Ibrutinib Prevents Acute Lung Injury via Multi-Targeting BTK, FLT3 and EGFR in Mice
Abstract
:1. Introduction
2. Results
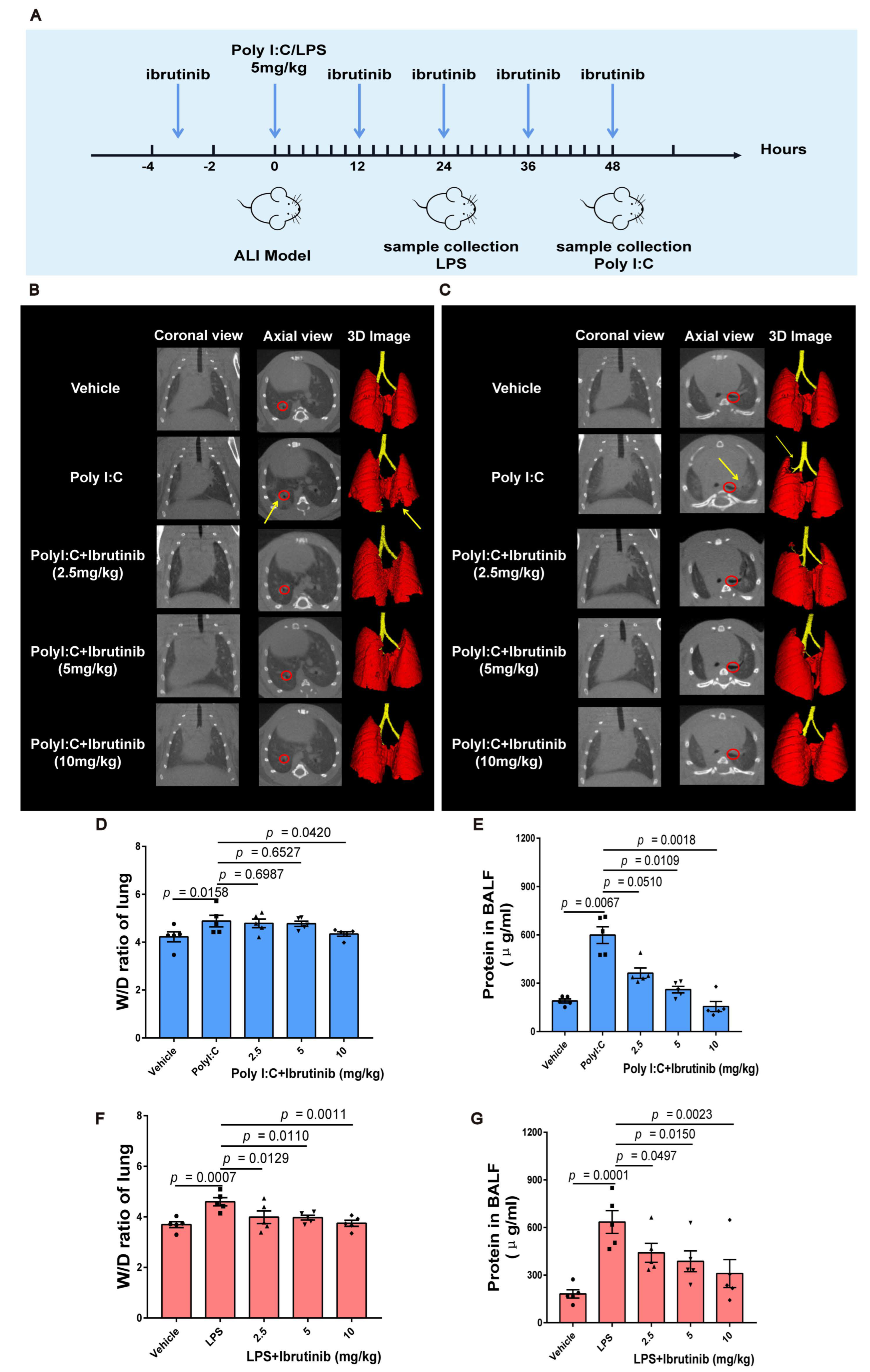
2.1. Ibrutinib Ameliorated Poly I:C and LPS-Induced Acute Lung Inflammation and Pulmonary Edema
2.2. Ibrutinib Suppressed Poly I:C- and LPS-Induced Inflammation in Lungs
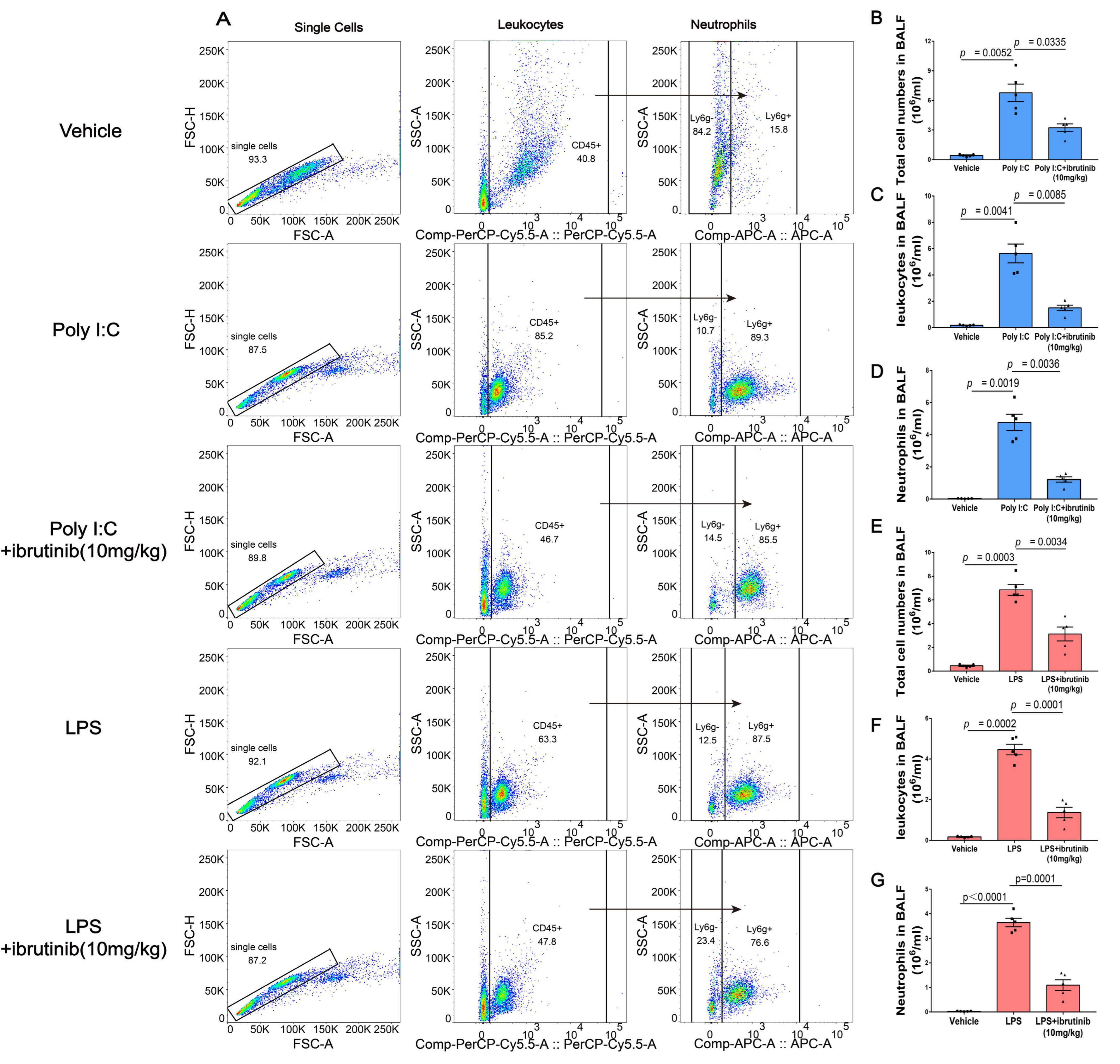
2.3. Ibrutinib Inhibited Poly I:C- and LPS-Induced Neutrophil Aggregation in BALF
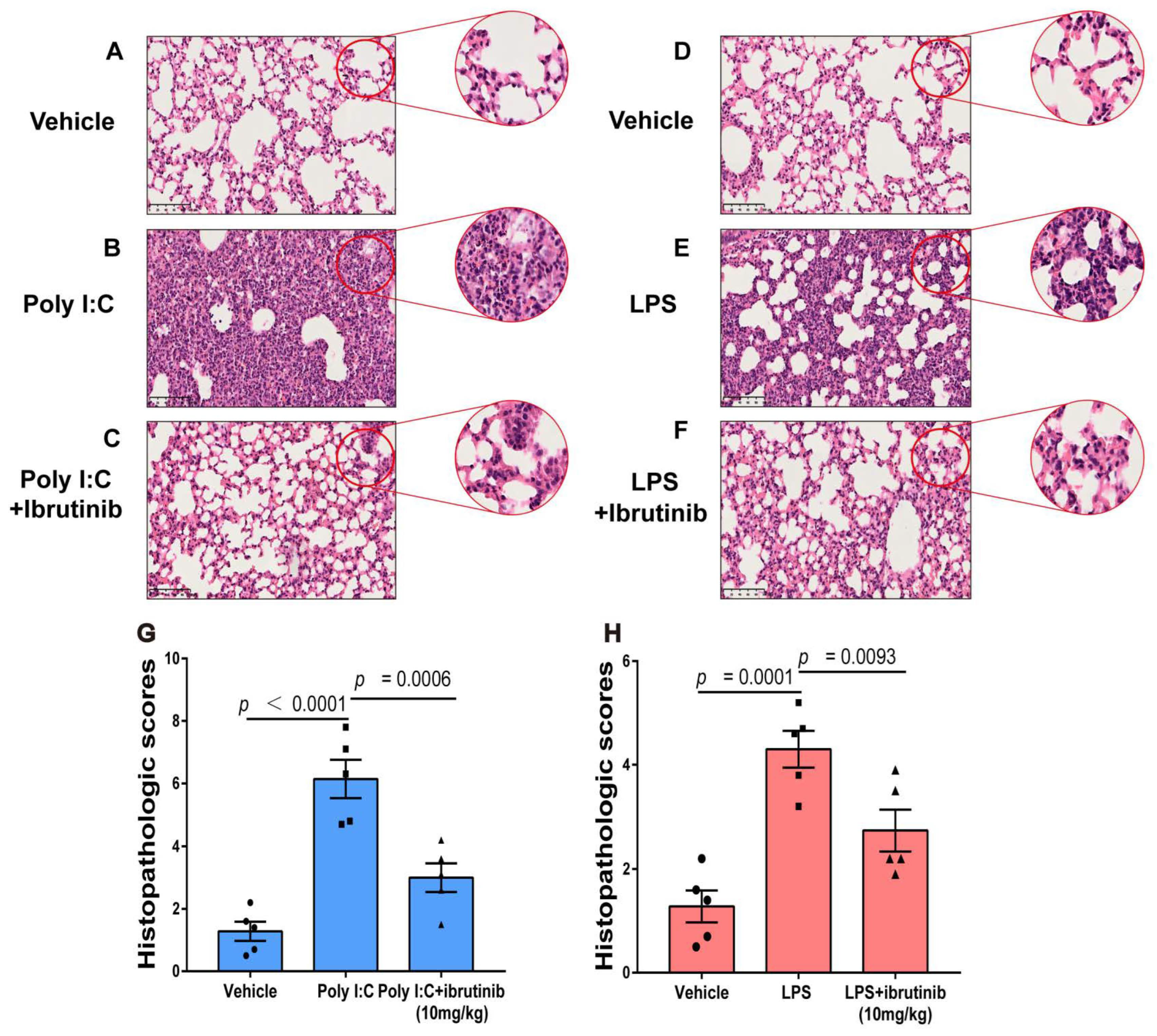
2.4. Ibrutinib Attenuated Poly I:C- and LPS-Induced Lung Histopathological Changes
2.5. Ibrutinib Suppressed Neutrophil and Inflammatory Factor Levels in Poly I:C- and LPS-Induced ALI
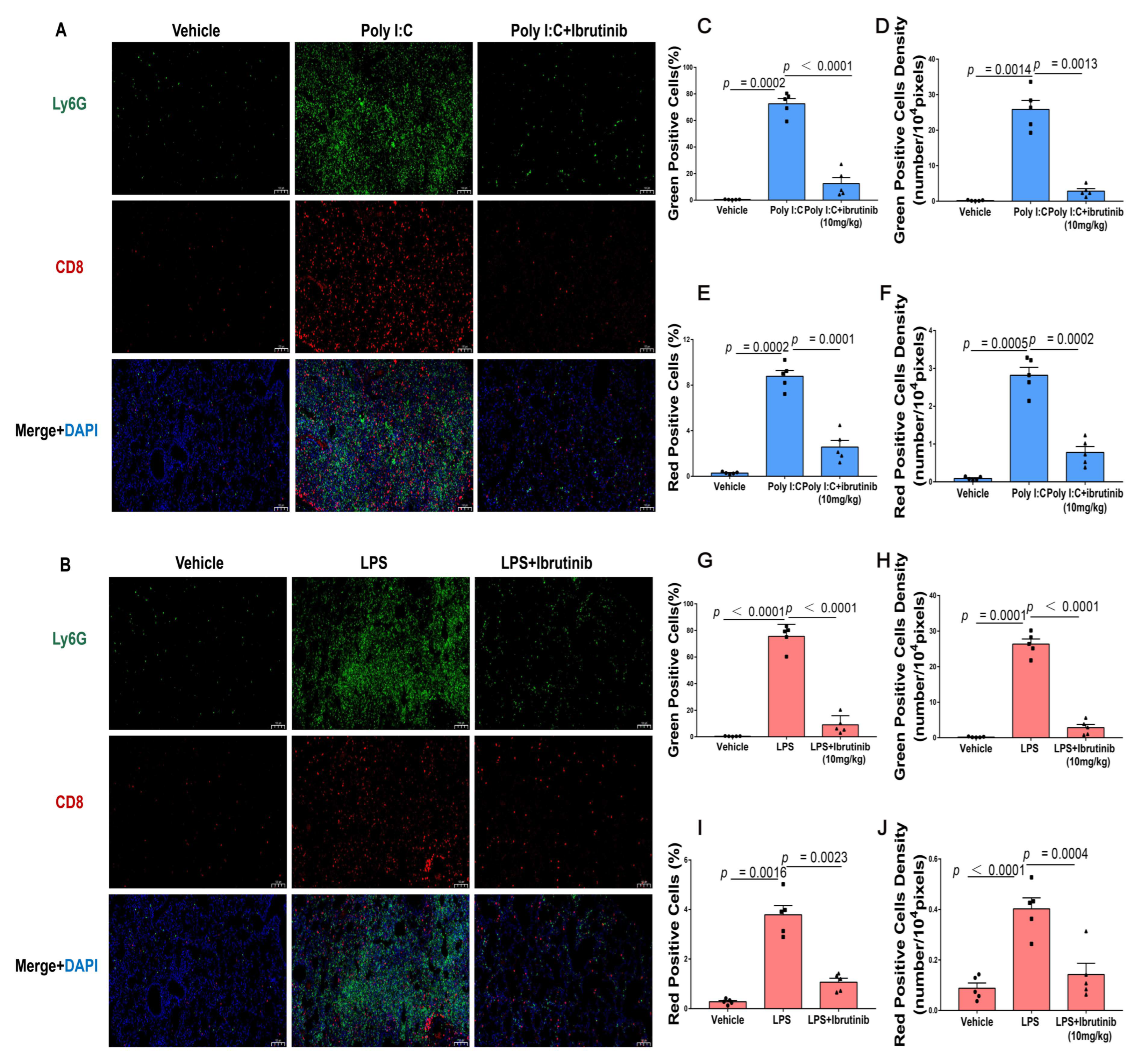
2.6. Neutrophil Infiltration (Ly6G) and CD8+ T-Cells Were Suppressed by Ibrutinib Treatment of Poly I:C- and LPS-Induced Lung Injury
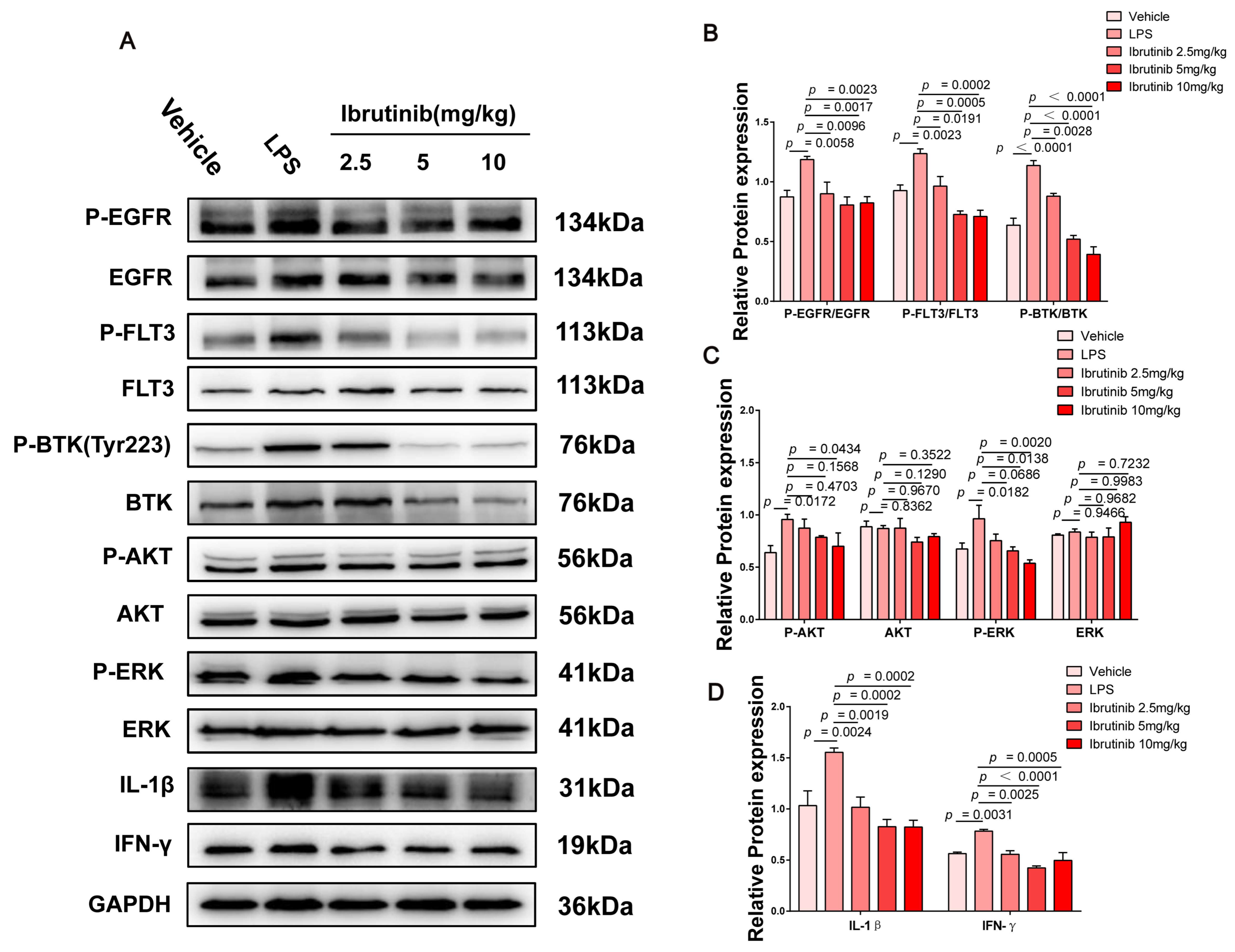
2.7. Ibrutinib Protected from Poly I:C- and LPS-Induced ALI by Inhibiting the BTK-, FLT3-, and EGFR-Related Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Establishment of Poly I:C and LPS-Induced ALI Model
4.3. In Vivo Micro-CT Imaging
4.4. Pulmonary Edema Assessment
4.5. Bronchoalveolar Lavage Fluid (BALF) Analysis
4.6. Assessment of Cytokine Levels
4.7. Pulmonary Histopathology
4.8. Immunohistochemical and Immunofluorescence Analysis
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Mishra, A.; Howland, E.; Zhao, C.; Shukla, D.; Weng, T.; Liu, L. Platelet-derived Wnt antagonist Dickkopf-1 is implicated in ICAM-1/VCAM-1-mediated neutrophilic acute lung inflammation. Blood 2015, 126, 2220–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, B.T.; Maioli, H.; Johnston, R.; Chaudhry, I.; Fink, S.L.; Xu, H.; Najafian, B.; Deutsch, G.; Lacy, J.M.; Williams, T.; et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet 2020, 396, 320–332. [Google Scholar] [CrossRef]
- Li, W.; Long, L.; Yang, X.; Tong, Z.; Southwood, M.; King, R.; Caruso, P.; Upton, P.D.; Yang, P.; Bocobo, G.A.; et al. Circulating BMP9 Protects the Pulmonary Endothelium during Inflammation-induced Lung Injury in Mice. Am. J. Respir. Crit. Care Med. 2021, 203, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Claser, C.; Nguee, S.Y.T.; Balachander, A.; Wu Howland, S.; Becht, E.; Gunasegaran, B.; Hartimath, S.V.; Lee, A.W.Q.; Theng Theng Ho, J.; Bing Ong, C.; et al. Lung endothelial cell antigen cross-presentation to CD8+T cells drives malaria-associated lung injury. Nat. Commun. 2019, 10, 4241, Correct in Nat. Commun. 2019, 10, 5066. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.Y.; Lin, M.S.; Kuo, T.C.; Chen, C.L.; Lin, C.C.; Chou, Y.C.; Chao, T.L.; Pang, Y.H.; Kao, H.C.; Huang, R.; et al. Humanized COVID-19 decoy antibody effectively blocks viral entry and prevents SARS-CoV-2 infection. EMBO Mol. Med. 2021, 13, e12828. [Google Scholar] [CrossRef]
- Chossière, G.P.; Xu, H.; Dixit, Y.; Isaacs, S.; Eastham, S.D.; Allroggen, F.; Speth, R.L.; Barrett, S.R.H. Air pollution impacts of COVID-19-related containment measures. Sci. Adv. 2021, 7, eabe1178. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhou, C.C.; Yu, L.Y.; Wang, L.; Deng, J.L.; Tao, Y.L.; Zhang, F.; Chen, W.S. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharm. Res. 2021, 163, 105224. [Google Scholar] [CrossRef]
- Zoulikha, M.; Xiao, Q.; Boafo, G.F.; Sallam, M.A.; Chen, Z.; He, W. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta Pharm. Sin. B 2022, 12, 600–620. [Google Scholar] [CrossRef]
