A Common Target of Nitrite and Nitric Oxide for Respiration Inhibition in Bacteria
Abstract
1. Introduction
2. Results
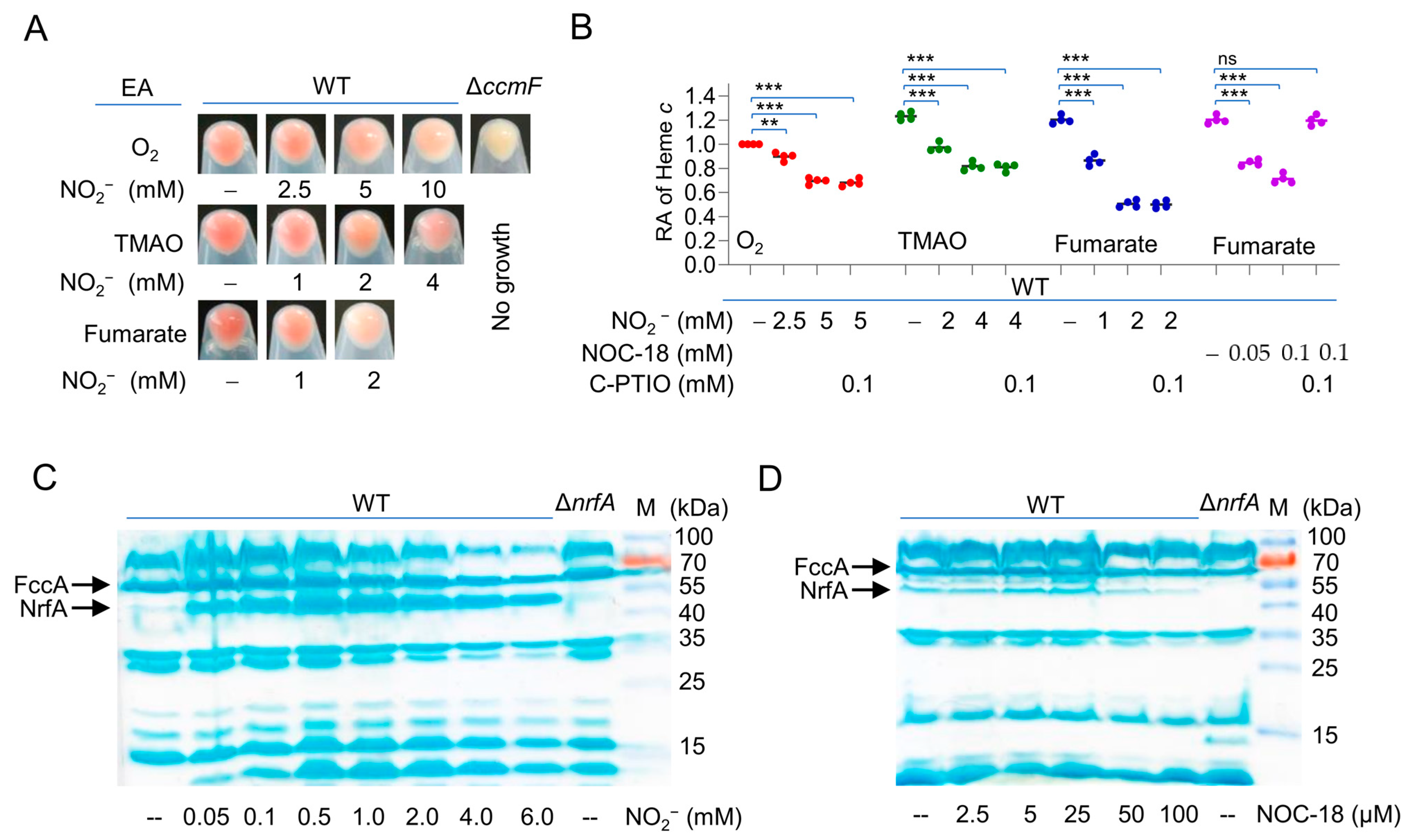
2.1. Nitrite and NO Down-Regulate cyt c Content of S. oneidensis
2.2. Nitrite/NO Compromise the cyt c Content in Growing Cells Only
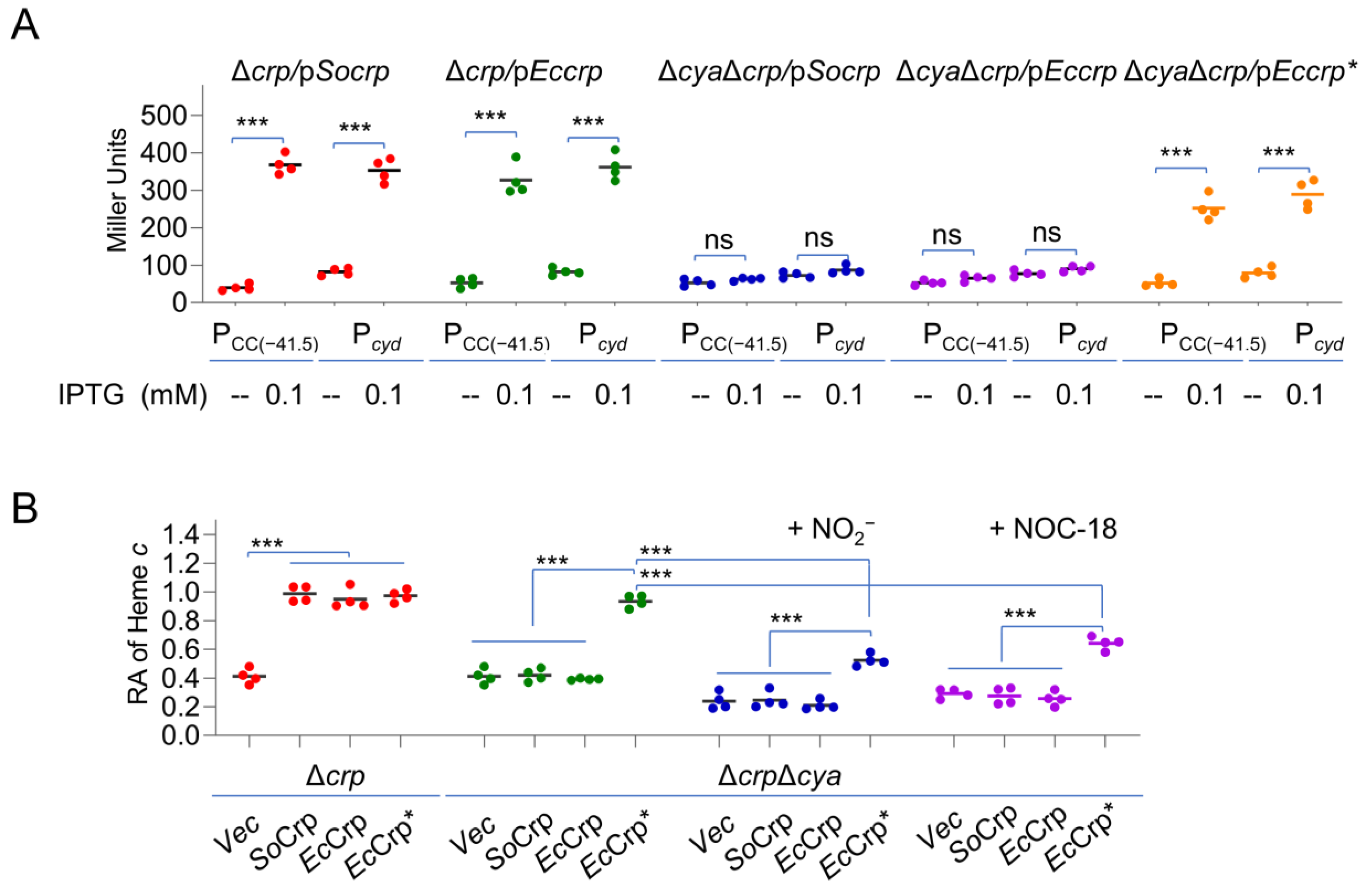
2.3. cAMP-CRP Is Not Exclusively Responsible for the Nitrite-/NO-Mediated Reduction in the cyt c Content
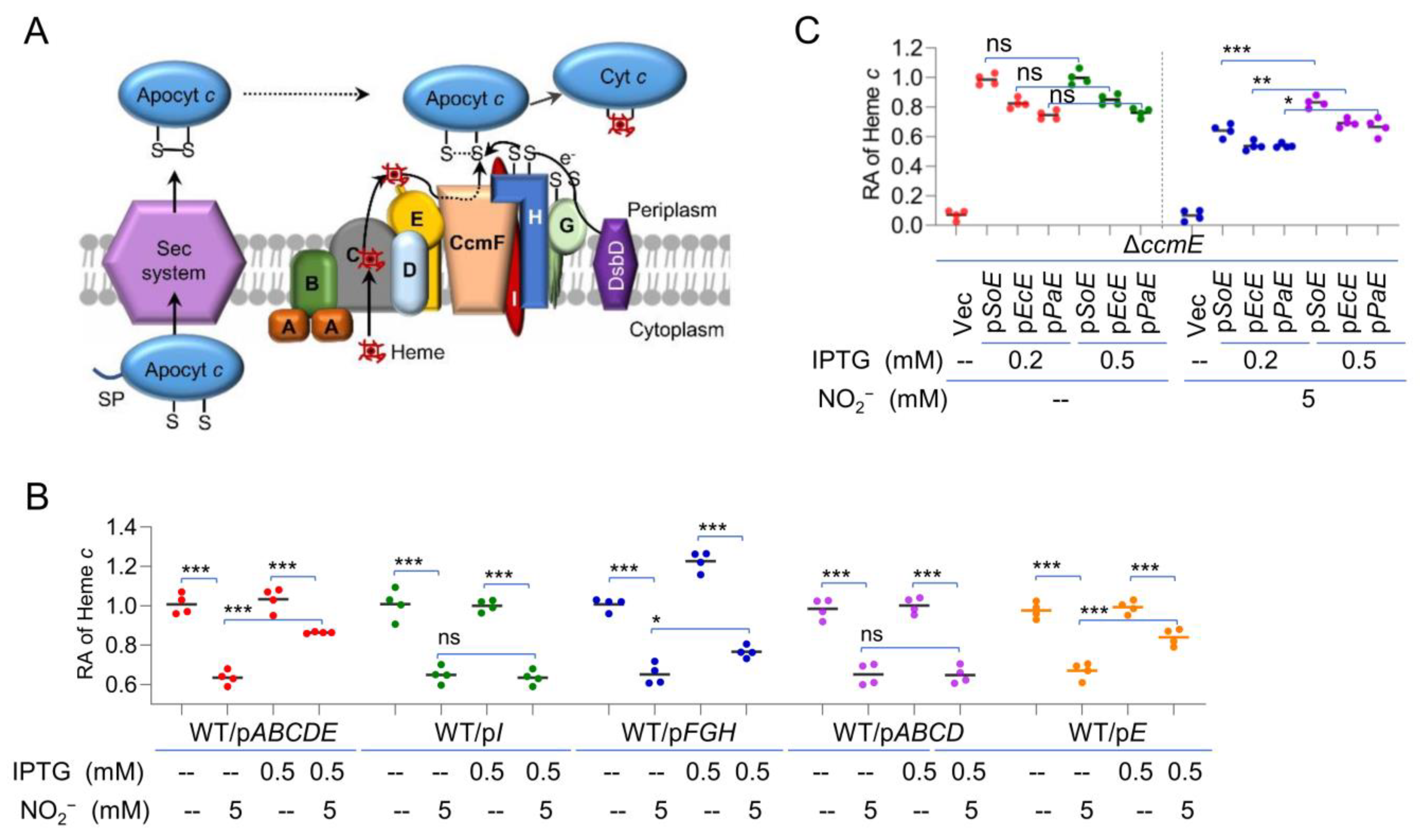
2.4. Increased Heme Levels Alleviate the Effect of Nitrite/NO on the cyt c Content
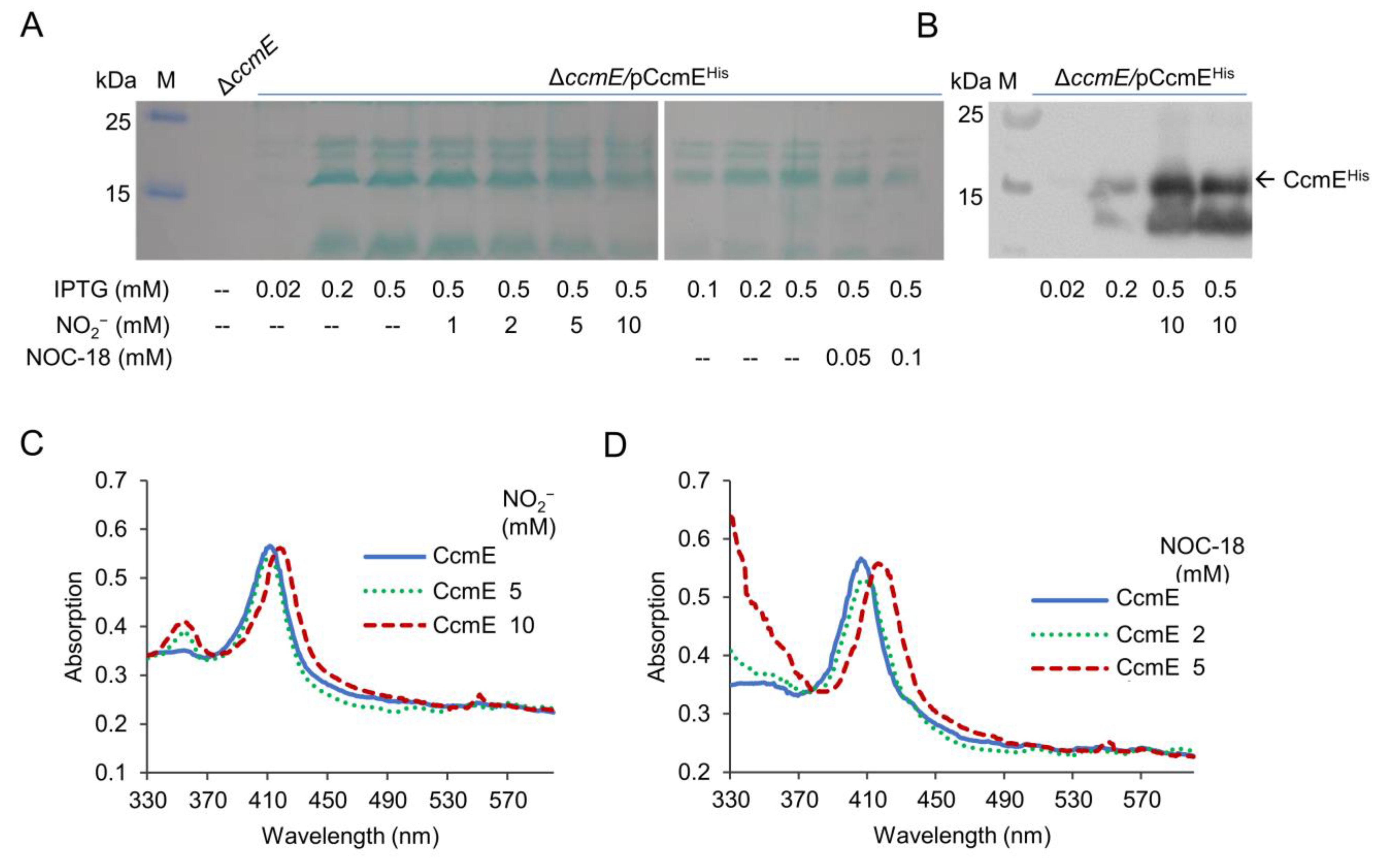
2.5. Identification of CcmE as a Likely Target of Nitrite/NO on cyt c Production
2.6. Nitrite/NO Impair CcmE Activity through a Complex Mechanism
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, and Culture Conditions
4.2. Construction of in-Frame Deletion Strains
4.3. Site-Directed Mutagenesis
4.4. Heme c Assays
4.5. Controlled Gene Expression and Complementation of Mutants
4.6. Preparation of Ether-Treated Bacterial Cells
4.7. Expression and Purification of Recombinant CcmE
4.8. SDS-PAGE, Heme-Staining, and Western Blotting
4.9. Spectroscopic Analysis
4.10. β-Galactosidase Activity Assay
4.11. Other Analyses
| Strain or Plasmid | Description | Source/Reference |
|---|---|---|
| E. coli | ||
| DH5α | Host strain for plasmids | Laboratory stock |
| WM3064 | Donor strain for conjugation; ΔdapA | W. Metcalf, UIUC a |
| S. oneidensis | ||
| MR-1 | Wild type | ATCC 700550 |
| HG0624 | Δcrp derived from MR-1 | [45] |
| HG0696 | ΔccmF derived from MR-1 | [5] |
| HG0958 | ΔnrfA derived from MR-1 | [58] |
| HG1070 | ΔccmE derived from MR-1 | This study |
| HG3901-0624 | ΔcyaΔcrp derived from MR-1 | This study |
| Plasmids | ||
| pHGM01 | Apr Gmr Cmr suicide vector | [5] |
| pHGEI01 | Integrative lacZ reporter vector | [75] |
| pHGEN-Ptac | IPTG-inducible Ptac expression vector | [71] |
| pHGEI01-PhemA | For measuring PhemA activity | [46] |
| pHGEI01-PhemC | For measuring PhemCactivity | [46] |
| pHGEI01-PhemH1 | For measuring PhemH1 activity | [77] |
| pHGEI01-PhemH2 | For measuring PhemH2 activity | [77] |
| pHGEI01-PCC(−41.5) | For measuring PCC(−41.5) activity | This study |
| pHGEI01-Pcyd | For measuring Pcyd activity | This study |
| pHGEN-Ptac-Socrp | For inducible production of S. oneidensis Crp | This study |
| pHGEN-Ptac-Eccrp | For inducible production of E. coli Crp | This study |
| pHGEN-Ptac-Eccrp* | For inducible production of cAMP-independent E. coli Crp | This study |
| pHGEN-Ptac-hemA | For inducible production of HemA | This study |
| pHGEN-Ptac-ccmI | For inducible production of CcmI | This study |
