MSClustering: A Cytoscape Tool for Multi-Level Clustering of Biological Networks
Abstract
1. Introduction
2. Results and Discussion
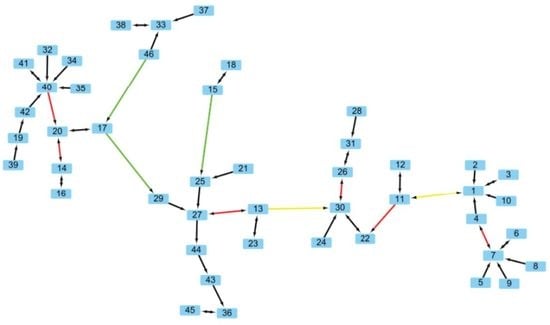
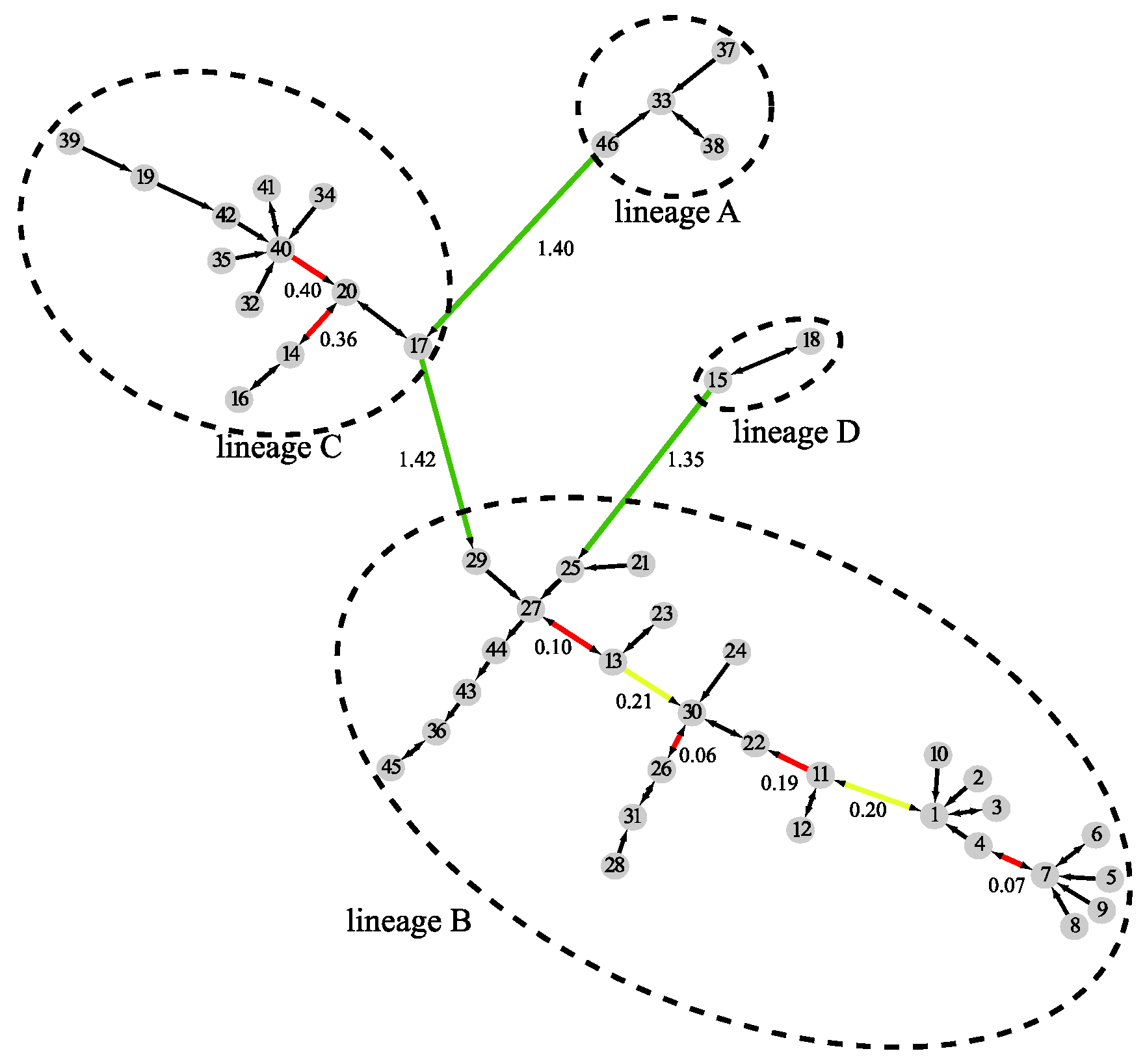
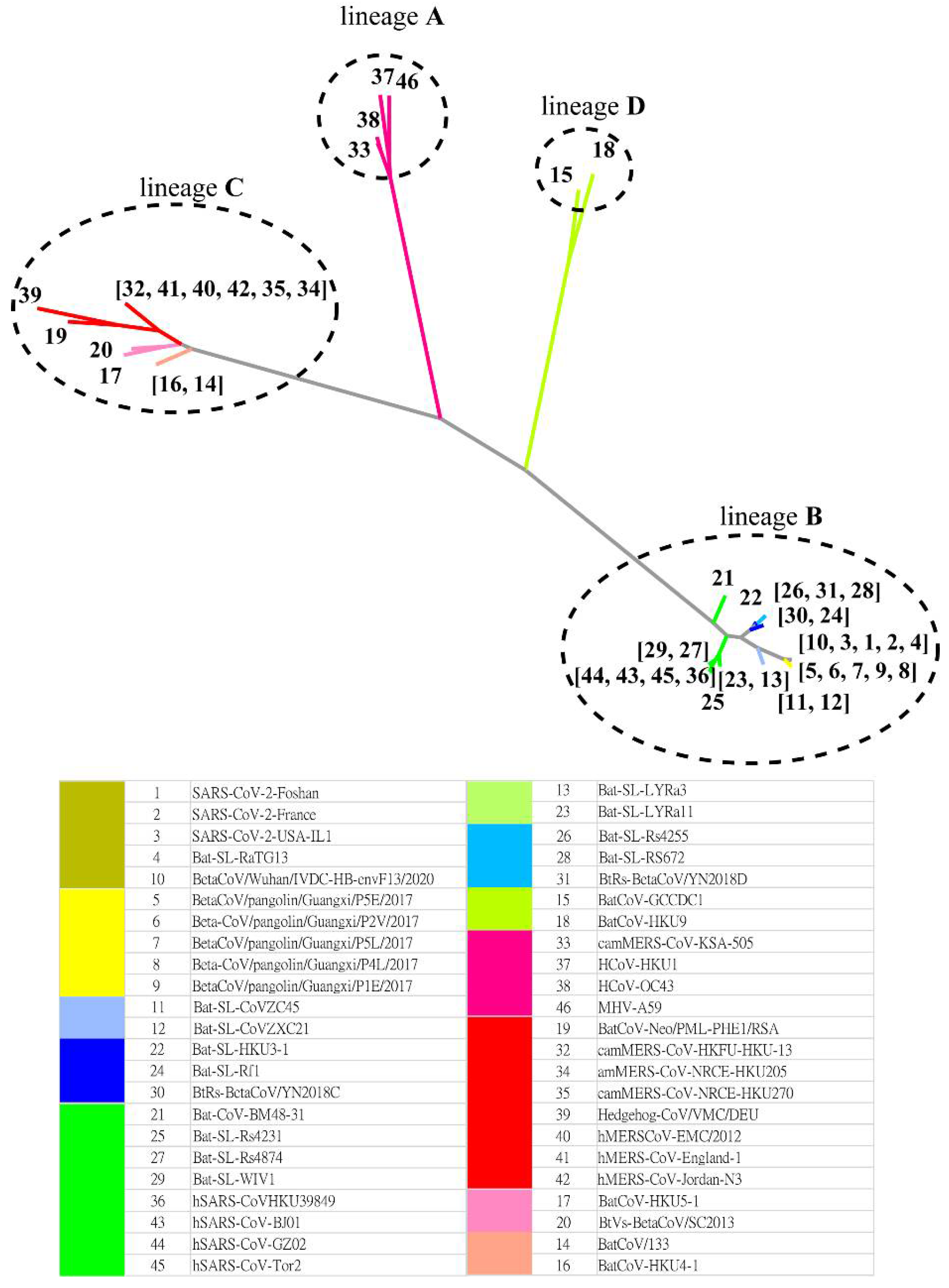
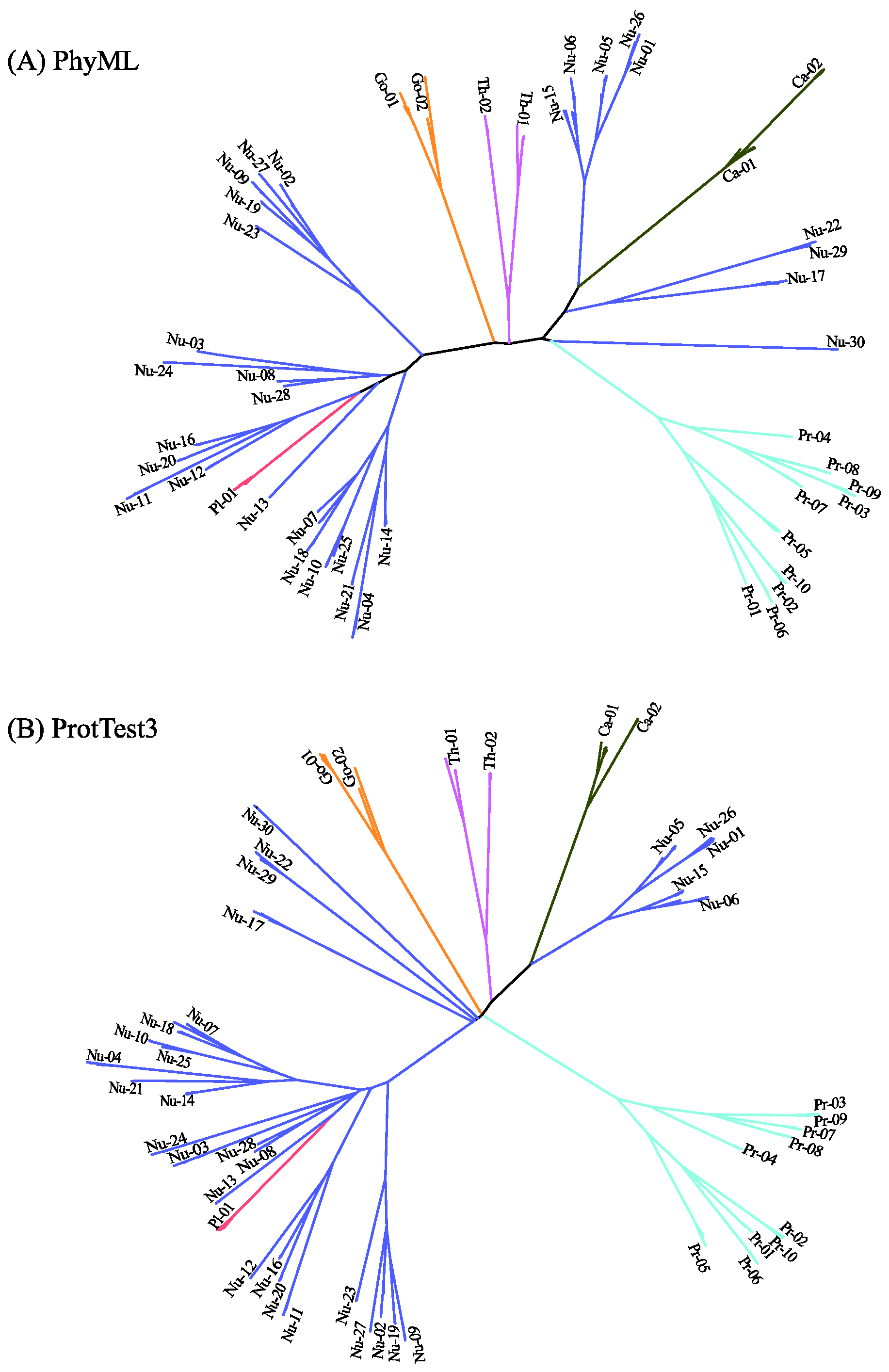
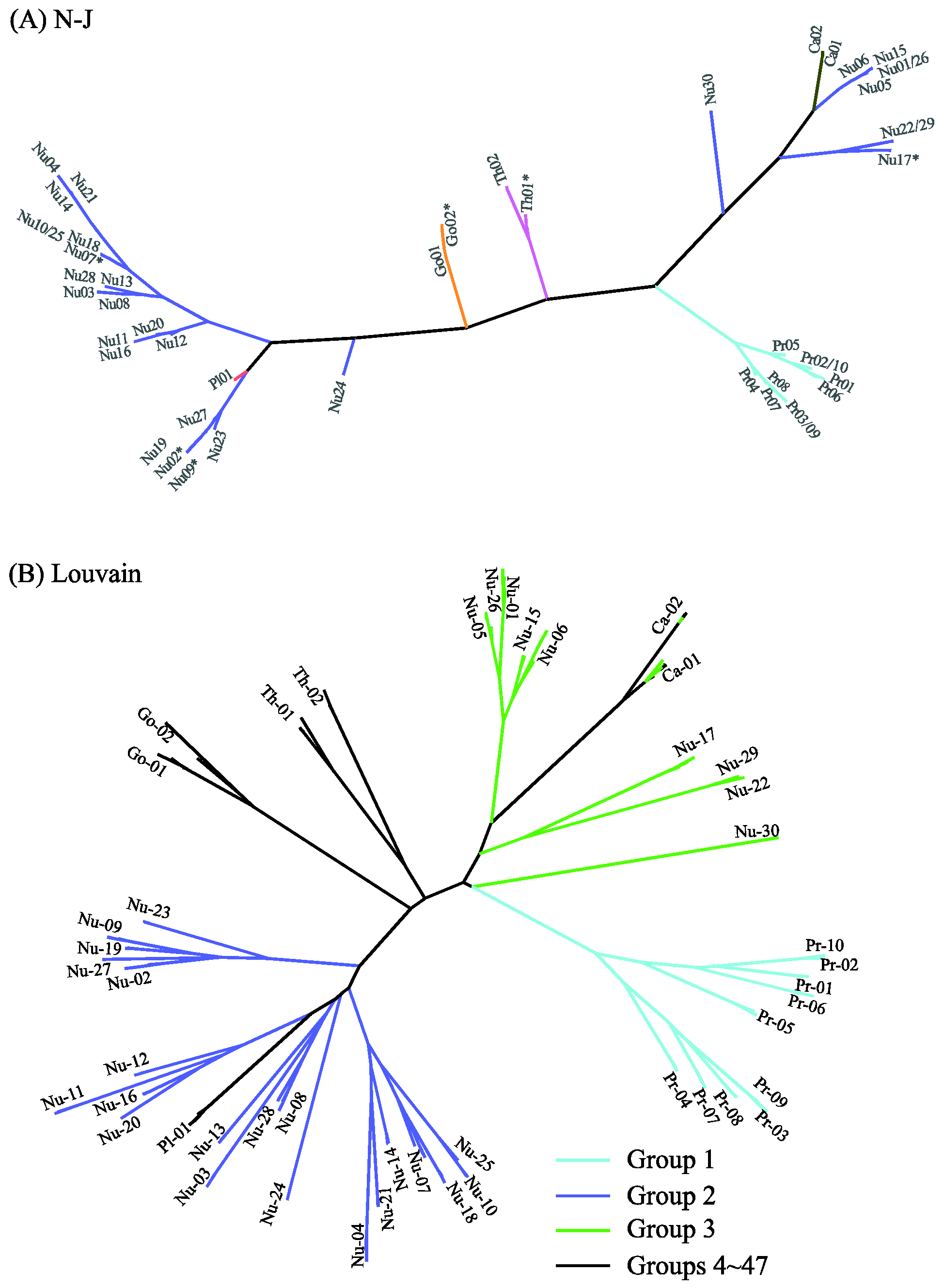
2.1. Comparing Phylogenetic Trees of Coronaviruses
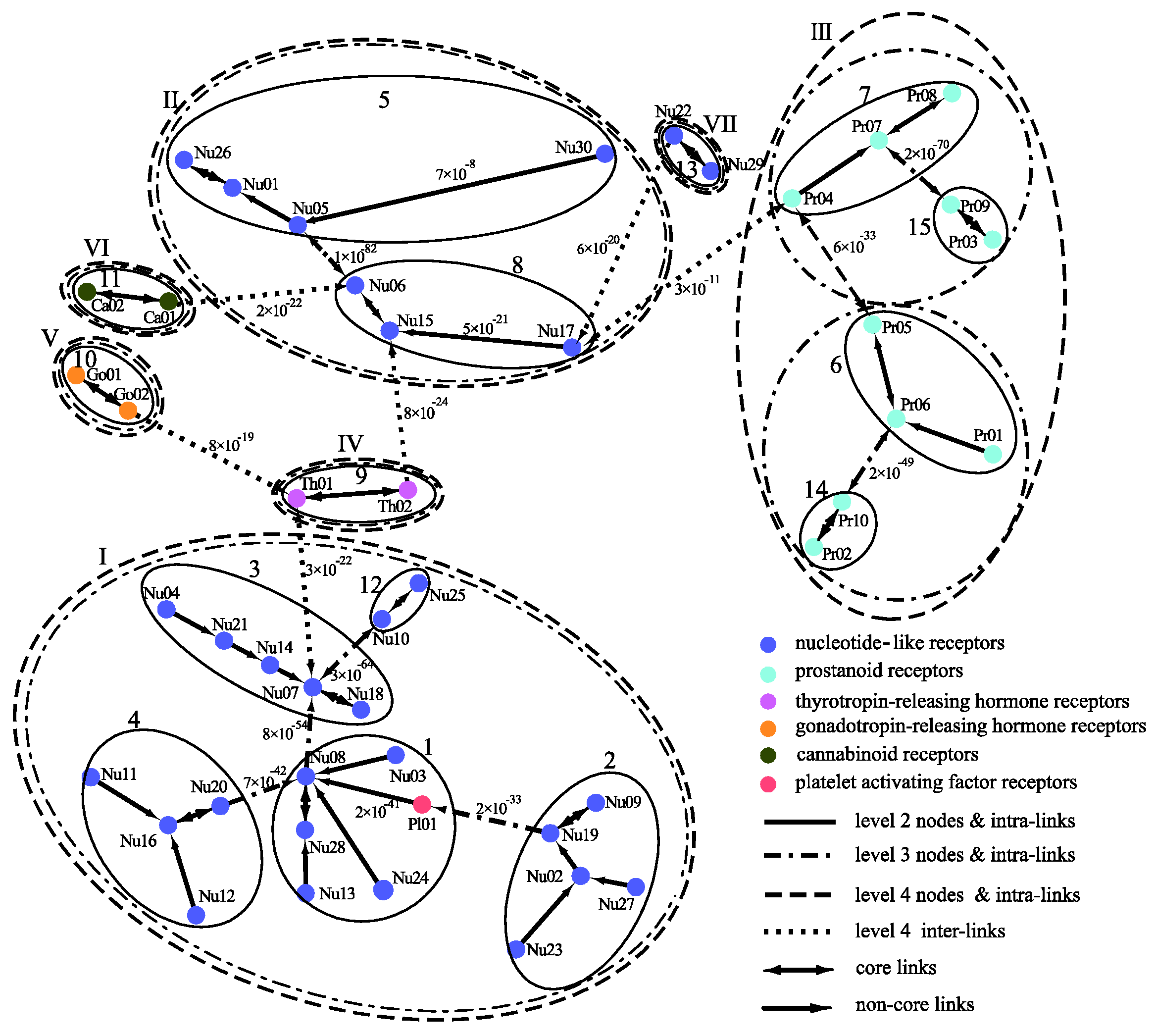
2.2. Comparing Phylogenetic Trees of GPCRs
2.3. Comparing Clustering Plugins in Cytoscape
3. Materials and Methods
3.1. Reading the Input File
3.2. Multi-Level Clustering
3.3. Network Visualization and Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paterlini, M. There shall be order. The legacy of Linnaeus in the age of molecular biology. EMBO Rep. 2007, 8, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Frigui, H. Clustering: Algorithms and applications. In Proceedings of the 2008 First Workshops on Image Processing Theory, Tools and Applications, Sousse, Tunisia, 23–26 November 2008; pp. 1–11. [Google Scholar]
