Wang, F.; Yu, Z.; Liu, J.; Cha, B.; Gong, Q.; et al. Alteration of the Intestinal Microbial Flora and the Serum IL-17 Level in Patients with Graves’ Disease Complicated with Vitamin D Deficiency. Int. Arch. Allergy Immunol. 2022, 183, 225–234. [Google Scholar] [CrossRef]
- Yang, M.; Sun, B.; Li, J.; Yang, B.; Xu, J.; Zhou, X.; Yu, J.; Zhang, X.; Zhang, Q.; Zhou, S.; et al. Alteration of the Intestinal Flora May Participate in the Development of Graves’ Disease: A Study Conducted among the Han Population in Southwest China. Endocr. Connect. 2019, 8, 822–828. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Ruiz-Limón, P.; Gómez-Pérez, A.M.; Molina-Vega, M.; Moreno-Indias, I.; Tinahones, F.J. Differential Microbial Pattern Description in Subjects with Autoimmune-Based Thyroid Diseases: A Pilot Study. J. Pers. Med. 2020, 10, 192. [Google Scholar] [CrossRef]
- El-Zawawy, H.T.; Ahmed, S.M.; El-Attar, E.A.; Ahmed, A.A.; Roshdy, Y.S.; Header, D.A. Study of Gut Microbiome in Egyptian Patients with Autoimmune Thyroid Diseases. Int. J. Clin. Pract. 2021, 75, e14038. [Google Scholar] [CrossRef]
- Cayres, L.C.d.F.; de Salis, L.V.V.; Rodrigues, G.S.P.; van Helvoort Lengert, A.; Biondi, A.P.C.; Sargentini, L.D.B.; Brisotti, J.L.; Gomes, E.; de Oliveira, G.L.V. Detection of Alterations in the Gut Microbiota and Intestinal Permeability in Patients With Hashimoto Thyroiditis. Front. Immunol. 2021, 12, 579140. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, H.M.; Mohammad, I.S.; Guo, H.; Shahzad, M.; Hou, Y.J.; Ma, C.; Naseem, Z.; Wu, X.; Shi, P.; Xu, J. Molecular Estimation of Alteration in Intestinal Microbial Composition in Hashimoto’s Thyroiditis Patients. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 95, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; An, Y.; Cao, B.; Sun, R.; Ke, J.; Zhao, D. The Composition of Gut Microbiota in Patients Bearing Hashimoto’s Thyroiditis with Euthyroidism and Hypothyroidism. Int. J. Endocrinol. 2020, 2020, 5036959. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Feng, J.; Li, J.; Zhao, L.; Liu, Y.; Chen, H.; Jin, Y.; Zhu, B.; Wei, Y. Alterations of the Gut Microbiota in Hashimoto’s Thyroiditis Patients. Thyroid Off. J. Am. Thyroid Assoc. 2018, 28, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The Resilience of the Intestinal Microbiota Influences Health and Disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef]
- Pinart, M.; Dötsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2021, 14, 12. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu. Rev. Microbiol. 2016, 70, 395–411. [Google Scholar] [CrossRef]
- Sultan, S.; El-Mowafy, M.; Elgaml, A.; Ahmed, T.A.E.; Hassan, H.; Mottawea, W. Metabolic Influences of Gut Microbiota Dysbiosis on Inflammatory Bowel Disease. Front. Physiol. 2021, 12, 715506. [Google Scholar] [CrossRef]
- Gaike, A.H.; Paul, D.; Bhute, S.; Dhotre, D.P.; Pande, P.; Upadhyaya, S.; Reddy, Y.; Sampath, R.; Ghosh, D.; Chandraprabha, D.; et al. The Gut Microbial Diversity of Newly Diagnosed Diabetics but Not of Prediabetics Is Significantly Different from That of Healthy Nondiabetics. mSystems 2020, 5, e00578-19. [Google Scholar] [CrossRef]
