Synthesis of an Anti-CD7 Recombinant Immunotoxin Based on PE24 in CHO and E. coli Cell-Free Systems
Abstract
1. Introduction
2. Results
2.1. E. coli-Based Cell-Free Production of PE24 RIT
2.2. CHO-Based Cell-Free Production of PE24 RIT
3. Discussion
3.1. Chaperone Screening for Soluble Yield and Activity of PE24 RIT in E. coli Cell-Free System
3.2. Production of PE24 RIT in CHO Cell-Free System
3.3. Comparison of CHO and E. coli Cell-Free-Produced PE24 RIT
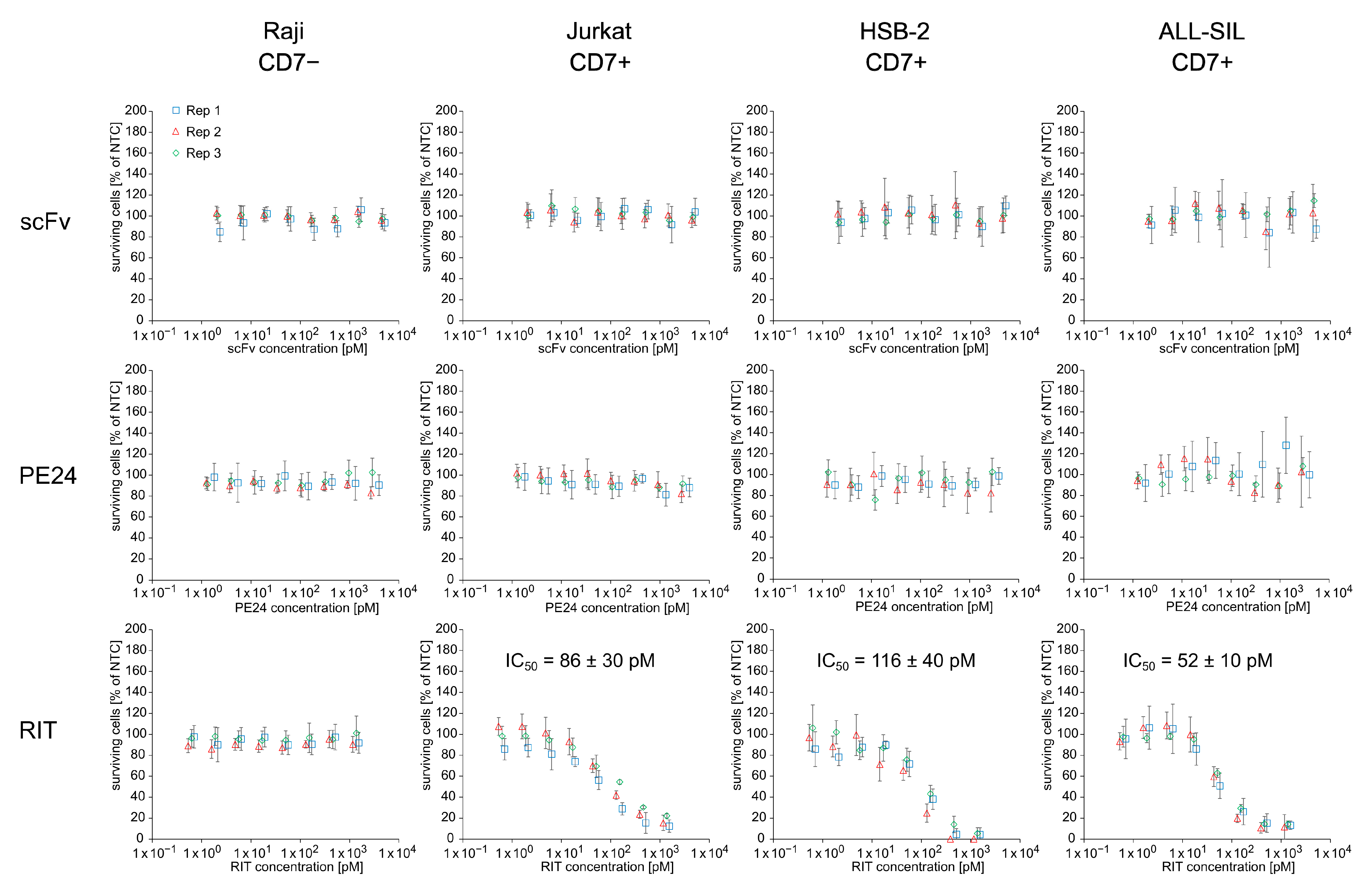
3.4. Anti-CD7-Targeted T Cell Depletion
4. Materials and Methods
4.1. DNA Templates for Cell-Free Protein Synthesis
4.1.1. Regulatory Elements
4.1.2. ScFv-Targeting Domain
4.1.3. PE24 Toxin Domain
4.1.4. Recombinant Immunotoxin
4.2. DNA Template Generation by Plasmid Prep and PCR
4.3. Cell-Free Protein Synthesis
4.3.1. E. coli-Based Cell-Free Protein Synthesis
4.3.2. CHO-Based Cell-Free Protein Synthesis
4.4. Determination of Total Protein Yields
4.5. SDS-PAGE, Autoradiography
4.6. ELISA
4.7. Cell Culture
4.8. MTT Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Gilad, Y.; Gellerman, G.; Lonard, D.M.; O’Malley, B.W. Drug Combination in Cancer Treatment—From Cocktails to Conjugated Combinations. Cancers 2021, 13, 669. [Google Scholar] [CrossRef] [PubMed]
- Pemmaraju, N.; Sweet, K.L.; Stein, A.S.; Wang, E.S.; Rizzieri, D.A.; Vasu, S.; Rosenblat, T.L.; Brooks, C.L.; Habboubi, N.; Mughal, T.I.; et al. Long-Term Benefits of Tagraxofusp for Patients with Blastic Plasmacytoid Dendritic Cell Neoplasm. J. Clin. Oncol. 2022, 40, 3032–3036. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.M.; Berkenblit, A. Antibody–Drug Conjugates for Cancer Treatment. Annu. Rev. Med. 2018, 69, 191–207. [Google Scholar] [CrossRef]
- Alewine, C.; Hassan, R.; Pastan, I. Advances in Anticancer Immunotoxin Therapy. Oncologist 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Khirehgesh, M.R.; Sharifi, J.; Safari, F.; Akbari, B. Immunotoxins and nanobody-based immunotoxins: Review and update. J. Drug Target. 2021, 29, 848–862. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.-S.; Liu, X.-L.; Hui, Q.; Lu, S.-Y.; Qu, L.-L.; Li, Y.-S.; Zhou, Y.; Ren, H.-L.; Hu, P. Clinical targeting recombinant immunotoxins for cancer therapy. OncoTargets Ther. 2017, 10, 3645–3665. [Google Scholar] [CrossRef]
- Bochennek, K.; Luckowitsch, M.; Lehrnbecher, T. Recent advances and future directions in the management of the immunocompromised host. Semin. Oncol. 2020, 47, 40–47. [Google Scholar] [CrossRef]
- Zuppone, S.; Fabbrini, M.S.; Vago, R. Hosts for Hostile Protein Production: The Challenge of Recombinant Immunotoxin Expression. Biomedicines 2019, 7, 38. [Google Scholar] [CrossRef]
- Rinas, U.; Garcia-Fruitós, E.; Corchero, J.L.; Vázquez, E.; Seras-Franzoso, J.; Villaverde, A. Bacterial Inclusion Bodies: Discovering Their Better Half. Trends Biochem. Sci. 2017, 42, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Onda, M. Recombinant immunotoxins with low endotoxins for clinical and animal studies. Methods Mol. Biol. 2012, 907, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Upadhyay, V.; Panda, A.K. Solubilization and Refolding of Inclusion Body Proteins. In Insoluble Proteins: Methods and Protocols; García-Fruitós, E., Ed.; Springer: New York, NY, USA, 2015; pp. 283–291. ISBN 978-1-4939-2205-5. [Google Scholar]
- LeMaistre, C.F.; Saleh, M.N.; Kuzel, T.M.; Foss, F.; Platanias, L.C.; Schwartz, G.; Ratain, M.; Rook, A.; Freytes, C.O.; Craig, F.; et al. Phase I Trial of a Ligand Fusion-Protein (DAB389IL-2) in Lymphomas Expressing the Receptor for Interkeukin-2. Blood 1998, 91, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.E.; Ramage, J.; Kiser, M.; Alexander, R.; Kucera, G.; Miller, M.S. Characterization of diphtheria fusion proteins targeted to the human interleukin-3 receptor. Protein Eng. 2000, 13, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Alderson, R.F.; Kreitman, R.J.; Chen, T.; Yeung, P.; Herbst, R.; Fox, J.A.; Pastan, I. CAT-8015: A Second-Generation Pseudomonas Exotoxin A–Based Immunotherapy Targeting CD22-Expressing Hematologic Malignancies. Clin. Cancer Res. 2009, 15, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Jun, S.-Y.; Kim, Y.-S. Critical Issues in the Development of Immunotoxins for Anticancer Therapy. J. Pharm. Sci. 2020, 109, 104–115. [Google Scholar] [CrossRef]