- Feng, Z.; Zhou, J.; Liu, Y.; Xia, R.; Li, Q.; Yan, L.; Chen, Q.; Chen, X.; Jiang, Y.; Chao, G.; et al. Epithelium- and endothelium-derived exosomes regulate the alveolar macrophages by targeting RGS1 mediated calcium signaling-dependent immune response. Cell Death Differ. 2021, 28, 2238–2256. [Google Scholar] [CrossRef]
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 diagnosis -A review of current methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef]
- Lewis, N.S.; Banyard, A.C.; Whittard, E.; Karibayev, T.; Al Kafagi, T.; Chvala, I.; Byrne, A.; Meruyert Akberovna, S.; King, J.; Harder, T.; et al. Emergence and spread of novel H5N8, H5N5 and H5N1 clade 2.3.4.4 highly pathogenic avian influenza in 2020. Emerg. Microbes Infect. 2021, 10, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Finkel, Y.; Mizrahi, O.; Nachshon, A.; Weingarten-Gabbay, S.; Morgenstern, D.; Yahalom-Ronen, Y.; Tamir, H.; Achdout, H.; Stein, D.; Israeli, O.; et al. The coding capacity of SARS-CoV-2. Nature 2021, 589, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; Zhang, X.; Yang, Y.; Zhu, J.; Du, L. Advances in mRNA and other vaccines against MERS-CoV. Transl Res. 2021, 242, 20–37. [Google Scholar] [CrossRef] [PubMed]
- West, R.; Kobokovich, A.; Connell, N.; Gronvall, G.K. COVID-19 Antibody Tests: A Valuable Public Health Tool with Limited Relevance to Individuals. Trends Microbiol. 2021, 29, 214–223. [Google Scholar] [CrossRef]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef]
- Nie, X.; Qian, L.; Sun, R.; Huang, B.; Dong, X.; Xiao, Q.; Zhang, Q.; Lu, T.; Yue, L.; Chen, S.; et al. Multi-organ proteomic landscape of COVID-19 autopsies. Cell 2021, 184, 775–791.e14. [Google Scholar] [CrossRef]
- Panda, R.; Castanheira, F.V.; Schlechte, J.M. A functionally distinct neutrophil landscape in severe COVID-19 reveals opportunities for adjunctive therapies. JCI Insight. 2022, 7, e152291. [Google Scholar] [CrossRef]
- Chen, E.; Chen, C.; Niu, Z.; Gan, L.; Wang, Q.; Li, M.; Cai, X.; Gao, R.; Katakam, S.; Chen, H.; et al. Poly(I:C) preconditioning protects the heart against myocardial ischemia/reperfusion injury through TLR3/PI3K/Akt-dependent pathway. Signal Transduct. Target. Ther. 2020, 5, 216. [Google Scholar] [CrossRef]
- Cai, X.; Panicker, S.R.; Biswas, I.; Giri, H.; Rezaie, A.R. Protective Role of Activated Protein C against Viral Mimetic Poly(I:C)-Induced Inflammation. Thromb. Haemost. 2021, 121, 1448–1463. [Google Scholar] [CrossRef]
- Tominari, T.; Akita, M.; Matsumoto, C.; Hirata, M.; Yoshinouchi, S.; Tanaka, Y.; Karouji, K.; Itoh, Y.; Maruyama, T.; Miyaura, C.; et al. Endosomal TLR3 signaling in stromal osteoblasts induces prostaglandin E2-mediated inflammatory periodontal bone resorption. J. Biol. Chem. 2022, 101603. [Google Scholar] [CrossRef]
- Jin, S.; Ding, X.; Yang, C.; Li, W.; Deng, M.; Liao, H.; Lv, X.; Pitt, B.R.; Billiar, T.R.; Zhang, L.M.; et al. Mechanical Ventilation Exacerbates Poly (I:C) Induced Acute Lung Injury: Central Role for Caspase-11 and Gut-Lung Axis. Front. Immunol. 2021, 12, 693874. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Yang, Y.; Hu, F.; Chen, X.; Zhou, J.; Li, Y.; Xu, Y.; Wang, H.; Chen, Y.; Zhang, M. TLR3 Regulated Poly I:C-Induced Neutrophil Extracellular Traps and Acute Lung Injury Partly Through p38 MAP Kinase. Front. Microbiol. 