- Torres, P.J.; Siakowska, M.; Banaszewska, B.; Pawelczyk, L.; Duleba, A.J.; Kelley, S.T.; Thackray, V.G. Gut Microbial Diversity in Women with Polycystic Ovary Syndrome Correlates With Hyperandrogenism. J. Clin. Endocrinol. Metab. 2018, 103, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, E.C.; Bilotta, A.L.; Gabrielli, M.; Scarpellini, E.; Lupascu, A.; Laginestra, A.; Novi, M.; Sottili, S.; Serricchio, M.; Cammarota, G.; et al. Association between Hypothyroidism and Small Intestinal Bacterial Overgrowth. J. Clin. Endocrinol. Metab. 2007, 92, 4180–4184. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Senghor, B.; Sokhna, C.; Ruimy, R.; Lagier, J.-C. Gut Microbiota Diversity According to Dietary Habits and Geographical Provenance. Hum. Microbiome J. 2018, 7–8, 1–9. [Google Scholar] [CrossRef]
- Losasso, C.; Eckert, E.M.; Mastrorilli, E.; Villiger, J.; Mancin, M.; Patuzzi, I.; Di Cesare, A.; Cibin, V.; Barrucci, F.; Pernthaler, J.; et al. Assessing the Influence of Vegan, Vegetarian and Omnivore Oriented Westernized Dietary Styles on Human Gut Microbiota: A Cross Sectional Study. Front. Microbiol. 2018, 9, 317. [Google Scholar] [CrossRef]
- Wu, G.D.; Compher, C.; Chen, E.Z.; Smith, S.A.; Shah, R.D.; Bittinger, K.; Chehoud, C.; Albenberg, L.G.; Nessel, L.; Gilroy, E.; et al. Comparative Metabolomics in Vegans and Omnivores Reveal Constraints on Diet-Dependent Gut Microbiota Metabolite Production. Gut 2016, 65, 63–72. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Wiersinga, W.M. Smoking and Thyroid. Clin. Endocrinol. 2013, 79, 145–151. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, Y.; Kim, S.J.; Lee, E.-J.; Chang, Y.; Ryu, S.; Shin, H.; Kim, H.-L.; Kim, H.-N.; Lee, J.H. Association between Cigarette Smoking Status and Composition of Gut Microbiota: Population-Based Cross-Sectional Study. J. Clin. Med. 2018, 7, 282. [Google Scholar] [CrossRef]
- Biedermann, L.; Zeitz, J.; Mwinyi, J.; Sutter-Minder, E.; Rehman, A.; Ott, S.J.; Steurer-Stey, C.; Frei, A.; Frei, P.; Scharl, M.; et al. Smoking Cessation Induces Profound Changes in the Composition of the Intestinal Microbiota in Humans. PLoS ONE 2013, 8, e59260. [Google Scholar] [CrossRef]
- Gui, X.; Yang, Z.; Li, M.D. Effect of Cigarette Smoke on Gut Microbiota: State of Knowledge. Front. Physiol. 2021, 12, 673341. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Masetti, G.; Moshkelgosha, S.; Köhling, H.-L.; Covelli, D.; Banga, J.P.; Berchner-Pfannschmidt, U.; Horstmann, M.; Diaz-Cano, S.; Goertz, G.-E.; Plummer, S.; et al. Gut Microbiota in Experimental Murine Model of Graves’ Orbitopathy Established in Different Environments May Modulate Clinical Presentation of Disease. Microbiome 2018, 6, 97. [Google Scholar] [CrossRef]
- Moshkelgosha, S.; Verhasselt, H.L.; Masetti, G.; Covelli, D.; Biscarini, F.; Horstmann, M.; Daser, A.; Westendorf, A.M.; Jesenek, C.; Philipp, S.; et al. Modulating Gut Microbiota in a Mouse Model of Graves’ Orbitopathy and Its Impact on Induced Disease. Microbiome 2021, 9, 45. [Google Scholar] [CrossRef]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut Microbiome Metagenomics Analysis Suggests a Functional Model for the Development of Autoimmunity for Type 1 Diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar] [CrossRef]
- Larsen, J.M. The Immune Response to Prevotella Bacteria in Chronic Inflammatory Disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef]
- Xu, Q.; Ni, J.-J.; Han, B.-X.; Yan, S.-S.; Wei, X.-T.; Feng, G.-J.; Zhang, H.; Zhang, L.; Li, B.; Pei, Y.-F. Causal Relationship Between Gut Microbiota and Autoimmune Diseases: A Two-Sample Mendelian Randomization Study. Front. Immunol. 2021, 12, 746998. [Google Scholar] [CrossRef]
- López, P.; Gueimonde, M.; Margolles, A.; Suárez, A. Distinct Bifidobacterium Strains Drive Different Immune Responses in Vitro. Int. J. Food Microbiol. 2010, 138, 157–165. [Google Scholar] [CrossRef]