- Weidel, U.H.; Tiefenthaler, G.; Schiller, C.; Weiss, E.H.; Georges, G.; Brinkmann, U. Prospects of bacterial and plant protein-based immunotoxins for treatment of cancer. Cancer Genom. Proteomics 2014, 11, 25–38. [Google Scholar]
- Nagata, S.; Pastan, I. Removal of B cell epitopes as a practical approach for reducing the immunogenicity of foreign protein-based therapeutics. Adv. Drug Deliv. Rev. 2009, 61, 977–985. [Google Scholar] [CrossRef]
- Kawa, S.; Onda, M.; Ho, M.; Kreitman, R.J.; Bera, T.K.; Pastan, I. The improvement of an anti-CD22 immunotoxin. mAbs 2011, 3, 479–486. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.-C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef]
- Dieffenbach, M.; Pastan, I. Mechanisms of Resistance to Immunotoxins Containing Pseudomonas Exotoxin A in Cancer Therapy. Biomolecules 2020, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Mazor, R.; Pastan, I. Immunogenicity of Immunotoxins Containing Pseudomonas Exotoxin A: Causes, Consequences, and Mitigation. Front. Immunol. 2020, 11, 1261. [Google Scholar] [CrossRef] [PubMed]
- Dondapati, S.K.; Stech, M.; Zemella, A.; Kubick, S. Cell-Free Protein Synthesis: A Promising Option for Future Drug Development. BioDrugs 2020, 34, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Silverman, A.D.; Karim, A.S.; Jewett, M.C. Cell-free gene expression: An expanded repertoire of applications. Nat. Rev. Genet. 2020, 21, 151–170. [Google Scholar] [CrossRef]
- Weldon, J.E.; Xiang, L.; Chertov, O.; Margulies, I.; Kreitman, R.J.; FitzGerald, D.J.; Pastan, I. A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood 2009, 113, 3792–3800. [Google Scholar] [CrossRef]
- Havaei, S.M.; Aucoin, M.G.; Jahanian-Najafabadi, A. Pseudomonas Exotoxin-Based Immunotoxins: Over Three Decades of Efforts on Targeting Cancer Cells with the Toxin. Front. Oncol. 2021, 11, 781800. [Google Scholar] [CrossRef]
- Sempowski, G.D.; Lee, D.M.; Kaufman, R.E.; Haynes, B.F. Structure and function of the CD7 molecule. Crit. Rev. Immunol. 1999, 19, 331–348. [Google Scholar]
- Campana, D.; van Dongen, J.J.; Mehta, A.; Coustan-Smith, E.; Wolvers-Tettero, I.L.; Ganeshaguru, K.; Janossy, G. Stages of T-Cell Receptor Protein Expression in T-Cell Acute Lymphoblastic Leukemia. Blood 1991, 77, 1546–1554. [Google Scholar] [CrossRef]
- Chang, H.; Yeung, J.; Brandwein, J.; Yi, Q. CD7 expression predicts poor disease free survival and post-remission survival in patients with acute myeloid leukemia and normal karyotype. Leuk. Res. 2007, 31, 157–162. [Google Scholar] [CrossRef]
- Hussein, S.; Gill, K.Z.; Sireci, A.N.; Colovai, A.I.; Small, T.; Emmons, F.N.; Murty, V.V.; Bhagat, G.; Alobeid, B. Aberrant T-cell antigen expression in B lymphoblastic leukaemia. Br. J. Haematol. 2011, 155, 449–456. [Google Scholar] [CrossRef]
- Vallera, D.A. Targeting T cells for GVHD therapy. Semin. Cancer Biol. 1996, 7, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, U.; Abken, H. CD4+CD7− T Cells: A Separate Subpopulation of Memory T Cells? J. Clin. Immunol. 1997, 17, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Récher, C. The beginning of a new therapeutic era in acute myeloid leukemia. eJHaem 2021, 2, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Komitopoulou, A.; Baltadakis, I.; Peristeri, I.; Goussetis, E. Immunotherapy and Allogeneic Bone Marrow Transplantation in B Acute Lymphoblastic Leukemia: How to Sequence? Clin. Hematol. Int. 2022, 4, 11–20. [Google Scholar] [CrossRef] [PubMed]
- McMahon, C.M.; Luger, S.M. Relapsed T Cell ALL: Current Approaches and New Directions. Curr. Hematol. Malig. Rep. 2019, 14, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Bayón-Calderón, F.; Toribio, M.L.; González-García, S. Facts and Challenges in Immunotherapy for T-Cell Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2020, 21, 7685. [Google Scholar] [CrossRef]
- Blazar, B.R.; Hill, G.R.; Murphy, W.J. Dissecting the biology of allogeneic HSCT to enhance the GvT effect whilst minimizing GvHD. Nat. Rev. Clin. Oncol. 2020, 17, 475–492. [Google Scholar] [CrossRef]
- Malard, F.; Huang, X.-J.; Sim, J.P.Y. Treatment and unmet needs in steroid-refractory acute graft-versus-host disease. Leukemia 2020, 34, 1229–1240. [Google Scholar] [CrossRef]
- Li, H.W.; Sykes, M. Emerging concepts in haematopoietic cell transplantation. Nat. Rev. Immunol. 2012, 12, 403–416. [Google Scholar] [CrossRef]