2018, 9, 3174. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Martín-Fernández, M.; Heredia-Rodríguez, M.; González-Jiménez, I.; Lorenzo-López, M.; Gómez-Pesquera, E.; Poves-Álvarez, R.; Álvarez, F.J.; Jorge-Monjas, P.; Beltrán-DeHeredia, J.; Gutiérrez-Abejón, E.; et al. Hyperoxemia in postsurgical sepsis/septic shock patients is associated with reduced mortality. Crit. Care 2022, 26, 4. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, M.P.; Tjahjono, N.; Light, Y.K.; Celebi, A.N.; Celebi, N.N.; Chintalapudi, P.; Butler, K.S.; Branda, S.S.; Krishnakumar, R. Upregulation of CD14 in mesenchymal stromal cells accelerates lipopolysaccharide-induced response and enhances antibacterial properties. iScience 2022, 25, 103759. [Google Scholar] [CrossRef]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef]
- Zheng, X.; Li, S.; Yang, H. Roles of Toll-Like Receptor 3 in Human Tumors. Front. Immunol. 2021, 12, 667454. [Google Scholar] [CrossRef]
- Francisco, S.; Billod, J.M.; Merino, J.; Punzón, C.; Gallego, A.; Arranz, A.; Martin-Santamaria, S.; Fresno, M. Induction of TLR4/TLR2 Interaction and Heterodimer Formation by Low Endotoxic Atypical LPS. Front. Immunol. 2022, 12, 748303. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Afzal, O.; Almalki, W.H.; Kazmi, I.; Javed Shaikh, M.A.; Thangavelu, L.; Gulati, M.; Singh, S.K.; Jha, N.K.; Gupta, P.K.; et al. Nuclear factor-kappa B (NF-κB) inhibition as a therapeutic Target. for plant nutraceuticals in mitigating inflammatory lung diseases. Chem. Biol. Interact. 2022, 354, 109842. [Google Scholar] [CrossRef]
- Smith, C.I.; Baskin, B.; Humire-Greiff, P.; Zhou, J.N.; Olsson, P.G.; Maniar, H.S.; Kjellén, P.; Lambris, J.D.; Christensson, B.; Hammarström, L.; et al. Expression of Bruton’s agammaglobulinemia tyrosine kinase gene, BTK, is selectively down-regulated in T lymphocytes and plasma cells. J. Immunol. 1994, 152, 557–565. [Google Scholar]
- Ran, F.; Liu, Y.; Wang, C.; Xu, Z.; Zhang, Y.; Liu, Y.; Zhao, G.; Ling, Y. Review of the development of BTK inhibitors in overcoming the clinical limitations of ibrutinib. Eur. J. Med. Chem. 2022, 229, 114009. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Mulder, T.A.; Österborg, A. BTK Inhibitors in Chronic Lymphocytic Leukemia: Biological Activity and Immune Effects. Front. Immunol. 2021, 12, 686768. [Google Scholar] [CrossRef] [PubMed]
- Vetrie, D.; Vořechovský, I.; Sideras, P.; Holland, J.; Davies, A.; Flinter, F.; Hammarström, L.; Kinnon, C.; Levinsky, R.; Bobrow, M.; et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature 1993, 361, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Wang, F.; Sun, T.; Xu, Z.; Zhang, J.; Bernard, D.; Xu, S.; Wang, S.; Kaminski, M.; Devata, S.; et al. Follicular Lymphoma-associated BTK Mutations are Inactivating Resulting in Augmented AKT Activation. Clin. Cancer Res. 2021, 27, 2301–2313. [Google Scholar] [CrossRef] [PubMed]