- Astbury, S.; Atallah, E.; Vijay, A.; Aithal, G.P.; Grove, J.I.; Valdes, A.M. Lower Gut Microbiome Diversity and Higher Abundance of Proinflammatory Genus Collinsella Are Associated with Biopsy-Proven Nonalcoholic Steatohepatitis. Gut Microbes 2020, 11, 569–580. [Google Scholar] [CrossRef]
- Jenq, R.R.; Taur, Y.; Devlin, S.M.; Ponce, D.M.; Goldberg, J.D.; Ahr, K.F.; Littmann, E.R.; Ling, L.; Gobourne, A.C.; Miller, L.C.; et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2015, 21, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K.; et al. Blautia Genus Associated with Visceral Fat Accumulation in Adults 20-76 Years of Age. NPJ Biofilms Microbiomes 2019, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean Diet Is Associated with the Gut Microbiota Pattern and Gastrointestinal Characteristics in an Adult Population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef]
- Lenkowski, M.; Nijakowski, K.; Kaczmarek, M.; Surdacka, A. The Loop-Mediated Isothermal Amplification Technique in Periodontal Diagnostics: A Systematic Review. J. Clin. Med. 2021, 10, 1189. [Google Scholar] [CrossRef]
- Van den Bogert, B.; Meijerink, M.; Zoetendal, E.G.; Wells, J.M.; Kleerebezem, M. Immunomodulatory Properties of Streptococcus and Veillonella Isolates from the Human Small Intestine Microbiota. PLoS ONE 2014, 9, e114277. [Google Scholar] [CrossRef]
- Kiseleva, E.P.; Mikhailopulo, K.I.; Sviridov, O.V.; Novik, G.I.; Knirel, Y.A.; Szwajcer Dey, E. The Role of Components of Bifidobacterium and Lactobacillus in Pathogenesis and Serologic Diagnosis of Autoimmune Thyroid Diseases. Benef. Microbes 2011, 2, 139–154. [Google Scholar] [CrossRef]
- Ferreira-Halder, C.V.; Faria, A.V.d.S.; Andrade, S.S. Action and Function of Faecalibacterium Prausnitzii in Health and Disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef]
- Wu, F.; Guo, X.; Zhang, J.; Zhang, M.; Ou, Z.; Peng, Y. Phascolarctobacterium Faecium Abundant Colonization in Human Gastrointestinal Tract. Exp. Ther. Med. 2017, 14, 3122–3126. [Google Scholar] [CrossRef]
- Omenetti, S.; Pizarro, T.T. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front. Immunol. 2015, 6, 639. [Google Scholar] [CrossRef]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive Impact of Non-Antibiotic Drugs on Human Gut Bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wen, S.; Zhang, J.; Peng, J.; Shen, X.; Xu, L. Systematic Review and Meta-Analysis: Changes of Gut Microbiota before and after Menopause. Dis. Markers 2022, 2022, 3767373. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, A.; Scambia, G.; Lello, S. Subclinical Hypothyroidism in Women’s Health: From Pre- to Post-Menopause. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2022, 38, 357–367. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Y.; Hou, X.; Li, J.; Cai, Y.; Hao, Y.; Ouyang, Q.; Wu, B.; Sun, Z.; Zhang, M.; et al. Small Intestinal Bacterial Overgrowth in Subclinical Hypothyroidism of Pregnant Women. Front. Endocrinol. 2021, 12, 604070. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yang, Y.; Fan, Y.; Guo, S.; Li, T.; Gu, M.; Zhang, T.; Gao, H.; Liu, R.; Yin, C. Characteristics of the Intestinal Flora of TPOAb-Positive Women with Subclinical Hypothyroidism in the Second Trimester of Pregnancy: A Single-Center Prospective Cohort Study. Front. Cell. Infect. Microbiol. 2022, 12, 794170. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Study Quality Assessment Tools | NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 22 August 2020).
- OCEBM Levels of Evidence. Available online: https://www.cebm.net/2016/05/ocebm-levels-of-evidence/ (accessed on 22 August 2020).