- Ferrari, G.; Thrasher, A.J.; Aiuti, A. Gene therapy using haematopoietic stem and progenitor cells. Nat. Rev. Genet. 2021, 22, 216–234. [Google Scholar] [CrossRef]
- McElwain, L.; Phair, K.; Kealey, C.; Brady, D. Current trends in biopharmaceuticals production in Escherichia coli. Biotechnol. Lett. 2022, 44, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, Y.-G.; Lee, G.M. CHO cells in biotechnology for production of recombinant proteins: Current state and further potential. Appl. Microbiol. Biotechnol. 2012, 93, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, N.E.; Levine, M.Z.; Oza, J.P. A User’s Guide to Cell-Free Protein Synthesis. Methods Protoc. 2019, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 2013, 14, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Fatima, K.; Naqvi, F.; Younas, H. A Review: Molecular Chaperone-mediated Folding, Unfolding and Disaggregation of Expressed Recombinant Proteins. Cell Biochem. Biophys. 2021, 79, 153–174. [Google Scholar] [CrossRef]
- Smolskaya, S.; Logashina, Y.A.; Andreev, Y.A. Escherichia coli Extract-Based Cell-Free Expression System as an Alternative for Difficult-to-Obtain Protein Biosynthesis. Int. J. Mol. Sci. 2020, 21, 928. [Google Scholar] [CrossRef]
- Brödel, A.K.; Sonnabend, A.; Kubick, S. Cell-free protein expression based on extracts from CHO cells. Biotechnol. Bioeng. 2014, 111, 25–36. [Google Scholar] [CrossRef]
- Stech, M.; Nikolaeva, O.; Thoring, L.; Stöcklein, W.F.M.; Wüstenhagen, D.A.; Hust, M.; Dübel, S.; Kubick, S. Cell-free synthesis of functional antibodies using a coupled in vitro transcription-translation system based on CHO cell lysates. Sci. Rep. 2017, 7, 12030. [Google Scholar] [CrossRef]
- Krebs, S.K.; Rakotoarinoro, N.; Stech, M.; Zemella, A.; Kubick, S. A CHO-Based Cell-Free Dual Fluorescence Reporter System for the Straightforward Assessment of Amber Suppression and scFv Functionality. Front. Bioeng. Biotechnol. 2022, 10, 873906. [Google Scholar] [CrossRef]
- Zemella, A.; Thoring, L.; Hoffmeister, C.; Šamalíková, M.; Ehren, P.; Wüstenhagen, D.A.; Kubick, S. Cell-free protein synthesis as a novel tool for directed glycoengineering of active erythropoietin. Sci. Rep. 2018, 8, 8514. [Google Scholar] [CrossRef] [PubMed]
- Iglewski, B.H.; Kabat, D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Proc. Natl. Acad. Sci. USA 1975, 72, 2284–2288. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Uchida, T. Highly frequent single amino acid substitution in mammalian elongation factor 2 (EF-2) results in expression of resistance to EF-2-ADP-ribosylating toxins. J. Biol. Chem. 1987, 262, 12298–12305. [Google Scholar] [CrossRef]
- Knödler, M.; Buyel, J.F. Plant-made immunotoxin building blocks: A roadmap for producing therapeutic antibody-toxin fusions. Biotechnol. Adv. 2021, 47, 107683. [Google Scholar] [CrossRef]
- Cole, S.D.; Miklos, A.E.; Chiao, A.C.; Sun, Z.Z.; Lux, M.W. Methodologies for preparation of prokaryotic extracts for cell-free expression systems. Synth. Syst. Biotechnol. 2020, 5, 252–267. [Google Scholar] [CrossRef]
- Francis, D.M.; Page, R. Strategies to Optimize Protein Expression in E. coli. Curr. Protoc. Protein Sci. 2010, 61, 5.24.1–5.24.29. [Google Scholar] [CrossRef]
- Kai, L.; Orbán, E.; Henrich, E.; Proverbio, D.; Dötsch, V.; Bernhard, F. Co-translational Stabilization of Insoluble Proteins in Cell-Free Expression Systems. In Insoluble Proteins: Methods and Protocols; García-Fruitós, E., Ed.; Springer: New York, NY, USA, 2015; pp. 125–143. ISBN 978-1-4939-2205-5. [Google Scholar]
- Gagoski, D.; Polinkovsky, M.E.; Mureev, S.; Kunert, A.; Johnston, W.; Gambin, Y.; Alexandrov, K. Performance benchmarking of four cell-free protein expression systems. Biotechnol. Bioeng. 2016, 113, 292–300. [Google Scholar] [CrossRef]
- Baum, W.; Steininger, H.; Bair, H.J.; Becker, W.; Hansen-Hagge, T.E.; Kressel, M.; Kremmer, E.; Kalden, J.R.; Gramatzki, M. Therapy with CD7 monoclonal antibody TH-69 is highly effective for xenografted human T-cell ALL. Br. J. Haematol. 1996, 95, 327–338. [Google Scholar] [CrossRef]
- Bhatwa, A.; Wang, W.; Hassan, Y.I.; Abraham, N.; Li, X.-Z.; Zhou, T. Challenges Associated With the Formation of Recombinant Protein Inclusion Bodies in Escherichia coli and Strategies to Address Them for Industrial Applications. Front. Bioeng. Biotechnol. 2021, 9, 630551. [Google Scholar] [CrossRef]