- Chen, R.H.G.; Chen, C.C.; Chen, C.M. Unsupervised cluster analyses of character networks in fiction: Community structure and centrality. Knowl. Based Syst. 2019, 163, 800–810. [Google Scholar] [CrossRef]
- Hu, G.-M.; Mai, T.-L.; Chen, C.-M. Visualizing the GPCR Network: Classification and Evolution. Sci. Rep. 2017, 7, 15495. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.H.-G.; Chen, C.-M. Visualizing the world’s scientific publications. J. Assoc. Inf. Sci. Technol. 2016, 67, 2477–2488. [Google Scholar] [CrossRef]
- McLachlan, G.J. Cluster analysis and related techniques in medical research. Stat. Methods Med. Res. 1992, 1, 27–48. [Google Scholar] [CrossRef]
- Yang, Z.; Rannala, B. Molecular phylogenetics: Principles and practice. Nat. Rev. Genet. 2012, 13, 303–314. [Google Scholar] [CrossRef]
- Schadt, E.E.; Sinsheimer, J.S.; Lange, K. Computational advances in maximum likelihood methods for molecular phylogeny. Genome Res. 1998, 8, 222–233. [Google Scholar] [CrossRef][Green Version]
- Nascimento, F.F.; Reis, M.D.; Yang, Z. A biologist’s guide to Bayesian phylogenetic analysis. Nat. Ecol. Evol. 2017, 1, 1446–1454. [Google Scholar] [CrossRef]
- Joost, P.; Methner, A. Phylogenetic analysis of 277 human G-protein-coupled receptors as a tool for the prediction of orphan receptor ligands. Genome Biol. 2002, 3, Research0063.1. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Whelan, S.; Goldman, N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 2001, 18, 691–699. [Google Scholar] [CrossRef]
- Hakim, M.S. SARS-CoV-2, COVID-19, and the debunking of conspiracy theories. Rev. Med. Virol. 2021, 31, e2222. [Google Scholar] [CrossRef]