| Author, Year | Setting | Study Group (F/M; Age) | Control Group (F/M; Age) | AITD Diagnosis | Inclusion Criteria | Exclusion Criteria | Thyroid Parameters Determined | Treatment |
|---|---|---|---|---|---|---|---|---|
| Chang et al., 2021 [30] | Taiwan | 55 (35/20); 45.09 ± 12.08 | 48 (30/18); 42.60 ± 9.78 | GD | Previously diagnosed with GD | Pregnancy, gastrointestinal disorders, gout, stroke, cancer, autoimmune diseases, history of gastrointestinal surgery, use of antibiotics, probiotics, prebiotics or symbiotics (<2 months), hormonal medication, Chinese herbal medicine (<3 months), pure vegetarians | TSH, FT4, TPOAb | PTU, MMI, CBZ |
| Chen et al., 2021 [31] | China | 15 (8/7); 28.87 ± 6.79 | 14 (8/6); 27.29 ± 5.73 | GD | Previously diagnosed GD | Untreated, transient hyperthyroidism, autoimmune diseases, long-term hormone treatment, diabetes, metabolic diseases, constipation, chronic diarrhea, inflammatory bowel disease, cancer, familial genetic disease, severe dysfunction of multiple organs, use of antibiotics, microbiological preparations (<1 month), vegetarians, pregnancy, lactation, history of gastrointestinal surgery, addiction to alcohol or drugs | TSH, FT3, FT4, T3, T4, TGAb, TPOAb, TRAb | MMI |
| Ishaq et al., 2018 [32] | China | 27 (17/10); range: 35–50 | 11 (7/4); age-matched | GD | Previously diagnosed GD | GD treatment (<6 months), gastrointestinal diseases, use of prebiotics, probiotics, or antibiotics (<2 months) | TSH, FT3, FT4, T3, T4, TGAb, TPOAb, TRAb | No treatment (<6 months) |
| Jiang et al., 2021 [33] | China | 45 (33/12); 37 (range: 16–65) | 59 (37/22); 43 (range: 22–71) | GD | Previously diagnosed GD | Malignancies, gastrointestinal diseases, endocrine system diseases, use of antibiotics, probiotics, or prebiotics (<1 month) | TSH, FT3, FT4, T3, T4, TGAb, TPOAb, TRAb, TMAb | Untreated |
| Shi et al., 2019 [34] | China | 33 (16/17); 46.0 ± 11.71 | 32 (16/16); 43.4 ± 9.7 | GO | Diagnosed with Graves’ orbitopathy, CAS ≥ 3/7, and NOSPECS score ≥IV | Age < 18 or >65 years, use of antibiotics or probiotics (<4 weeks), use of hormonal medication, Chinese herbal medicine (<3 months), chronic diarrhea or constipation, inflammatory bowel disease, acute infections, diabetes, stroke, heart diseases, renal or hepatic dysfunction, cancer, autoimmune diseases, gastrointestinal surgery, pure vegetarians, pregnancy, lactation, alcohol or substance addiction | TSH, FT3, FT4, T3, T4, TGAb, TPOAb, TRAb | MMI |
| Shi et al., 2021 [35] | China | GO: 33 (16/17); 46.0 ± 11.7; GD: 30 (20/10); 45.0 ± 12.8 | 32 (16/16); 43.4 ± 9.7 | GD/GO | Diagnosed with GD/GO | Age < 18 or >65 years, use of antibiotics or probiotics (<4 weeks), use of hormonal medication, Chinese herbal medicine (<3 months), chronic diarrhea or constipation, inflammatory bowel disease, acute infections, diabetes, stroke, heart diseases, renal or hepatic dysfunction, cancer, autoimmune diseases, gastrointestinal surgery, pure vegetarians, pregnancy, lactation, alcohol or substance addiction | TSH, FT3, FT4, T3, T4, TGAb, TPOAb, TRAb | MMI |
| Su et al., 2020 [36] | China | 58 (35/23); 42.07 ± 10.22 | 63 (35/28); 43.86 ± 9.20 | GD | Diagnosed with GD | Pregnancy, smoking, alcohol addiction, diarrhea, hypertension, diabetes, lipid dysregulation, BMI > 27, use of antibiotics, probiotics, prebiotics, symbiotics, hormonal medication, laxatives, proton pump inhibitors, insulin sensitizers, Chinese herbal medicine (<3 months), autoimmune diseases, malignancy, history of gastrointestinal surgery | TSH, FT3, FT4, TGAb, TPOAb | Untreated |