- Tsalkova, T.; Zardeneta, G.; Kudlicki, W.; Kramer, G.; Horowitz, P.M.; Hardesty, B. GroEL and GroES increase the specific enzymic activity of newly-synthesized rhodanese if present during in vitro transcription/translation. Biochemistry 1993, 32, 3377–3380. [Google Scholar] [CrossRef]
- Ryabova, L.A.; Desplancq, D.; Spirin, A.S.; Plückthun, A. Functional antibody production using cell-free translation: Effects of protein disulfide isomerase and chaperones. Nat. Biotechnol. 1997, 15, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Merk, H.; Stiege, W.; Tsumoto, K.; Kumagai, I.; Erdmann, V.A. Cell-Free Expression of Two Single-Chain Monoclonal Antibodies against Lysozyme: Effect of Domain Arrangement on the Expression1. J. Biochem. 1999, 125, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, J.R.; Torella, C.; Iriarte, A.; Martinez–Carrion, M. Conformation of Aspartate Aminotransferase Isozymes Folding under Different Conditions Probed by Limited Proteolysis. J. Biol. Chem. 1998, 273, 23191–23202. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ookubo, Y.; Fujii, I.; Nakano, H.; Yamane, T. Expression of Fab fragment of catalytic antibody 6D9 in an Escherichia coli in vitro coupled transcription/translation system. FEBS Lett. 2002, 514, 290–294. [Google Scholar] [CrossRef]
- Yin, G.; Swartz, J.R. Enhancing multiple disulfide bonded protein folding in a cell-free system. Biotechnol. Bioeng. 2004, 86, 188–195. [Google Scholar] [CrossRef]
- Frey, S.; Haslbeck, M.; Hainzl, O.; Buchner, J. Synthesis and characterization of a functional intact IgG in a prokaryotic cell-free expression system. Biol. Chem. 2008, 389, 37–45. [Google Scholar] [CrossRef]
- Park, C.-G.; Kim, T.-W.; Oh, I.-S.; Song, J.K.; Kim, D.-M. Expression of functional Candida antarctica lipase B in a cell-free protein synthesis system derived from Escherichia coli. Biotechnol. Progress 2009, 25, 589–593. [Google Scholar] [CrossRef]
- Welsh, J.P.; Bonomo, J.; Swartz, J.R. Localization of BiP to translating ribosomes increases soluble accumulation of secreted eukaryotic proteins in an Escherichia coli cell-free system. Biotechnol. Bioeng. 2011, 108, 1739–1748. [Google Scholar] [CrossRef]
- Groff, D.; Armstrong, S.; Rivers, P.J.; Zhang, J.; Yang, J.; Green, E.; Rozzelle, J.; Liang, S.; Kittle, J.D.; Steiner, A.R.; et al. Engineering toward a bacterial “endoplasmic reticulum” for the rapid expression of immunoglobulin proteins. mAbs 2014, 6, 671–678. [Google Scholar] [CrossRef]
- Chi, H.; Wang, X.; Li, J.; Ren, H.; Huang, F. Folding of newly translated membrane protein CCR5 is assisted by the chaperonin GroEL-GroES. Sci. Rep. 2015, 5, 17037. [Google Scholar] [CrossRef]
- Yang, H.-J.; Lee, K.-H.; Lim, H.J.; Kim, D.-M. Tandem Cell-Free Protein Synthesis as a Tool for Rapid Screening of Optimal Molecular Chaperones. Biotechnol. J. 2019, 14, 1800523. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, K.; Yabuki, T.; Ito, M.; Kigawa, T. Cold shock proteins improve E. coli cell-free synthesis in terms of soluble yields of aggregation-prone proteins. Biotechnol. Bioeng. 2020, 117, 1628–1639. [Google Scholar] [CrossRef]
- Ying, B.-W.; Taguchi, H.; Ueda, H.; Ueda, T. Chaperone-assisted folding of a single-chain antibody in a reconstituted translation system. Biochem. Biophys. Res. Commun. 2004, 320, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Kanamori, T.; Ueda, T.; Taguchi, H. Global analysis of chaperone effects using a reconstituted cell-free translation system. Proc. Natl. Acad. Sci. USA 2012, 109, 8937–8942. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gu, L.; Aach, J.; Church, G.M. Improved cell-free RNA and protein synthesis system. PLoS ONE 2014, 9, e106232. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Fujiwara, K.; Taguchi, H. Identification of novel in vivo obligate GroEL/ES substrates based on data from a cell-free proteomics approach. FEBS Lett. 2016, 590, 251–257. [Google Scholar] [CrossRef]
- Murakami, S.; Matsumoto, R.; Kanamori, T. Constructive approach for synthesis of a functional IgG using a reconstituted cell-free protein synthesis system. Sci. Rep. 2019, 9, 671. [Google Scholar] [CrossRef]
- Kang, S.-H.; Kim, D.-M.; Kim, H.-J.; Jun, S.-Y.; Lee, K.-Y.; Kim, H.-J. Cell-Free Production of Aggregation-Prone Proteins in Soluble and Active Forms. Biotechnol. Progress 2005, 21, 1412–1419. [Google Scholar] [CrossRef]