- Purvis, G.S.D.; Aranda-Tavio, H.; Channon, K.M.; Greaves, D.R. Bruton’s tyrosine kinase (BTK) regulatesmyeloid cell recruitment during acute inflammation. Br. J. Pharmacol. 2021, 179, 2754–2770. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.Y.; Nam, J.H.; Yoon, G.; Lee, J.Y.; Nam, Y.; Kang, H.J.; Cho, H.J.; Kim, J.; Hoe, H.S. Ibrutinib suppresses LPS-induced neuroinflammatory responses in BV2 microglial cells and wild-type mice. J. Neuroinflamm. 2018, 15, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murali, I.; Kasar, S.; Naeem, A.; Tyekucheva, S.; Khalsa, J.K.; Thrash, E.M.; Itchaki, G.; Livitz, D.; Leshchiner, I.; Dong, S.; et al. Activation of the MAPK pathway mediates resistance to PI3K inhibitors in chronic lymphocytic leukemia. Blood 2021, 138, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.I.; Chen, P.J.; Wang, Y.H.; Tseng, H.H.; Chang, S.H.; Wu, T.S.; Yang, S.H.; Lee, Y.T.; Hwang, T.L. Bletinib ameliorates neutrophilic inflammation and lung injury by inhibiting Src family kinase phosphorylation and activity. Br. J. Pharmacol. 2021, 178, 4069–4084. [Google Scholar] [CrossRef]
- Benner, B.; Carson, W.E. Observations on the use of Bruton’s tyrosine kinase inhibitors in SAR-CoV-2 and cancer. J. Hematol Oncol. 2021, 14, 15. [Google Scholar] [CrossRef]
- Flinsenberg, T.W.H.; Tromedjo, C.C.; Hu, N.; Liu, Y.; Guo, Y.; Thia, K.Y.T.; Noori, T.; Song, X.; Aw Yeang, H.X.; Tantalo, D.G.; et al. Differential effects of BTK inhibitors ibrutinib and zanubrutinib on NK-cell effector function in patients with mantle cell lymphoma. Haematologica 2020, 105, e76–e79. [Google Scholar] [CrossRef] [Green Version]
- Hänel, G.; Angerer, C.; Petry, K.; Lichtenegger, F.S.; Subklewe, M. Blood DCs activated with R848 and poly(I:C) induce antigen-specific immune responses against viral and tumor-associated antigens. Cancer Immunol. Immunother. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.S.; Hoffman, E.A.; Stokes, J.; Kummet, N.; Smith, K.A.; Baker, F.; Zúñiga, T.M.; Simpson, R.J.; Katsanis, E. Regulatory Dendritic Cells Induced by Bendamustine Are Associated With Enhanced Flt3 Expression and Alloreactive T-Cell Death. Front. Immunol. 2021, 12, 699128. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; He, H.L.; Lu, X.M.; Yang, Y.; Qiu, H.B. Modulation of FLT3 signaling targets conventional dendritic cells to attenuate acute lung injury. APMIS 2012, 120, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.C.; Chen, L.C.; Huang, H.L.; HuangFu, W.C.; Chen, Y.Y.; Eight Lin, T.; Lien, S.T.; Tseng, H.J.; Sung, T.Y.; Hsieh, J.H.; et al. Identification of a dual FLT3 and MNK2 inhibitor for acute myeloid leukemia treatment using a structure-based virtual screening approach. Bioorg. Chem. 2022, 121, 105675. [Google Scholar] [CrossRef]
- Zhu, W.J.; Lin, L.P.; Liu, D.; Qian, J.C.; Zhou, B.B.; Yuan, D.D.; Tan, R.X. Pharmacophore-inspired discovery of FLT3 inhibitor from kimchi. Food Chem. 2021, 361, 130139. [Google Scholar] [CrossRef]
- Wang, F.; Huang, J.; Guo, T.; Zheng, Y.; Zhang, L.; Zhang, D.; Wang, F.; Naren, D.; Cui, Y.; Liu, X.; et al. Homoharringtonine synergizes with quizartinib in FLT3-ITD acute myeloid leukemia by targeting FLT3-AKT-c-Myc pathway. BioChem. Pharmacol. 2021, 188, 114538. [Google Scholar] [CrossRef]
- Liu, X.; Yue, C.; Shi, L.; Liu, G.; Cao, Q.; Shan, Q.; Wang, Y.; Chen, X.; Li, H.; Wang, J.; et al. MALT1 is a potential therapeutic Target. in glioblastoma and plays a crucial role in EGFR-induced NF-κB activation. J. Cell Mol. Med. 2020, 24, 7550–7562. [Google Scholar] [CrossRef]
- Tao, H.; Li, N.; Zhang, Z.; Mu, H.; Meng, C.; Xia, H.; Fu, L.; Xu, Y.; Zhang, S. Erlotinib Protects LPS-Induced Acute Lung Injury in Mice by Inhibiting EGFR/TLR4 Signaling Pathway. Shock 2019, 51, 131–138. [Google Scholar] [CrossRef]