| pHGEN-Ptac-ccmABCDE | For inducible production of CcmABCDE | This study |
| pHGEN-Ptac-ccmABCD | For inducible production of CcmABCD | This study |
| pHGEN-Ptac-ccmFGH ccmABCD | For inducible production of CcmFGH | This study |
| pHGEN-Ptac-ccmE | For inducible production of S. oneidensis CcmE | This study |
| pHGEN-Ptac-EcccmE | For inducible production of E. coli CcmE | This study |
| pHGEN-Ptac-PaccmE | For inducible production of P. aeruginosa CcmE | This study |
| pHGEN-Ptac-ccmEhis | For inducible expression of His-tagged CcmE | This study |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alvarez-Paggi, D.; Hannibal, L.; Castro, M.A.; Oviedo-Rouco, S.; Demicheli, V.; Tórtora, V.; Tomasina, F.; Radi, R.; Murgida, D.H. Multifunctional cytochrome c: Learning new tricks from an old dog. Chem. Rev. 2017, 117, 13382–13460. [Google Scholar] [CrossRef] [PubMed]
- Kranz, R.G.; Richard-Fogal, C.; Taylor, J.-S.; Frawley, E.R. Cytochrome c biogenesis: Mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control. Microbiol. Mol. Biol. Rev. 2009, 73, 510–528. [Google Scholar] [CrossRef] [PubMed]
- Verissimo, A.F.; Daldal, F. Cytochrome c biogenesis System I: An intricate process catalyzed by a maturase supercomplex. Biochim. Biophys. Acta 2014, 1837, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Cianciotto, N.P.; Cornelis, P.; Baysse, C. Impact of the bacterial type I cytochrome c maturation system on different biological processes. Mol. Microbiol. 2005, 56, 1408–1415. [Google Scholar] [CrossRef]
- Jin, M.; Jiang, Y.; Sun, L.; Yin, J.; Fu, H.; Wu, G.; Gao, H. Unique organizational and functional features of the cytochrome c maturation system in Shewanella oneidensis. PLoS ONE 2013, 8, e75610. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Jin, M.; Wan, F.; Gao, H. Shewanella oneidensis cytochrome c maturation component CcmI is essential for heme attachment at the non-canonical motif of nitrite reductase NrfA. Mol. Microbiol. 2015, 95, 410–425. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Nakajima An, D.; Parks, J.M.; Skolnick, J. AF2Complex predicts direct physical interactions in multimeric proteins with deep learning. Nat. Commun. 2022, 13, 1744. [Google Scholar] [CrossRef]
- Schulz, H.; Hennecke, H.; Thöny-Meyer, L. Prototype of a heme chaperone essential for cytochrome c maturation. Science 1998, 281, 1197–1200. [Google Scholar] [CrossRef]
- Feissner, R.E.; Richard-Fogal, C.L.; Frawley, E.R.; Kranz, R.G. ABC transporter-mediated release of a haem chaperone allows cytochrome c biogenesis. Mol. Microbiol. 2006, 61, 219–231. [Google Scholar] [CrossRef]