| Yan et al., 2020 [37] | China | 39 (28/11); 37.49 ± 12.95 | 17 (11/6); 33.42 ± 9.13 | GD | Diagnosed with GD | Pregnancy, smoking, alcohol addiction, use of antibiotics, hormonal medication, Chinese herbal medicine (3 months), use of medicine for the treatment of GD (<6 months), gastrointestinal diseases | TSH, T3, T4, TGAb, TPOAb, TRAb | No treatment (<6 months) |
| Yang et al., 2022 [38] | China | 191 (116/75); mean: 45.8 | 30 (NR); NR | GD | Newly diagnosed with GD, without receiving any treatment, participating voluntarily with compliance, and written informed consent | Use of probiotics, prebiotics, antibiotics, Chinese herbal medicine (<1 month), complications of infection-associated diseases or other diseases (<1 month), chronic stress | TSH, FT3, FT4, TPOAb, TGAb TRAb | Untreated |
| Yang et al., 2019 [39] | China | 15 (NR); range: 46–55 | 15 (age- and sex-matched) | GD | Diagnosed with GD | Osteoporosis, pregnancy, autoimmune diseases, infectious diseases, use of antibiotics, probiotics, metformin, acarbose, herbal preparations, use of any antithyroid treatment, chronic stress | NR | NR |
| Cornejo-Pareja et al., 2020 [40] | Spain | GD: 9 (7/2); 46.2 ± 8.6; HT: 9 (9/0); 40.3 ± 9.6 | 11 (7/4); 48.8 ± 6.2 | GD and HT | Diagnosed with GD or HT | Pregnancy, diabetes, autoimmune diseases, gastrointestinal disorders, extreme diets, use of antibiotics, probiotics (<3 months), nonacceptance of informed consent | TSH, FT3, FT4, TPOAb, TSIAb | GD: CBZ; HT: LT4 |
| El-Zawawy et al., 2021 [41] | Egypt | GD: 13 (4/9); 38.2; HT: 7 (6/1); 39.4 | 30 (17/13); 39.7 ± 10.9 | GD and HT | Newly diagnosed and uncontrolled AITD (GD or HT) | Malignancy, recent gastrointestinal surgery (<6 months), recent hospitalization, use of antibiotics, nonsteroidal anti-inflammatory drugs, corticosteroids (<3 months), infectious diarrhea, other comorbidities, autoimmune diseases, pregnancy, severe burn, sepsis, pure vegetarians, smoking, alcohol or substance addiction, unable to give consent as mentally challenged, children | TSH, FT3, FT4, TPOAb, TRAb | NR |
| Cayres et al., 2021 [42] | Brazil | 40 (36/4); 48.9 ± 13.3 | 53 (NR); 45.6 ± 16.7 | HT | Diagnosed with HT | Use of anti-inflammatories, immunosuppressant drugs, antibiotics, vaccination (<30 days), gastrointestinal surgeries, inflammatory bowel diseases, chronic diarrhea | TSH, FT4, TPOAb, TGAb | LT4 |
| Ishaq et al., 2017 [43] | China | 29 (20/9); range: 40–60 | 12 (8/4); range: 40–60 | HT | Diagnosed with HT | Gastrointestinal diseases, use of antibiotics, probiotics, and prebiotics (<60 days) | TSH, T3, T4, TPOAb, TGAb | NR |
| Liu et al., 2020 [44] | China | HTE: 45 (45/0); 34.6 ± 1.0 HTH: 18 (18/0); 36.3 ± 2.1 | 34 (34/0); 29.6 ± 0.6 | HT | Diagnosed with HT | Autoimmune diseases, thyroid surgeries, pregnancy, use of antibiotics (<1 week) | TSH, FT3, FT4, T3, T4, TPOAb, TGAb | LT4 |
| Zhao et al., 2018 [45] | China | Exploration cohort: 28 (25/3); 44.29 ± 12.25; validation cohort: 22 (19/3); 45.82 ± 10.7 | Exploration cohort: 16 (14/2); 44.63 ± 10.33; validation cohort: 11 (9/2); 43.73 ± 10.95 | HT | Diagnosed with HT, the presence of euthyroidism | Pregnancy, lactation, smoking, alcohol addiction, hypertension, diabetes, lipid dysregulation, BMI > 27, use of antibiotics, probiotics, prebiotics, symbiotics, hormonal medication, laxatives, proton pump inhibitors, insulin sensitizers, Chinese herbal medicine (<3 months), autoimmune diseases, malignancy, history of gastrointestinal surgery | TSH, FT3, FT4, TPOAb, TGAb | NR |
| Study | AITD Diagnosis | Type of Laboratory Material | Methods of Microbiological Analysis | Altered Microbiota Composition | Richness | Diversity | ||