- De, S.; Zhou, H.; DeSantis, D.; Croniger, C.M.; Li, X.; Stark, G.R. Erlotinib protects against LPS-induced endotoxicity because TLR4 needs EGFR to signal. Proc. Natl. Acad. Sci. USA 2015, 112, 9680–9685. [Google Scholar] [CrossRef] [Green Version]
- Ondrisova, L.; Mraz, M. Genetic and Non-Genetic Mechanisms of Resistance to BCR Signaling Inhibitors in B Cell Malignancies. Front. Oncol. 2020, 10, 591577. [Google Scholar] [CrossRef]
- Treon, S.P.; Castillo, J.J.; Skarbnik, A.P.; Soumerai, J.D.; Ghobrial, I.M.; Guerrera, M.L.; Meid, K.; Yang, G. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood 2020, 135, 1912–1915. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.Y.; Zhou, S.N.; Pan, W.T.; Sun, J.; Yang, L.Q.; Zhang, L.; Qiu, M.Z.; Yang, D.J. A multi-kinase inhibitor APG-2449 enhances the antitumor effect of ibrutinib in esophageal squamous cell carcinoma via EGFR/FAK pathway inhibition. Biochem. Pharmacol. 2021, 183, 114318. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Huang, Y.; Zhang, B.; Lin, N. The effect of ibrutinib on radiosensitivity in pancreatic cancer cells by targeting EGFR/AKT/mTOR signaling pathway. Biomed. Pharmacother. 2020, 128, 110133. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Elkholy, K.H.; Wani, N.A.; Li, D.; Hu, P.; Barajas, J.M.; Yu, L.; Zhang, X.; Jacob, S.T.; Khan, W.N.; et al. Ibrutinib Potentiates Antihepatocarcinogenic Efficacy of Sorafenib by Targeting EGFR in Tumor Cells and BTK in Immune Cells in the Stroma. Mol. Cancer Ther. 2020, 19, 384–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Zhang, H.; Cao, M.; Wang, L.; Wu, S.; Fang, B. Auranofin Enhances Ibrutinib’s Anticancer Activity in EGFR-Mutant Lung Adenocarcinoma. Mol. Cancer Ther. 2018, 17, 2156–2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillinger, G.; Abdul-Aziz, A.; Zaitseva, L.; Lawes, M.; MacEwan, D.J.; Bowles, K.M.; Rushworth, S.A. Targeting BTK for the treatment of FLT3-ITD mutated acute myeloid leukemia. Sci. Rep. 2015, 5, 12949. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Wang, H.; Liu, J.; Zhang, Y.; Luo, J.; Li, Y.; Yang, C.; Jiang, J. Ganoderic acid B attenuates LPS-induced lung injury. Int. Immunopharmacol. 2020, 88, 106990. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, Z.; Jiang, A.; Wu, D.; Li, S.; Liu, Z.; Wei, Z.; Yang, Z.; Guo, C. Protective Effects of Pterostilbene on Lipopolysaccharide-Induced Acute Lung Injury in Mice by Inhibiting NF-κB and Activating Nrf2/HO-1 Signaling Pathways. Front. Pharmacol. 2021, 11, 591836. [Google Scholar] [CrossRef]
- Jiang, L.; Shen, Y.; Guo, D.; Yang, D.; Liu, J.; Fei, X.; Yang, Y.; Zhang, B.; Lin, Z.; Yang, F.; et al. EpCAM-dependent extracellular vesicles from intestinal epithelial cells maintain intestinal tract immune balance. Nat. Commun. 2016, 7, 13045, Correct in Nat. Commun. 2017, 8, 16006; Correct in Nat. Commun. 2020, 11, 3655. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.Y.; Li, X.Q.; Hu, W.P.; Cu, S.C.; Dai, J.J.; Gao, Y.N.; Zhang, Y.T.; Bai, X.Y.; Shi, D.Y. Self-developed NF-κB inhibitor 270 protects against LPS-induced acute kidney injury and lung injury through improving inflammation. Biomed. Pharmacother. 2022, 147, 112615. [Google Scholar] [CrossRef]