- Richard-Fogal, C.L.; Frawley, E.R.; Bonner, E.R.; Zhu, H.; San Francisco, B.; Kranz, R.G. A conserved haem redox and trafficking pathway for cofactor attachment. EMBO J. 2009, 28, 2349–2359. [Google Scholar] [CrossRef]
- Mendez, D.L.; Lowder, E.P.; Tillman, D.E.; Sutherland, M.C.; Collier, A.L.; Rau, M.J.; Fitzpatrick, J.A.J.; Kranz, R.G. Cryo-EM of CcsBA reveals the basis for cytochrome c biogenesis and heme transport. Nat. Chem. Biol. 2022, 18, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Pervushin, K.; Bischof, D.; Braun, M.; Thöny-Meyer, L. Unusual heme−Histidine bond in the active site of a chaperone. J. Am. Chem. Soc. 2005, 127, 3716–3717. [Google Scholar] [CrossRef] [PubMed]
- Harvat, E.M.; Redfield, C.; Stevens, J.M.; Ferguson, S.J. Probing the heme-binding site of the cytochrome c maturation protein CcmE. Biochemistry 2009, 48, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.M.; Mavridou, D.A.I.; Hamer, R.; Kritsiligkou, P.; Goddard, A.D.; Ferguson, S.J. Cytochrome c biogenesis System I. FEBS J. 2011, 278, 4170–4178. [Google Scholar] [CrossRef]
- Brausemann, A.; Zhang, L.; Ilcu, L.; Einsle, O. Architecture of the membrane-bound cytochrome c heme lyase CcmF. Nat. Chem. Biol. 2021, 17, 800–805. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Grubina, R.; Doyle, M.P. The new chemical biology of nitrite reactions with hemoglobin: R-state catalysis, oxidative denitrosylation, and nitrite reductase/anhydrase. Acc. Chem. Res. 2008, 42, 157–167. [Google Scholar] [CrossRef]
- Ford, P.C. Reactions of NO and nitrite with heme models and proteins. Inorg. Chem. 2010, 49, 6226–6239. [Google Scholar] [CrossRef]
- Bowman, L.A.H.; McLean, S.; Poole, R.K.; Fukuto, J.M. The diversity of microbial responses to nitric oxide and agents of nitrosative stress: Close cousins but not identical twins. In Advances in Microbial Physiology; Robert, K.P., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 59, pp. 135–219. [Google Scholar]
- Maia, L.B.; Moura, J.J.G. How biology handles nitrite. Chem. Rev. 2014, 114, 5273–5357. [Google Scholar] [CrossRef]
- Reddy, D.; Lancaster, J.; Cornforth, D. Nitrite inhibition of Clostridium botulinum: Electron spin resonance detection of iron-nitric oxide complexes. Science 1983, 221, 769–770. [Google Scholar] [CrossRef]
- Hyduke, D.R.; Jarboe, L.R.; Tran, L.M.; Chou, K.J.Y.; Liao, J.C. Integrated network analysis identifies nitric oxide response networks and dihydroxyacid dehydratase as a crucial target in Escherichia coli. Proc. Natl. Acad. Sci. USA 2007, 104, 8484–8489. [Google Scholar] [CrossRef]
- Richardson, A.R.; Payne, E.C.; Younger, N.; Karlinsey, J.E.; Thomas, V.C.; Becker, L.A.; Navarre, W.W.; Castor, M.E.; Libby, S.J.; Fang, F.C. Multiple targets of nitric oxide in the tricarboxylic acid cycle of Salmonella enterica Serovar Typhimurium. Cell Host Microbe 2011, 10, 33–43. [Google Scholar] [CrossRef]