|---|---|---|---|---|---|---|---|---|
| ACE | Chao1 | Simpson | Shannon | |||||
| Chang et al., 2021 [30] | GD | Fecal samples collected in a clean container, aliquoted, immediately frozen, and stored at −80 °C | 16S rRNA gene sequencing | up: Bacteroidetes, Actinobacteria/Bacteroides, Prevotella_9; down: Firmicutes/Faecalibacterium, Lachnospiraceae_NK4A136_group | ↑ | ↑ | ↑ | ↑ |
| Chen et al., 2021 [31] | GD | Fecal samples collected on dry and clean paper, placed in sterile containers, transported at <4 °C, divided into portions, frozen, and stored at −80 °C | 16S rDNA gene sequencing | up: Lactobacillus, Veillonella, Streptococcus; down: Proteobacteria, Synergistetes | - | ↓ | ↓ * | ↓ * |
| Ishaq et al., 2018 [32] | GD | Fecal samples collected in an icebox, transported within 1 h of defecation, and stored at −80 °C | 16S rRNA gene sequencing | up: Prevotella_9, Haemophilus; down: Alistipes, Faecalibacterium, Dialister, Bifidobacterium, Lactobacillus | ↓ * | ↓ * | ↓ | ↓ |
| Jiang et al., 2021 [33] | GD | Fecal samples collected and stored at −80 °C | 16S rRNA gene sequencing | up: Bacteroidetes/Bacteroides, Lactobacillus; down: Firmicutes/Blautia, Eubacterium_hallii_group, Anaerostipes, Collinsella, Dorea, unclassified_f_Peptostreptococcaceae, Ruminococcus_torques_group | ↓ * | ↓ * | ↑ * | ↓ * |
| Shi et al., 2019 [34] | GO | Fecal samples (2.5 g) collected in tubes prefilled with fecal DNA stabilizer and stored at −80 °C | 16S rRNA gene sequencing | up: Bacteroidetes/unidentified_Prevotellaceae; down: Firmicutes/Blautia, Fusicatenibacter, Butyricicoccus, Anaerostipes, Collinsella | ns | ns | ↓ * | ↓ * |
| Shi et al., 2021 [35] | GD/GO | Fecal samples (2.5 g) collected in tubes prefilled with fecal DNA stabilizer and stored at −80 °C | 16S rRNA gene sequencing | GO vs. GD: up: Subdoligranulum, Bilophila; down: Deinococcus-Thermus, Chloroflexi/Blautia, Anaerostipes, Dorea, Butyricicoccus, Romboutsia, Fusicatenibacter, unidentified_Lachnospiraceae, unidentified_Clostridiales, Collinsella, Intestinibacter, Phascolarctobacterium | ns | ns | - | ↓ * |
| Su et al., 2020 [36] | GD | Fecal samples stored at −80 °C after liquid nitrogen freezing | 16S rRNA gene sequencing | up: Spirochaetae, Saccharibacteria, Bacteroidetes; down: Firmicutes, Proteobacteria, Synergistetes, Tenericutes, Verrucomicrobia | ↓ * | ↓ * | ↓ * | ↓ * |
| Yan et al., 2020 [37] | GD | Fecal samples stored at −80 °C | 16S rRNA gene sequencing | up: Bacilli, Lactobacillales, Prevotella, Megamonas, Veillonella; down: Ruminococcus, Rikenellaceae, Alistipes | - | ns | ns | ↓ * |
| Yang et al., 2022 [38] | GD | Fecal samples (10 g) immediately stored in sterile iceboxes and stored at −80 °C | 16S rRNA gene sequencing | up: Actinobacteria/Bifidobacterium, Collinsella, Pediococcus; down: Firmicutes/Roseburia, Dialister | ns | ns | ns | ns |
| Yang et al., 2019 [39] | GD | Fecal samples (2 g) stored at −80 °C | 16S rRNA gene sequencing | up: Firmicutes, Proteobacteria, Actinobacillus/Oribacterium, Mogibacterium, Lactobacillus, Aggregatibacter; down: Bacteroidetes | ↓ | ↓ | ↓ | ↓ |
| Cornejo-Pareja et al., 2020 [40] | GD | Fecal samples immediately refrigerated and stored at −80 °C | 16S rRNA gene sequencing | up: Fusobacterium, Sutterella; down: Faecalibacterium | - | - | - | ns |
| HT | up: Victivallaceae | - | - | - | ns | |||
| El-Zawawy et al., 2021 [41] | GD | Fecal samples kept at −20 °C upon defecation at home and stored at −80 °C | 16S rRNA gene sequencing | up: Bacteroidetes/Prevotella; down: Firmicutes | - | - | - | ↓ |
| HT | - | - | - | ↓ | ||||