- Oh, I.-S.; Lee, J.-C.; Lee, M.; Chung, J.; Kim, D.-M. Cell-free production of functional antibody fragments. Bioprocess Biosyst. Eng. 2009, 33, 127. [Google Scholar] [CrossRef]
- Jin, X.; Kightlinger, W.; Hong, S.H. Optimizing Cell-Free Protein Synthesis for Increased Yield and Activity of Colicins. Methods Protoc. 2019, 2, 28. [Google Scholar] [CrossRef]
- Soltani, M.; Hunt, J.P.; Bundy, B.C. Rapid RNase inhibitor production to enable low-cost, on-demand cell-free protein synthesis biosensor use in human body fluids. Biotechnol. Bioeng. 2021, 118, 3973–3983. [Google Scholar] [CrossRef] [PubMed]
- Groff, D.; Carlos, N.A.; Chen, R.; Hanson, J.A.; Liang, S.; Armstrong, S.; Li, X.; Zhou, S.; Steiner, A.; Hallam, T.J.; et al. Development of an E. coli strain for cell-free ADC manufacturing. Biotechnol. Bioeng. 2022, 119, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Koubek, J.; Schmitt, J.; Galmozzi, C.V.; Kramer, G. Mechanisms of Cotranslational Protein Maturation in Bacteria. Front. Mol. Biosci. 2021, 8, 689755. [Google Scholar] [CrossRef] [PubMed]
- de Marco, A. Protocol for preparing proteins with improved solubility by co-expressing with molecular chaperones in Escherichia coli. Nat. Protoc. 2007, 2, 2632–2639. [Google Scholar] [CrossRef]
- de Marco, A.; Deuerling, E.; Mogk, A.; Tomoyasu, T.; Bukau, B. Chaperone-based procedure to increase yields of soluble recombinant proteins produced in E. coli. BMC Biotechnol. 2007, 7, 32. [Google Scholar] [CrossRef]
- Liu, C.; Kobashigawa, Y.; Yamauchi, S.; Toyota, Y.; Teramoto, M.; Ikeguchi, Y.; Fukuda, N.; Sato, T.; Sato, Y.; Kimura, H.; et al. Preparation of single-chain Fv antibodies in the cytoplasm of Escherichia coli by simplified and systematic chaperone optimization. J. Biochem. 2019, 166, 455–462. [Google Scholar] [CrossRef]
- Yano, N.; Emi, T.; Gregory, D.J.; Fedulov, A.V. Consideration on Efficient Recombinant Protein Production: Focus on Substrate Protein-Specific Compatibility Patterns of Molecular Chaperones. Protein J. 2021, 40, 756–764. [Google Scholar] [CrossRef]
- Haacke, A.; Fendrich, G.; Ramage, P.; Geiser, M. Chaperone over-expression in Escherichia coli: Apparent increased yields of soluble recombinant protein kinases are due mainly to soluble aggregates. Protein Expr. Purif. 2009, 64, 185–193. [Google Scholar] [CrossRef]
- Weldon, J.E.; Pastan, I. A guide to taming a toxin—Recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS J. 2011, 278, 4683–4700. [Google Scholar] [CrossRef]
- Liaci, A.M.; Förster, F. Take Me Home, Protein Roads: Structural Insights into Signal Peptide Interactions during ER Translocation. Int. J. Mol. Sci. 2021, 22, 11871. [Google Scholar] [CrossRef]
- Armstrong, S.; Li, J.-H.; Zhang, J.; Rod Merrill, A. Characterization of Competitive Inhibitors for the Transferase Activity of Pseudomonas aeruginosa Exotoxin A. J. Enzyme Inhib. Med. Chem. 2002, 17, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Merrill, A.R.; Mangroo, D. Identification of peptide inhibitors of Pseudomonas aeruginosa exotoxin A function using a yeast two-hybrid approach. FEMS Microbiol. Lett. 2003, 218, 85–92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yates, S.P.; Taylor, P.L.; Jørgensen, R.; Ferraris, D.; Zhang, J.; Andersen, G.R.; Merrill, A.R. Structure–function analysis of water-soluble inhibitors of the catalytic domain of exotoxin A from Pseudomonas aeruginosa. Biochem. J. 2005, 385, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Slavinskaya, Z.; Merrill, A.R.; Kaufmann, S.H.E. Human α-defensins neutralize toxins of the mono-ADP-ribosyltransferase family. Biochem. J. 2006, 399, 225–229. [Google Scholar] [CrossRef]
- Zou, G.; de Leeuw, E. Neutralization of Pseudomonas auruginosa Exotoxin A by human neutrophil peptide 1. Biochem. Biophys. Res. Commun. 2018, 501, 454–457. [Google Scholar] [CrossRef]
- Santajit, S.; Seesuay, W.; Mahasongkram, K.; Sookrung, N.; Ampawong, S.; Reamtong, O.; Diraphat, P.; Chaicumpa, W.; Indrawattana, N. Human single-chain antibodies that neutralize Pseudomonas aeruginosa-exotoxin A-mediated cellular apoptosis. Sci. Rep. 2019, 9, 14928. [Google Scholar] [CrossRef]
- Lugo, M.R.; Merrill, A.R. Development of Anti-Virulence Therapeutics against Mono-ADP-Ribosyltransferase Toxins. Toxins 2021, 13, 16. [Google Scholar] [CrossRef]