- Meng, Q.; Yin, J.; Jin, M.; Gao, H. Distinct nitrite and nitric oxide physiologies in Escherichia coli and Shewanella oneidensis. Appl. Environ. Microbiol. 2018, 84, e00559-18. [Google Scholar] [CrossRef]
- Meng, Q.; Sun, Y.; Gao, H. Cytochromes c constitute a layer of protection against nitric oxide but not nitrite. Appl. Environ. Microbiol. 2018, 84, e01255-18. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Gao, H. Physiological roles of nitrite and nitric oxide in bacteria: Similar consequences from distinct cell targets, protection, and Sensing Systems. Adv. Biol. 2021, 5, 2100773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, K.; Meng, Q.; Gao, H. Nitrite modulates aminoglycoside tolerance by inhibiting cytochrome heme-copper oxidase in bacteria. Commun. Biol. 2020, 3, 269. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Romine, M.F.; Beliaev, A.S.; Auchtung, J.M.; Driscoll, M.E.; Gardner, T.S.; Nealson, K.H.; Osterman, A.L.; Grigoriy, P.; Reed, J.L. Towards environmental systems biology of Shewanella. Nat. Rev. Microbiol. 2008, 6, 592–603. [Google Scholar] [CrossRef]
- Meyer, T.E.; Tsapin, A.I.; Vandenberghe, I.; De Smet, L.; Frishman, D.; Nealson, K.H.; Cusanovich, M.A.; Van Beeumen, J.J. Identification of 42 possible cytochrome c genes in the Shewanella oneidensis genome and characterization of six soluble cytochromes. OMICS J. Integr. Biol. 2004, 8, 57–77. [Google Scholar] [CrossRef]
- Gao, H.; Barua, S.; Liang, Y.; Wu, L.; Dong, Y.; Reed, S.; Chen, J.; Culley, D.; Kennedy, D.; Yang, Y.; et al. Impacts of Shewanella oneidensis c-type cytochromes on aerobic and anaerobic respiration. Microb. Biotechnol. 2010, 3, 455–466. [Google Scholar] [CrossRef]
- Guo, K.; Wang, W.; Wang, H.; Lu, Z.; Gao, H. Complex oxidation of apocytochromes c during bacterial cytochrome c maturation. Appl. Environ. Microbiol. 2019, 85, e01989-19. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Sun, W.; Kong, L.; Gao, H. Distinct roles of Shewanella oneidensis thioredoxin in regulation of cellular responses to hydrogen and organic peroxides. Appl. Environ. Microbiol. 2019, 85, e01700-19. [Google Scholar] [CrossRef]
- Fu, H.; Chen, H.; Wang, J.; Zhou, G.; Zhang, H.; Zhang, L.; Gao, H. Crp-dependent cytochrome bd oxidase confers nitrite resistance to Shewanella oneidensis. Environ. Microbiol. 2013, 15, 2198–2212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Yin, J.; Chen, H.; Hua, Y.; Sun, L.; Gao, H. Combined effect of loss of the caa3 oxidase and Crp regulation drives Shewanella to thrive in redox-stratified environments. ISME J. 2013, 7, 1752–1763. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Fu, H.; Yin, J.; Yuan, J.; Gao, H. Molecular underpinnings of nitrite effect on CymA-dependent respiration in Shewanella oneidensis. Front. Microbiol. 2016, 7, 1154. [Google Scholar] [CrossRef]