- Hu, Q.; Lyon, C.J.; Fletcher, J.K.; Tang, W.; Wan, M.; Hu, T.Y. Extracellular vesicle activities regulating macrophage- and tissue-mediated injury and repair responses. Acta Pharm. Sin. B 2021, 11, 1493–1512. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Xu, M.M.; Fan, C.; Feng, C.L.; Lu, Q.K.; Lu, H.M.; Xiang, C.G.; Bai, F.; Wang, H.Y.; Wu, Y.W.; et al. STING inhibitor ameliorates LPS-induced ALI by preventing vascular endothelial cells-mediated immune cells chemotaxis and adhesion. Acta Pharmacol. Sin. 2021, 43, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Peritore, A.F.; D’Amico, R.; Siracusa, R.; Cordaro, M.; Fusco, R.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Di Paola, R.; Cuzzocrea, S.; et al. Management of Acute Lung Injury: Palmitoylethanolamide as a New Approach. Int. J. Mol. Sci. 2021, 22, 5533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Ye, S.B.; Ni, J.J.; Cai, T.T.; Liu, Y.N.; Huang, D.J.; Mai, H.Q.; Chen, Q.Y.; He, J.; Zhang, X.S.; et al. STING signaling remodels the tumor microenvironment by antagonizing myeloid-derived suppressor cell expansion. Cell Death Differ. 2019, 26, 2314–2328. [Google Scholar] [CrossRef]
- Shang, A.; Gu, C.; Wang, W.; Wang, X.; Sun, J.; Zeng, B.; Chen, C.; Chang, W.; Ping, Y.; Ji, P.; et al. Exosomal circPACRGL promotes progression of colorectal cancer via the miR-142-3p/miR-506-3p- TGF-β1 axis. Mol. Cancer 2020, 19, 117. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, J.; Feng, B.; Lin, F.; Zhou, J.; Liu, J.; Shi, X.; Lu, X.; Pan, Q.; Yu, J.; et al. Immunosuppressive effect of mesenchymal stem cells on lung and gut CD8+ T cells in lipopolysaccharide-induced acute lung injury in mice. Cell Prolif. 2021, 54, e13028. [Google Scholar] [CrossRef]
- Lee, G.C.; Restrepo, M.I.; Harper, N.; Manoharan, M.S.; Smith, A.M.; Meunier, J.A.; Sanchez-Reilly, S.; Ehsan, A.; Branum, A.P.; Winter, C.; et al. Immunologic resilience and COVID-19 survival advantage. J. Allergy Clin. Immunol. 2021, 148, 1176–1191. [Google Scholar] [CrossRef]
- Yang, C.C.; Wu, C.J.; Chien, C.Y.; Chien, C.T. Green Tea Polyphenol Catechins Inhibit Coronavirus Replication and Potentiate the Adaptive Immunity and Autophagy-Dependent Protective Mechanism to Improve Acute Lung Injury in Mice. Antioxidants 2021, 10, 928. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Irfan, M.; Quah, Y.; Saba, E.; Kim, S.D.; Park, S.C.; Jeong, M.G.; Kwak, Y.S.; Rhee, M.H. The increasing hematopoietic effect of the combined treatment ofKorean Red Ginseng and Colla corii asini on cyclophosphamide-induced immunosuppression in mice. J. Ginseng Res. 2021, 45, 591–598. [Google Scholar] [CrossRef]
- Wilson, A.J.; Troy-Barnes, E.; Subhan, M.; Clark, F.; Gupta, R.; Fielding, A.K.; Kottaridis, P.; Mansour, M.R.; O’Nions, J.; Payne, E.; et al. Successful remission induction therapy with gilteritinib in a patient with de novo FLT3-mutated acute myeloid leukaemia and severe COVID-19. Br. J. Haematol. 2020, 190, e189–e191. [Google Scholar] [CrossRef]
- Meng, C.; Wang, S.; Wang, X.; Lv, J.; Zeng, W.; Chang, R.; Li, Q.; Wang, X. Amphiregulin inhibits TNF-α-induced alveolar epithelial cell death through EGFR signaling pathway. Biomed. Pharmacother. 2020, 125, 109995. [Google Scholar] [CrossRef] [PubMed]
- Vagapova, E.R.; Lebedev, T.D.; Prassolov, V.S. Viral fibrotic scoring and drug screen based on MAPKactivity uncovers EGFR as a key regulator of COVID-19 fibrosis. Sci. Rep. 2021, 11, 11234. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Li, Z.Y.; Dong, L.L.; Li, W.J.; Wu, Y.P.; Wang, J.; Chen, H.P.; Liu, H.W.; Li, M.; Jin, C.L.; et al. Inactivation of MTOR promotes autophagy-mediated epithelial injury in particulate matter-induced airway inflammation. Autophagy 2020, 16, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef]