| Cayres et al., 2021 [42] | HT | Fecal samples (200 mg) | 16S rRNA gene sequencing | up: Bacteroides; down: Bifidobacterium | - | - | - | - |
| Ishaq et al., 2017 [43] | HT | Fecal samples collected in a sterile cup, transported within 4 h of defecation, and stored at −80 °C | 16S rRNA gene sequencing | down: Bifidobacterium, Lactobacillus, Dialister | ↑ * | ↑ * | ↑ | ↑ |
| Liu et al., 2020 [44] | HT | Fecal samples collected in tubes prefilled with fecal DNA stabilizer and stored at −80 °C | 16S rRNA gene sequencing | HTH: up: Phascolarctobacterium; HTE: up: Lachnospiraceae_incertae_sedis, Lactonifactor, Alistipes, Subdoligranulum | - | - | - | ↓ * |
| Zhao et al., 2018 [45] | HT | Fecal samples immediately divided into aliquots, frozen on dry ice, and stored at −80 °C | 16S rRNA gene sequencing | up: Firmicutes/Blautia, Roseburia, Ruminococcus_torques_group, Romboutsia, Dorea, Fusicatenibacter, Eubacterium_hallii_group; down: Bacteroidetes/Faecalibacterium, Bacteroides, Prevotella_9, Lachnoclostridium | ↑ | ↑ | ↓ | ↑ |
| Study | AITD Diagnosis | Bacterial Phylum | Bacterial Genus | Relative Abundance | ||
|---|---|---|---|---|---|---|
| Study | Control | p-Value | ||||
| Chang et al., 2021 [30] | GD | Bacteroidetes | 0.4210 | 0.2690 | <0.01 | |
| Actinobacteria | 0.0288 | 0.0111 | <0.01 | |||
| Firmicutes | 0.5020 | 0.6630 | <0.01 | |||
| Collinsella | 0.0052 | 0.0014 | <0.01 | |||
| Parabacteroides | 0.0110 | 0.0009 | <0.01 | |||
| Prevotella_9 | 0.0767 | 0.0017 | <0.01 | |||
| Chen et al., 2021 [31] | GD | Synergistetes | 0.000012 | 0.0024 | 0.028 | |
| Veillonella | 0.0172 | 0.0006 | 0.039 | |||
| Ishaq et al., 2018 [32] | GD | Prevotella_9 | 0.4970 | 0.1952 | 0.034 | |
| Haemophilus | 0.1358 | 0.0099 | 0.049 | |||
| Dialister | 0.0110 | 0.0445 | 0.047 | |||
| Alistipes | 0.0180 | 0.0474 | 0.025 | |||
| Faecalibacterium | 0.0289 | 0.0562 | 0.014 | |||
| Yang et al., 2022 [38] | GD | Actinobacteria | 0.1229 | 0.0175 | 0.003 | |
| TM7 | 0.0001 | 0.00001 | 0.011 | |||
| Firmicutes | 0.6088 | 0.7522 | 0.044 | |||
| Cyanobacteria | 0.0002 | 0.00003 | 0.050 | |||
| El-Zawawy et al., 2021 [41] | GD and HT | Bacteroidetes | 0.738 | 0.326 | <0.001 | |
| Firmicutes | 0.248 | 0.543 | <0.001 | |||
| Prevotella | 0.4000 | 0.0161 | 0.006 | |||
| Ishaq et al., 2017 [43] | HT | Dialister | 0.0091 | 0.0446 | 0.029 | |
| Zhao et al., 2018 [45] | HT | Firmicutes | 0.826 | 0.691 | <0.001 | |
| Bacteroidetes | 0.099 | 0.227 | <0.001 | |||
| Faecalibacterium | 0.0987 | 0.1510 | 0.004 | |||
| Bacteroides | 0.0613 | 0.1330 | <0.001 | |||
| Prevotella_9 | 0.0183 | 0.0601 | <0.001 | |||
| Blautia | 0.0977 | 0.0586 | <0.001 | |||
| Roseburia | 0.0398 | 0.0312 | 0.010 | |||
| Lachnoclostridium | 0.0241 | 0.0285 | 0.013 | |||
| Ruminococcus_torques_group | 0.0306 | 0.0200 | 0.002 | |||
| Romboutsia | 0.0235 | 0.0146 | 0.006 | |||
| Dorea | 0.0200 | 0.0138 | 0.006 | |||
| Fusicatenibacter | 0.0186 | 0.0100 | <0.001 | |||
| Eubacterium_hallii_group | 0.0258 | 0.0110 | <0.001 | |||
| Microbial Phylum/Genus | Graves’ Disease (A) | Hashimoto’s Thyroiditis (B) | Healthy Controls (C) | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean, % | −95% CI | +95% CI | Mean, % | −95% CI | +95% CI | Mean, % | −95% CI | +95% CI | A vs. B | A vs. C | B vs. C | |
| Bacteroidetes | 48.964 | 31.446 | 66.615 | 39.038 | 19.009 | 61.224 | 33.948 | 26.366 | 41.968 | 0.487 | 0.089 | 0.628 |
| Bacteroides | 24.806 | 6.786 | 49.378 | 19.629 | 10.027 | 31.489 | 19.498 | 12.611 | 27.466 | 0.653 | 0.560 | 0.985 |
| Prevotella | 28.911 | 5.242 | 61.787 | 14.965 | 3.338 | 32.884 | 9.451 | 2.638 | 19.853 | 0.360 | 0.127 | 0.513 |