- Saffarini, D.A.; Schultz, R.; Beliaev, A. Involvement of cyclic AMP (cAMP) and cAMP receptor protein in anaerobic respiration of Shewanella oneidensis. J. Bacteriol. 2003, 185, 3668–3671. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, Q.; Sun, Y.; Gao, H. NapB in excess inhibits growth of Shewanella oneidensis by dissipating electrons of the quinol pool. Sci. Rep. 2016, 6, 37456. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, P.; Huang, Y.; Gao, H. Complex interplay of heme-copper oxidases with nitrite and nitric oxide. Int. J. Mol. Sci. 2022, 23, 979. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, B.; Schicklberger, M.; Kuermann, J.; Spormann, A.M.; Gescher, J. Periplasmic electron transfer via the c-type cytochromes MtrA and FccA of MR-1. Appl. Environ. Microbiol. 2009, 75, 7789–7796. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, J.; Fu, H.; Zhou, G.; Shi, M.; Gao, H. A Crp-dependent two-component system regulates nitrate and nitrite respiration in Shewanella oneidensis. PLoS ONE 2012, 7, e51643. [Google Scholar] [CrossRef]
- Korobko, V.M.; Melnikova, N.B.; Panteleev, D.A.; Martusevich, A.K.; Peretyagin, S.P. The study of the complexes of nitromedicine with cytochrome c and NO-containing aqueous dosage form in the wound treatment of rats. Nitric Oxide 2014, 42, 62–69. [Google Scholar] [CrossRef][Green Version]
- Nilsson, Z.N.; Mandella, B.L.; Sen, K.; Kekilli, D.; Hough, M.A.; Moënne-Loccoz, P.; Strange, R.W.; Andrew, C.R. Distinguishing nitro vs. nitrito coordination in cytochrome c’ using vibrational spectroscopy and density functional theory. Inorg. Chem. 2017, 56, 13205–13213. [Google Scholar] [CrossRef]
- Vosberg, H.-P.; Hoffmann-Berling, H. DNA synthesis in nucleotide-permeable Escherichia coli cells: I. Preparation and properties of ether-treated cells. J. Mol. Biol. 1971, 58, 739–753. [Google Scholar] [CrossRef]
- Paradis-Bleau, C.; Markovski, M.; Uehara, T.; Lupoli, T.J.; Walker, S.; Kahne, D.E.; Bernhardt, T.G. Lipoprotein cofactors located in the outer membrane activate bacterial cell wall polymerases. Cell 2010, 143, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Charania, M.A.; Brockman, K.L.; Zhang, Y.; Banerjee, A.; Pinchuk, G.E.; Fredrickson, J.K.; Beliaev, A.S.; Saffarini, D.A. Involvement of a membrane-bound class III adenylate cyclase in regulation of anaerobic respiration in Shewanella oneidensis MR-1. J. Bacteriol. 2009, 191, 4298–4306. [Google Scholar] [CrossRef]