- Lam, T.T.-Y.; Jia, N.; Zhang, Y.-W.; Shum, M.H.-H.; Jiang, J.-F.; Zhu, H.-C.; Tong, Y.-G.; Shi, Y.-X.; Ni, X.-B.; Liao, Y.-S.; et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature 2020, 583, 282–285. [Google Scholar] [CrossRef]
- Jaimes, J.A.; André, N.M.; Chappie, J.S.; Millet, J.K.; Whittaker, G.R. Phylogenetic analysis and structural modeling of SARS-CoV-2 spike protein reveals an evolutionary distinct and proteolytically sensitive activation loop. J. Mol. Biol. 2020, 432, 3309–3325. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Blondel, V.D.; Guillaume, J.-L.; Lambiotte, R.; Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, 2008, P10008. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. ProtTest 3: Fast selection of best-fit models of protein evolution. Bioinformatics 2011, 27, 1164–1165. [Google Scholar] [CrossRef]
- Pándy-Szekeres, G.; Munk, C.; Tsonkov, T.M.; Mordalski, S.; Harpsøe, K.; Hauser, A.S.; Bojarski, A.J.; Gloriam, D.E. GPCRdb in 2018: Adding GPCR structure models and ligands. Nucleic Acids Res. 2017, 46, D440–D446. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2020, 49, D480–D489. [Google Scholar] [CrossRef]