- Roy, V.; Ghani, K.; Caruso, M. A Dominant-Negative Approach That Prevents Diphthamide Formation Confers Resistance to Pseudomonas Exotoxin A and Diphtheria Toxin. PLoS ONE 2010, 5, e15753. [Google Scholar] [CrossRef]
- Wei, H.; Bera, T.K.; Wayne, A.S.; Xiang, L.; Colantonio, S.; Chertov, O.; Pastan, I. A Modified Form of Diphthamide Causes Immunotoxin Resistance in a Lymphoma Cell Line with a Deletion of the WDR85 Gene. J. Biol. Chem. 2013, 288, 12305–12312. [Google Scholar] [CrossRef]
- Mayer, K.; Schröder, A.; Schnitger, J.; Stahl, S.; Brinkmann, U. Influence of DPH1 and DPH5 Protein Variants on the Synthesis of Diphthamide, the Target of ADPRibosylating Toxins. Toxins 2017, 9, 78. [Google Scholar] [CrossRef]
- Gupta, P.K.; Liu, S.; Leppla, S.H. Characterization of a Chinese Hamster Ovary Cell Mutant Having a Mutation in Elongation Factor-2. PLoS ONE 2010, 5, e9078. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mateus-Seidl, R.; Stahl, S.; Dengl, S.; Birzele, F.; Herrmuth, H.; Mayer, K.; Niederfellner, G.; Liu, X.-F.; Pastan, I.; Brinkmann, U. Interplay between reversible phosphorylation and irreversible ADP-ribosylation of eukaryotic translation elongation factor 2. Biol. Chem. 2019, 400, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, P.J.; Johnson, V.G.; Andrew, S.M.; Hoogenboom, H.R.; Raus, J.C.; Youle, R.J. Characterization of single-chain antibody (sFv)-toxin fusion proteins produced in vitro in rabbit reticulocyte lysate. J. Biol. Chem. 1993, 268, 5302–5308. [Google Scholar] [CrossRef]
- Pelham, H.R.B.; Jackson, R.J. An Efficient mRNA-Dependent Translation System from Reticulocyte Lysates. Eur. J. Biochem. 1976, 67, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Thoring, L.; Kubick, S. Versatile Cell-Free Protein Synthesis Systems Based on Chinese Hamster Ovary Cells. In Recombinant Protein Expression in Mammalian Cells: Methods and Protocols; Hacker, D.L., Ed.; Springer: New York, NY, USA, 2018; pp. 289–308. ISBN 978-1-4939-8730-6. [Google Scholar]
- Chowdhury, P.S.; Pastan, I. Improving antibody affinity by mimicking somatic hypermutation in vitro. Nat. Biotechnol. 1999, 17, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kim, G.-B.; Woo, J.-H.; Liu, Y.Y.; Mathias, A.; Stavrou, S.; Neville, D.M. Improvement of a Recombinant Anti-Monkey Anti-CD3 Diphtheria Toxin Based Immunotoxin by Yeast Display Affinity Maturation of the scFv. Bioconjugate Chem. 2007, 18, 947–955. [Google Scholar] [CrossRef]
- Kuan, C.-T.; Wakiya, K.; Keir, S.T.; Li, J.; Herndon II, J.E.; Pastan, I.; Bigner, D.D. Affinity-matured anti-glycoprotein NMB recombinant immunotoxins targeting malignant gliomas and melanomas. Int. J. Cancer 2011, 129, 111–121. [Google Scholar] [CrossRef]
- Fujimori, K.; Covell, D.G.; Fletcher, J.E.; Weinstein, J.N. A Modeling Analysis of Monoclonal Antibody Percolation Through Tumors: A Binding-Site Barrier. J. Nucl. Med. 1990, 31, 1191. [Google Scholar]
- Kim, D.M.; Swartz, J.R. Regeneration of adenosine triphosphate from glycolytic intermediates for cell-free protein synthesis. Biotechnol. Bioeng. 2001, 74, 309–316. [Google Scholar] [CrossRef]
- Jewett, M.C.; Swartz, J.R. Substrate replenishment extends protein synthesis with an in vitro translation system designed to mimic the cytoplasm. Biotechnol. Bioeng. 2004, 87, 465–472. [Google Scholar] [CrossRef]
- Bommannan, K.; Arumugam, J.R.; Radhakrishnan, V.; Kalaiyarasi, J.P.; Mehra, N.; Sagar, T.G.; Sundersingh, S. Precursor B-lineage acute lymphoblastic leukemia patients with aberrant natural killer cell and T cell-lineage antigen expression: Experience from a tertiary cancer care center. Hematol. Transfus. Cell. Ther. 2022, 44, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Batlevi, C.L.; Matsuki, E.; Brentjens, R.J.; Younes, A. Novel immunotherapies in lymphoid malignancies. Nat. Rev. Clin. Oncol. 2016, 13, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, H. Emerging agents and regimens for AML. J. Hematol. Oncol. 2021, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Kasikis, S.; Etra, A.; Levine, J.E. Current and Emerging Targeted Therapies for Acute Graft-Versus-Host Disease. BioDrugs 2021, 35, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Iżykowska, K.; Rassek, K.; Korsak, D.; Przybylski, G.K. Novel targeted therapies of T cell lymphomas. J. Hematol. Oncol. 2020, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tan, Y.; Wang, G.; Deng, B.; Ling, Z.; Song, W.; Seery, S.; Zhang, Y.; Peng, S.; Xu, J.; et al. Donor-Derived CD7 Chimeric Antigen Receptor T Cells for T-Cell Acute Lymphoblastic Leukemia: First-in-Human, Phase I Trial. J. Clin. Oncol. 2021, 39, 3340–3351. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Yuan, Z.; Liu, L.; Luo, L.; Li, Y.; Wu, K.; Liu, J.; Yang, C.; Li, Z.; et al. Eradication of T-ALL Cells by CD7-targeted Universal CAR-T Cells and Initial Test of Ruxolitinib-based CRS Management. Clin. Cancer Res. 2021, 27, 1242–1246. [Google Scholar] [CrossRef]