- Gao, H.; Wang, X.; Yang, Z.; Chen, J.; Liang, Y.; Chen, H.; Palzkill, T.; Zhou, J. Physiological roles of ArcA, Crp, and EtrA and their interactive control on aerobic and anaerobic respiration in Shewanella oneidensis. PLoS ONE 2010, 5, e15295. [Google Scholar] [CrossRef]
- Yin, J.; Meng, Q.; Fu, H.; Gao, H. Reduced expression of cytochrome oxidases largely explains cAMP inhibition of aerobic growth in Shewanella oneidensis. Sci. Rep. 2016, 6, 24449. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.; Kerby, R.L.; Conrad, M.; Roberts, G.P. Study of highly constitutively active mutants suggests how cAMP activates cAMP receptor protein*. J. Biol. Chem. 2006, 281, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Savery, N.J.; Lloyd, G.S.; Kainz, M.; Gaal, T.; Ross, W.; Ebright, R.H.; Gourse, R.L.; Busby, S.J.W. Transcription activation at class II CRP-dependent promoters: Identification of determinants in the C-terminal domain of the RNA polymerase α subunit. EMBO J. 1998, 17, 3439–3447. [Google Scholar] [CrossRef]
- Luo, Q.; Dong, Y.; Chen, H.; Gao, H. Mislocalization of rieske Protein PetA predominantly accounts for the aerobic growth defect of tat mutants in Shewanella oneidensis. PLoS ONE 2013, 8, e62064. [Google Scholar] [CrossRef]
- Layer, G. Heme biosynthesis in prokaryotes. Biochim. Biophys. Acta Mol. Cell. Res. 2021, 1868, 118861. [Google Scholar] [CrossRef]
- Dailey, H.A.; Dailey, T.A.; Gerdes, S.; Jahn, D.; Jahn, M.; O’Brian, M.R.; Warren, M.J. Prokaryotic heme biosynthesis: Multiple pathways to a common essential product. Microbiol. Mol. Biol. Rev. 2017, 81, e00048-16. [Google Scholar] [CrossRef]
- Brennan, C.M.; Mazzucca, N.Q.; Mezoian, T.; Hunt, T.M.; Keane, M.L.; Leonard, J.N.; Scola, S.E.; Beer, E.N.; Perdue, S.; Pellock, B.J. Reduced heme levels underlie the exponential growth defect of the Shewanella oneidensis hfq mutant. PLoS ONE 2014, 9, e109879. [Google Scholar] [CrossRef]
- Yang, T. Mechanism of nitrite inhibition of cellular respiration in Pseudomonas aeruginosa. Curr. Microbiol. 1985, 12, 35–39. [Google Scholar] [CrossRef]
- Tonzetich, Z.J.; McQuade, L.E.; Lippard, S.J. Detecting and understanding the roles of nitric oxide in biology. Inorg. Chem. 2010, 49, 6338–6348. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.M.; Zhu, J. Chapter Five-An introduction to nitric oxide sensing and response in bacteria. In Advances in Applied Microbiology; Sima, S., Geoffrey, M.G., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 87, pp. 187–220. [Google Scholar]