- Bjarnadóttir, T.K.; Gloriam, D.E.; Hellstrand, S.H.; Kristiansson, H.; Fredriksson, R.; Schiöth, H.B. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics 2006, 88, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Gao, Z.-G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, M.; Wang, J.; Pan, Y.; Wu, F.X. CytoNCA: A cytoscape plugin for centrality analysis and evaluation of protein interaction networks. Biosystems 2015, 127, 67–72. [Google Scholar] [CrossRef] [PubMed]



| Methods (Without Visualization) | Computation Time (s) | |
|---|---|---|
| Distance-based | MSClustering | 0.0021 |
| Neighbor-joining | 0.036 | |
| Louvain | 2.5 | |
| Character-based | IQ-TREE | 1462 |
| PhyML | 4243 | |
| ProtTest3 | 29,760 |
| Plugin | Features | Clustering Algorithms | Input |
|---|---|---|---|
| AutoAnnotate | finds clusters and visually annotates them with labels and groups | MCL, AP, CF, CC, CCC, and SCPS | selected Cytoscape network |
| clusterMaker2 | unifies a variety of algorithms for clustering networks and attributes as well as for ranking clusters based on potentially orthogonal data | AP and 20 others | selected Cytoscape network |
| ClusterViz | found cluster can be subjected to GO enrichment analysis | FAG-EC, MCODE, and EAGLE | selected Cytoscape network |
| CytoCluster | analyzes and visualizes clusters from a selected Cytoscape network | HC-PIN, DCU, IPCA, OH-PIN, IPC-MCE, and ClusterONE | selected Cytoscape network |
| MCODE | clusters a given network based on the topology to find densely connected regions | MCODE | selected Cytoscape network |
| MSClustering | an efficient app for hierarchical clustering and phylogenetics of large complex networks | MSC | distance matrix |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, B.-K.; Hu, G.-M.; Chen, R.; Chen, C.-M. MSClustering: A Cytoscape Tool for Multi-Level Clustering of Biological Networks. Int. J. Mol. Sci. 2022, 23, 14240. https://doi.org/10.3390/ijms232214240
Ge B-K, Hu G-M, Chen R, Chen C-M. MSClustering: A Cytoscape Tool for Multi-Level Clustering of Biological Networks. International Journal of Molecular Sciences. 2022; 23(22):14240. https://doi.org/10.3390/ijms232214240
Chicago/Turabian StyleGe, Bo-Kai, Geng-Ming Hu, Rex Chen, and Chi-Ming Chen. 2022. "MSClustering: A Cytoscape Tool for Multi-Level Clustering of Biological Networks" International Journal of Molecular Sciences 23, no. 22: 14240. https://doi.org/10.3390/ijms232214240
APA StyleGe, B.-K., Hu, G.-M., Chen, R., & Chen, C.-M. (2022). MSClustering: A Cytoscape Tool for Multi-Level Clustering of Biological Networks. International Journal of Molecular Sciences, 23(22), 14240. https://doi.org/10.3390/ijms232214240