- Letsiou, E.; Sammani, S.; Wang, H.; Belvitch, P.; Dudek, S.M. Parkin regulates lipopolysaccharide-induced proinflammatory responses in acute lung injury. Transl. Res. 2017, 181, 71–82. [Google Scholar] [CrossRef]
- Guo, L.; Li, S.; Zhao, Y.; Qian, P.; Ji, F.; Qian, L.; Wu, X.; Qian, G. Silencing Angiopoietin-Like Protein 4 (ANGPTL4) Protects Against Lipopolysaccharide-Induced Acute Lung Injury Via Regulating SIRT1 /NF-kB Pathway. J. Cell Physiol. 2015, 230, 2390–2402. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Wang, C.; He, C.; Ma, Q.; Li, J.; Wang, W.; Xu, Y.T.; Wang, T. Qingwenzhike Prescription Alleviates Acute Lung Injury Induced by LPS via Inhibiting TLR4/NF-kB Pathway and NLRP3 Inflammasome Activation. Front. Pharmacol. 2021, 12, 790072. [Google Scholar] [CrossRef]
- Galvão-Filho, B.; de Castro, J.T.; Figueiredo, M.M.; Rosmaninho, C.G.; Antonelli, L.R.D.V.; Gazzinelli, R.T. The emergence of pathogenic TNF/iNOS producing dendritic cells (Tip-DCs) in a malaria model of acute respiratory distress syndrome (ARDS) is dependent on CCR4. Mucosal Immunol. 2019, 12, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef]
- Heuberger, J.; Trimpert, J.; Vladimirova, D.; Goosmann, C.; Lin, M.; Schmuck, R.; Mollenkopf, H.J.; Brinkmann, V.; Tacke, F.; Osterrieder, N.; et al. Epithelial response to IFN-γ promotes SARS-CoV-2 infection. EMBO Mol. Med. 2021, 13, e13191. [Google Scholar] [CrossRef]
- Ye, B.; Zhou, C.; Guo, H.; Zheng, M. Effects of BTK signalling in pathogenic microorganism infections. J. Cell Mol. Med. 2019, 23, 6522–6529. [Google Scholar] [CrossRef] [PubMed]
- Florence, J.M.; Krupa, A.; Booshehri, L.M.; Davis, S.A.; Matthay, M.A.; Kurdowska, A.K. Inhibiting Bruton’s tyrosine kinase rescues mice from lethal influenza-induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L52–L58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maynard, S.; Ros-Soto, J.; Chaidos, A.; Innes, A.; Paleja, K.; Mirvis, E.; Buti, N.; Sharp, H.; Palanicawandar, R.; Milojkovic, D. The role of ibrutinib in COVID-19 hyperinflammation: A case report. Int. J. Infect Dis. 2021, 105, 274–276. [Google Scholar] [CrossRef]
- Wu, H.; Hu, C.; Wang, A.; Weisberg, E.L.; Wang, W.; Chen, C.; Zhao, Z.; Yu, K.; Liu, J.; Wu, J.; et al. Ibrutinib selectively targets FLT3-ITD in mutant FLT3-positive AML. Leukemia 2016, 30, 754–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Liu, C.; Tsui, S.T.; Liu, D. Second-generation inhibitors of Bruton tyrosine kinase. J. Hematol Oncol. 2016, 9, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budak, C.; Mençik, V.; Gider, V. Determining similarities of COVID-19—Lung cancer drugs and affinity binding mode analysis by graph neural network-based GEFA method. J. Biomol. Struct. Dyn. 2021, 1–13. [Google Scholar] [CrossRef]
- Shan, X.; Zhang, Y.; Chen, H.; Dong, L.; Wu, B.; Xu, T.; Hu, J.; Liu, Z.; Wang, W.; Wu, L.; et al. Inhibition of epidermal growth factor receptor attenuates LPS-induced inflammation and acute lung injury in rats. Oncotarget 2017, 8, 26648–26661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musie, E.; Moore, C.C.; Martin, E.N.; Scheld, W.M. Toll-like receptor 4 stimulation before or after Streptococcus pneumoniae induced sepsis improves survival and is dependent on T-cells. PLoS ONE 2014, 9, e86015. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, S.M.; Peer, C.J.; Figg, W.D. Ibrutinib’s off-Target. mechanism: Cause for dose optimization. Cancer Biol. Ther. 2021, 22, 529–531. [Google Scholar] [CrossRef]