- Samouilov, A.; Woldman, Y.Y.; Zweier, J.; Khramtsov, V. Magnetic resonance study of the transmembrane nitrite diffusion. Nitric Oxide 2007, 16, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Lü, W.; Du, J.; Schwarzer, N.J.; Wacker, T.; Andrade, S.L.; Einsle, O. The formate/nitrite transporter family of anion channels. Biol. Chem. 2013, 394, 715–727. [Google Scholar] [CrossRef]
- Gao, H.; Yang, Z.K.; Barua, S.; Reed, S.B.; Romine, M.F.; Nealson, K.H.; Fredrickson, J.K.; Tiedje, J.M.; Zhou, J. Reduction of nitrate in Shewanella oneidensis depends on atypical NAP and NRF systems with NapB as a preferred electron transport protein from CymA to NapA. ISME J. 2009, 3, 966–976. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, H.; Wang, J.; Sun, L.; Jiang, Y.; Zhang, L.; Gao, H. Impacts of nitrate and nitrite on physiology of Shewanella oneidensis. PLoS ONE 2013, 8, e62629. [Google Scholar] [CrossRef]
- Albakri, Q.A.; Stuehr, D.J. Intracellular assembly of inducible NO synthase is limited by nitric oxide-mediated changes in heme insertion and availability. J. Biol. Chem. 1996, 271, 5414–5421. [Google Scholar] [CrossRef]
- Waheed, S.M.; Ghosh, A.; Chakravarti, R.; Biswas, A.; Haque, M.M.; Panda, K.; Stuehr, D.J. Nitric oxide blocks cellular heme insertion into a broad range of heme proteins. Free Radic. Biol. Med. 2010, 48, 1548–1558. [Google Scholar] [CrossRef]
- Richard-Fogal, C.; Kranz, R.G. The CcmC:heme:CcmE complex in heme trafficking and cytochrome c biosynthesis. J. Mol. Biol. 2010, 401, 350–362. [Google Scholar] [CrossRef]
- Shevket, S.H.; Gonzalez, D.; Cartwright, J.L.; Kleanthous, C.; Ferguson, S.J.; Redfield, C.; Mavridou, D.A.I. The CcmC–CcmE interaction during cytochrome c maturation by System I is driven by protein–protein and not protein–heme contacts. J. Biol. Chem. 2018, 293, 16778–16790. [Google Scholar] [CrossRef] [PubMed]
- Gallio, A.E.; Fung, S.S.P.; Cammack-Najera, A.; Hudson, A.J.; Raven, E.L. Understanding the logistics for the distribution of heme in cells. JACS Au 2021, 1, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.E. Nitric oxide and iron proteins. Biochim. Biophys. Acta 1999, 1411, 290–309. [Google Scholar] [CrossRef]
- Daltrop, O.; Allen, J.W.A.; Willis, A.C.; Ferguson, S.J. In vitro formation of a c-type cytochrome. Proc. Natl. Acad. Sci. USA 2002, 99, 7872–7876. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Stevens, J.M.; Daltrop, O.; Harvat, E.M.; Hong, L.; Ferguson, S.J.; Kitagawa, T. The interaction of covalently bound heme with the cytochrome c maturation protein CcmE. J. Biol. Chem. 2004, 279, 51981–51988. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wan, F.; Mao, Y.; Gao, H. Unraveling the mechanism for the viability deficiency of null mutant. J. Bacteriol. 2015, 197, 2179–2189. [Google Scholar] [CrossRef]