| Firmicutes | 47.207 | 34.033 | 60.582 | 49.955 | 27.895 | 72.025 | 50.185 | 35.499 | 64.854 | 0.835 | 0.779 | 0.986 |
| Actinobacteria | 4.692 | 1.087 | 10.638 | 3.641 | 0.824 | 8.347 | 2.77 | 0.632 | 6.356 | 0.737 | 0.482 | 0.713 |
| Bifidobacterium | 9.027 | 0.759 | 24.955 | 4.354 | 0.894 | 10.238 | 3.54 | 0.656 | 8.593 | 0.390 | 0.274 | 0.794 |
| TPOAb | TRAb | TGAb | TSH | |||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |
| Phyla | Firmicutes [41], Bacteroidetes [30,36,41], Actinobacteria [30] | Firmicutes [30], Proteobacteria [36], Synergistetes [31,36] | Firmicutes [41], Bacteroidetes [41] | Proteobacteria [36], Synergistetes [31,36] | Actinobacteria [36] | Synergistetes [31] | Firmicutes [30,36], Proteobacteria [36], Synergistetes [31,36] | Bacteroidetes [30,36] |
| Genera | Blautia [33,45], Lactobacillus [31], Streptococcus [36,45], Veillonella [36], Alistipes [40], Prevotella [30,36,41], Bifidobacterium [38] | Blautia [36], Faecalibacterium [30,40,45], Phascolarctobacterium [36,45], Alistipes [36], Bacteroides [33,36,45], Prevotella [45] | Lactobacillus [31], Streptococcus [36], Veillonella [36], Prevotella [36], Bifidobacterium [38] | Blautia [36], Lactobacillus [36], Phascolarctobacterium [31,36], Alistipes [36], Bacteroides [36] | Blautia [45], Streptococcus [36,45], Prevotella [36], Bifidobacterium [38] | Phascolarctobacterium [36,45], Bacteroides [45], Prevotella [45] | Blautia [36], Faecalibacterium [30], Lactobacillus [36], Phascolarctobacterium [31,36], Alistipes [36], Bacteroides [36] | Lactobacillus [31], Streptococcus [36], Veillonella [36], Prevotella [30,36] |
| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Patients with autoimmune thyroid diseases, including Graves’ disease and Hashimoto’s thyroiditis, aged 0–99 years, both sexes | Patients with other autoimmune diseases |
| Intervention | Not applicable | |
| Comparison | Not applicable | |
| Outcomes | Alterations in microbiota composition, including richness, diversity, and abundance indices | Alterations in microbiota composition without determined diversity indices |
| Study design | Case–control, cohort, and cross-sectional studies | Literature reviews, case reports, expert opinions, letters to the editor, conference reports |
| Published after 2000 | Not published in English |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawicka-Gutaj, N.; Gruszczyński, D.; Zawalna, N.; Nijakowski, K.; Muller, I.; Karpiński, T.; Salvi, M.; Ruchała, M. Microbiota Alterations in Patients with Autoimmune Thyroid Diseases: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 13450. https://doi.org/10.3390/ijms232113450
Sawicka-Gutaj N, Gruszczyński D, Zawalna N, Nijakowski K, Muller I, Karpiński T, Salvi M, Ruchała M. Microbiota Alterations in Patients with Autoimmune Thyroid Diseases: A Systematic Review. International Journal of Molecular Sciences. 2022; 23(21):13450. https://doi.org/10.3390/ijms232113450
Chicago/Turabian StyleSawicka-Gutaj, Nadia, Dawid Gruszczyński, Natalia Zawalna, Kacper Nijakowski, Ilaria Muller, Tomasz Karpiński, Mario Salvi, and Marek Ruchała. 2022. "Microbiota Alterations in Patients with Autoimmune Thyroid Diseases: A Systematic Review" International Journal of Molecular Sciences 23, no. 21: 13450. https://doi.org/10.3390/ijms232113450
APA StyleSawicka-Gutaj, N., Gruszczyński, D., Zawalna, N., Nijakowski, K., Muller, I., Karpiński, T., Salvi, M., & Ruchała, M. (2022). Microbiota Alterations in Patients with Autoimmune Thyroid Diseases: A Systematic Review. International Journal of Molecular Sciences, 23(21), 13450. https://doi.org/10.3390/ijms232113450