- Shatzel, J.J.; Olson, S.R.; Tao, D.L.; McCarty, O.J.T.; Danilov, A.V.; DeLoughery, T.G. Ibrutinib-associated bleeding: Pathogenesis, management and risk reduction strategies. J. Thromb. Haemost. 2017, 15, 835–847. [Google Scholar] [CrossRef] [Green Version]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K. Baricitinib plus Remdesivir for Hospitalized Adults withCovid-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Marconi, V.C.; Ramanan, A.V.; de Bono, S.; Kartman, C.E.; Krishnan, V.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Alatorre-Alexander, J.; de Cassia Pellegrini, R.; et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021, 9, 1407–1418. [Google Scholar] [CrossRef]
- Smith, D.P.; Oechsle, O.; Rawling, M.J.; Savory, E.; Lacoste, A.M.B.; Richardson, P.J. Expert-Augmented Computational Drug Repurposing Identified Baricitinib as a Treatment for COVID-19. Front. Pharmacol. 2021, 12, 709856. [Google Scholar] [CrossRef] [PubMed]
- Roschewski, M.; Lionakis, M.S.; Sharman, J.P.; Roswarski, J.; Goy, A.; Monticelli, M.A.; Roshon, M.; Wrzesinski, S.H.; Desai, J.V.; Zarakas, M.A.; et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci. Immunol. 2020, 5, eabd0110. [Google Scholar] [CrossRef]
- von Hundelshausen, P.; Siess, W. Bleeding by Bruton Tyrosine Kinase-Inhibitors: Dependency on Drug Type and Disease. Cancers 2021, 13, 1103. [Google Scholar] [CrossRef]
- Zdżalik-Bielecka, D.; Kozik, K.; Poświata, A.; Jastrzębski, K.; Jakubik, M.; Miączyńska, M. Bemcentinib and Gilteritinib Inhibit Cell Growth and Impair the Endo-Lysosomal and Autophagy Systems in an AXL-Independent Manner. Mol. Cancer Res. 2022, 20, 446–455. [Google Scholar] [CrossRef]
- Schein, C.H. Repurposing approved drugs for cancer therapy. Br. Med. Bull. 2021, 137, 13–27. [Google Scholar] [CrossRef]
- Liu, M.; Shi, L.; Zou, X.; Zheng, X.; Zhang, F.; Ding, X.; Zhu, H.; Shen, Y. Caspase inhibitor zVAD-fmk protects against acute pancreatitis-associated lung injury via inhibiting inflammation and apoptosis. Pancreatology 2016, 16, 733–738. [Google Scholar] [CrossRef]
- Shen, W.; Gan, J.; Xu, S.; Jiang, G.; Wu, H. Penehyclidine hydrochloride attenuates LPS-induced acute lung injury involvement of NF-kappaB pathway. Pharm. Res. 2009, 60, 296–302. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, H.; Song, X.; Lei, J.; Lu, P.; Zhao, G.; Kang, X.; Zhang, D.; Zhang, T.; Ren, Y.; Peng, C.; et al. Ibrutinib Prevents Acute Lung Injury via Multi-Targeting BTK, FLT3 and EGFR in Mice. Int. J. Mol. Sci. 2022, 23, 13478. https://doi.org/10.3390/ijms232113478
Rao H, Song X, Lei J, Lu P, Zhao G, Kang X, Zhang D, Zhang T, Ren Y, Peng C, et al. Ibrutinib Prevents Acute Lung Injury via Multi-Targeting BTK, FLT3 and EGFR in Mice. International Journal of Molecular Sciences. 2022; 23(21):13478. https://doi.org/10.3390/ijms232113478
Chicago/Turabian StyleRao, Huanan, Xiaominting Song, Jieting Lei, Peng Lu, Guiying Zhao, Xin Kang, Duanna Zhang, Tingrui Zhang, Yali Ren, Cheng Peng, and et al. 2022. "Ibrutinib Prevents Acute Lung Injury via Multi-Targeting BTK, FLT3 and EGFR in Mice" International Journal of Molecular Sciences 23, no. 21: 13478. https://doi.org/10.3390/ijms232113478