- Sun, L.; Jin, M.; Ding, W.; Yuan, J.; Kelly, J.; Gao, H. Posttranslational modification of flagellin FlaB in Shewanella oneidensis. J. Bacteriol. 2013, 195, 2550–2561. [Google Scholar] [CrossRef]
- Sivaraman, T.; Kumar, T.; Jayaraman, G.; Yu, C. The mechanism of 2, 2, 2-trichloroacetic acid-induced protein precipitation. J. Protein Chem. 1997, 16, 291–297. [Google Scholar] [CrossRef]
- Meng, Q.; Liang, H.; Gao, H. Roles of multiple KASIII homologues of Shewanella oneidensis in initiation of fatty acid synthesis and in cerulenin resistance. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1153–1163. [Google Scholar] [CrossRef]
- Mirelman, D.; Yashouv-Gan, Y.; Schwarz, U. Peptidoglycan biosynthesis in a thermosensitive division mutant of Escherichia coli. Biochemistry 1976, 15, 1781–1790. [Google Scholar] [CrossRef]
- Thomas, P.E.; Ryan, D.; Levin, W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal. Biochem. 1976, 75, 168–176. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, Y.; Wang, S.; Gao, H. Mutual interplay between ArcA and σE orchestrates envelope stress response in Shewanella oneidensis. Environ. Microbiol. 2021, 23, 652–668. [Google Scholar] [CrossRef]
- Fu, H.; Jin, M.; Ju, L.; Mao, Y.; Gao, H. Evidence for function overlapping of CymA and the cytochrome bc1 complex in the Shewanella oneidensis nitrate and nitrite respiration. Environ. Microbiol. 2014, 16, 3181–3195. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Feng, X.; Wang, W.; Chen, Y.; Chen, Z.; Gao, H. Free rather than total iron content is critically linked to the Fur physiology in Shewanella oneidensis. Front. Microbiol. 2020, 11, 593246. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wang, J.; Feng, X.; Gao, H. A Common Target of Nitrite and Nitric Oxide for Respiration Inhibition in Bacteria. Int. J. Mol. Sci. 2022, 23, 13841. https://doi.org/10.3390/ijms232213841
Wang W, Wang J, Feng X, Gao H. A Common Target of Nitrite and Nitric Oxide for Respiration Inhibition in Bacteria. International Journal of Molecular Sciences. 2022; 23(22):13841. https://doi.org/10.3390/ijms232213841
Chicago/Turabian StyleWang, Wei, Jiahao Wang, Xue Feng, and Haichun Gao. 2022. "A Common Target of Nitrite and Nitric Oxide for Respiration Inhibition in Bacteria" International Journal of Molecular Sciences 23, no. 22: 13841. https://doi.org/10.3390/ijms232213841
APA StyleWang, W., Wang, J., Feng, X., & Gao, H. (2022). A Common Target of Nitrite and Nitric Oxide for Respiration Inhibition in Bacteria. International Journal of Molecular Sciences, 23(22), 13841. https://doi.org/10.3390/ijms232213841