- Lu, P.; Liu, Y.; Yang, J.; Zhang, X.; Yang, X.; Wang, H.; Wang, L.; Wang, Q.; Jin, D.; Li, J.; et al. Naturally selected CD7 CAR-T therapy without genetic manipulations for T-ALL/LBL: First-in-human phase 1 clinical trial. Blood 2022, 140, 321–334. [Google Scholar] [CrossRef]
- You, F.; Wang, Y.; Jiang, L.; Zhu, X.; Chen, D.; Yuan, L.; An, G.; Meng, H.; Yang, L. A novel CD7 chimeric antigen receptor-modified NK-92MI cell line targeting T-cell acute lymphoblastic leukemia. Am. J. Cancer Res. 2019, 9, 64–78. [Google Scholar]
- Groth, C.; van Groningen, L.F.; Matos, T.R.; Bremmers, M.E.; Preijers, F.W.; Dolstra, H.; Reicherts, C.; Schaap, N.P.; van Hooren, E.H.; IntHout, J.; et al. Phase I/II Trial of a Combination of Anti-CD3/CD7 Immunotoxins for Steroid-Refractory Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2019, 25, 712–719. [Google Scholar] [CrossRef]
- Frankel, A.E.; Laver, J.H.; Willingham, M.C.; Burns, L.J.; Kersey, J.H.; Vallera, D.A. Therapy of Patients with T-cell Lymphomas and Leukemias Using an Anti-CD7 Monoclonal Antibody-Rich a Chain Immunotoxin. Leuk. Lymphoma 1997, 26, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Katz, F.E.; Janossy, G.; Cumber, A.; Ross, W.; Blacklock, H.A.; Tax, W.; Thorpe, P.E. Elimination of T cells from human peripheral blood and bone marrow using a cocktail of three anti-T cell immunotoxins. Br. J. Haematol. 1987, 67, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Fishwild, D.M.; Staskawicz, M.O.; Wu, H.-M.; Carroll, S.F. Cytotoxicity against human peripheral blood mononuclear cells and T cell lines mediated by anti-T cell immunotoxins in the absence of added potentiator. Clin. Exp. Immunol. 1991, 86, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.; Vallera, D.A.; Jaszcz, W.B.; Nguyen, D.; Kersey, J.H. Successful treatment of human acute T-cell leukemia in SCID mice using the anti-CD7-deglycosylated ricin A-chain immunotoxin DA7. Cancer Res. 1992, 52, 1314–1321. [Google Scholar] [PubMed]
- Morland, B.J.; Barley, J.; Boehm, D.; Flavell, S.U.; Ghaleb, N.; Kohler, J.A.; Okayama, K.; Wilkins, B.; Flavell, D.J. Effectiveness of HB2 (anti-CD7)—Saporin immunotoxin in an in vivo model of human T-cell leukaemia developed in severe combined immunodeficient mice. Br. J. Cancer 1994, 69, 279–285. [Google Scholar] [CrossRef]
- Pauza, M.E.; Doumbia, S.O.; Pennell, C.A. Construction and characterization of human CD7-specific single-chain Fv immunotoxins. J. Immunol. 1997, 158, 3259. [Google Scholar]
- Flavell, D.J.; Boehm, D.A.; Noss, A.; Flavell, S.U. Comparison of the potency and therapeutic efficacy of the anti-CD7 immunotoxin HB2-saporin constructed with one or two saporin moieties per immunotoxin molecule. Br. J. Cancer 1997, 75, 1035–1043. [Google Scholar] [CrossRef]
- Waurzyniak, B.; Schneider, E.A.; Tumer, N.; Yanishevski, Y.; Gunther, R.; Chelstrom, L.M.; Wendorf, H.; Myers, D.E.; Irvin, J.D.; Messinger, Y.; et al. In vivo toxicity, pharmacokinetics, and antileukemic activity of TXU (anti-CD7)-pokeweed antiviral protein immunotoxin. Clin. Cancer Res. 1997, 3, 881–890. [Google Scholar]
- Peipp, M.; Küpers, H.; Saul, D.; Schlierf, B.; Greil, J.; Zunino, S.J.; Gramatzki, M.; Fey, G.H. A Recombinant CD7-specific Single-Chain Immunotoxin Is a Potent Inducer of Apoptosis in Acute Leukemic T Cells. Cancer Res. 2002, 62, 2848–2855. [Google Scholar]
- Bremer, E.; Samplonius, D.F.; Peipp, M.; van Genne, L.; Kroesen, B.-J.; Fey, G.H.; Gramatzki, M.; de Leij, L.F.; Helfrich, W. Target Cell–Restricted Apoptosis Induction of Acute Leukemic T Cells by a Recombinant Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand Fusion Protein with Specificity for Human CD7. Cancer Res. 2005, 65, 3380–3388. [Google Scholar] [CrossRef]
- Bremer, E.; Cate, B.T.; Samplonius, D.F.; de Leij, L.F.M.H.; Helfrich, W. CD7-restricted activation of Fas-mediated apoptosis: A novel therapeutic approach for acute T-cell leukemia. Blood 2006, 107, 2863–2870. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, J.; Zhu, X.; Yu, Y.; Chen, D.; Yuan, L.; Gu, Z.; Zhang, X.; Qi, L.; Gong, Z.; et al. Novel CD7-specific nanobody-based immunotoxins potently enhanced apoptosis of CD7-positive malignant cells. Oncotarget 2016, 7, 34070–34083. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, J.; Zhu, X.; Tang, X.; Bao, Y.; Sun, X.; Huang, Y.; Tian, F.; Liu, X.; Yang, L. Humanized CD7 nanobody-based immunotoxins exhibit promising anti-T-cell acute lymphoblastic leukemia potential. Int. J. Nanomed. 2017, 12, 1969–1983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jain, A.; Milhas, S.; Williamson, D.J.; Mysliwy, J.; Lodge, A.; Thirlway, J.; Al Nakeeb, M.; Miller, A.; Rabbitts, T.H. An antibody-drug conjugate with intracellular drug release properties showing specific cytotoxicity against CD7-positive cells. Leuk. Res. 2021, 108, 106626. [Google Scholar] [CrossRef]
- Simons, L.; Cavazzana, M.; André, I. Concise Review: Boosting T-Cell Reconstitution Following Allogeneic Transplantation—Current Concepts and Future Perspectives. Stem Cells Transl. Med. 2019, 8, 650–657. [Google Scholar] [CrossRef]
- Raab, D.; Graf, M.; Notka, F.; Schödl, T.; Wagner, R. The GeneOptimizer Algorithm: Using a sliding window approach to cope with the vast sequence space in multiparameter DNA sequence optimization. Syst. Biol. Synth. Biol. 2010, 4, 215–225. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Pastan, I. Importance of the glutamate residue of KDEL in increasing the cytotoxicity of Pseudomonas exotoxin derivatives and for increased binding to the KDEL receptor. Biochem J. 1995, 307, 29–37. [Google Scholar] [CrossRef]
- Kwon, Y.-C.; Jewett, M.C. High-throughput preparation methods of crude extract for robust cell-free protein synthesis. Sci. Rep. 2015, 5, 8663. [Google Scholar] [CrossRef]
- Dopp, J.L.; Jo, Y.R.; Reuel, N.F. Methods to reduce variability in E. coli-based cell-free protein expression experiments. Synth. Syst. Biotechnol. 2019, 4, 204–211. [Google Scholar] [CrossRef]
- Stech, M.; Rakotoarinoro, N.; Teichmann, T.; Zemella, A.; Thoring, L.; Kubick, S. Synthesis of Fluorescently Labeled Antibodies Using Non-Canonical Amino Acids in Eukaryotic Cell-Free Systems. In Structural Proteomics: High-Throughput Methods; Owens, R.J., Ed.; Springer: New York, NY, USA, 2021; pp. 175–190. ISBN 978-1-0716-1406-8. [Google Scholar]
- Laemmli, U.K.; Beguin, F.; Gujer-Kellenberger, G. A factor preventing the major head protein of bacteriophage T4 from random aggregation. J. Mol. Biol. 1970, 47, 69–85. [Google Scholar] [CrossRef]
- Lin, A.V. Indirect ELISA. Methods Mol. Biol. 2015, 1318, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General Cytotoxicity Assessment by Means of the MTT Assay. Methods Mol. Biol. 2015, 1250, 333–348. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krebs, S.K.; Stech, M.; Jorde, F.; Rakotoarinoro, N.; Ramm, F.; Marinoff, S.; Bahrke, S.; Danielczyk, A.; Wüstenhagen, D.A.; Kubick, S. Synthesis of an Anti-CD7 Recombinant Immunotoxin Based on PE24 in CHO and E. coli Cell-Free Systems. Int. J. Mol. Sci. 2022, 23, 13697. https://doi.org/10.3390/ijms232213697
Krebs SK, Stech M, Jorde F, Rakotoarinoro N, Ramm F, Marinoff S, Bahrke S, Danielczyk A, Wüstenhagen DA, Kubick S. Synthesis of an Anti-CD7 Recombinant Immunotoxin Based on PE24 in CHO and E. coli Cell-Free Systems. International Journal of Molecular Sciences. 2022; 23(22):13697. https://doi.org/10.3390/ijms232213697
Chicago/Turabian StyleKrebs, Simon K., Marlitt Stech, Felix Jorde, Nathanaël Rakotoarinoro, Franziska Ramm, Sophie Marinoff, Sven Bahrke, Antje Danielczyk, Doreen A. Wüstenhagen, and Stefan Kubick. 2022. "Synthesis of an Anti-CD7 Recombinant Immunotoxin Based on PE24 in CHO and E. coli Cell-Free Systems" International Journal of Molecular Sciences 23, no. 22: 13697. https://doi.org/10.3390/ijms232213697
APA StyleKrebs, S. K., Stech, M., Jorde, F., Rakotoarinoro, N., Ramm, F., Marinoff, S., Bahrke, S., Danielczyk, A., Wüstenhagen, D. A., & Kubick, S. (2022). Synthesis of an Anti-CD7 Recombinant Immunotoxin Based on PE24 in CHO and E. coli Cell-Free Systems. International Journal of Molecular Sciences, 23(22), 13697. https://doi.org/10.3390/ijms232213697





