Genome-Wide Identification of ATG Gene Family Members in Fagopyrum tataricum and Their Expression during Stress Responses
Abstract
:1. Introduction
2. Results
2.1. Autophagy Occurs in TB under Drought Stress
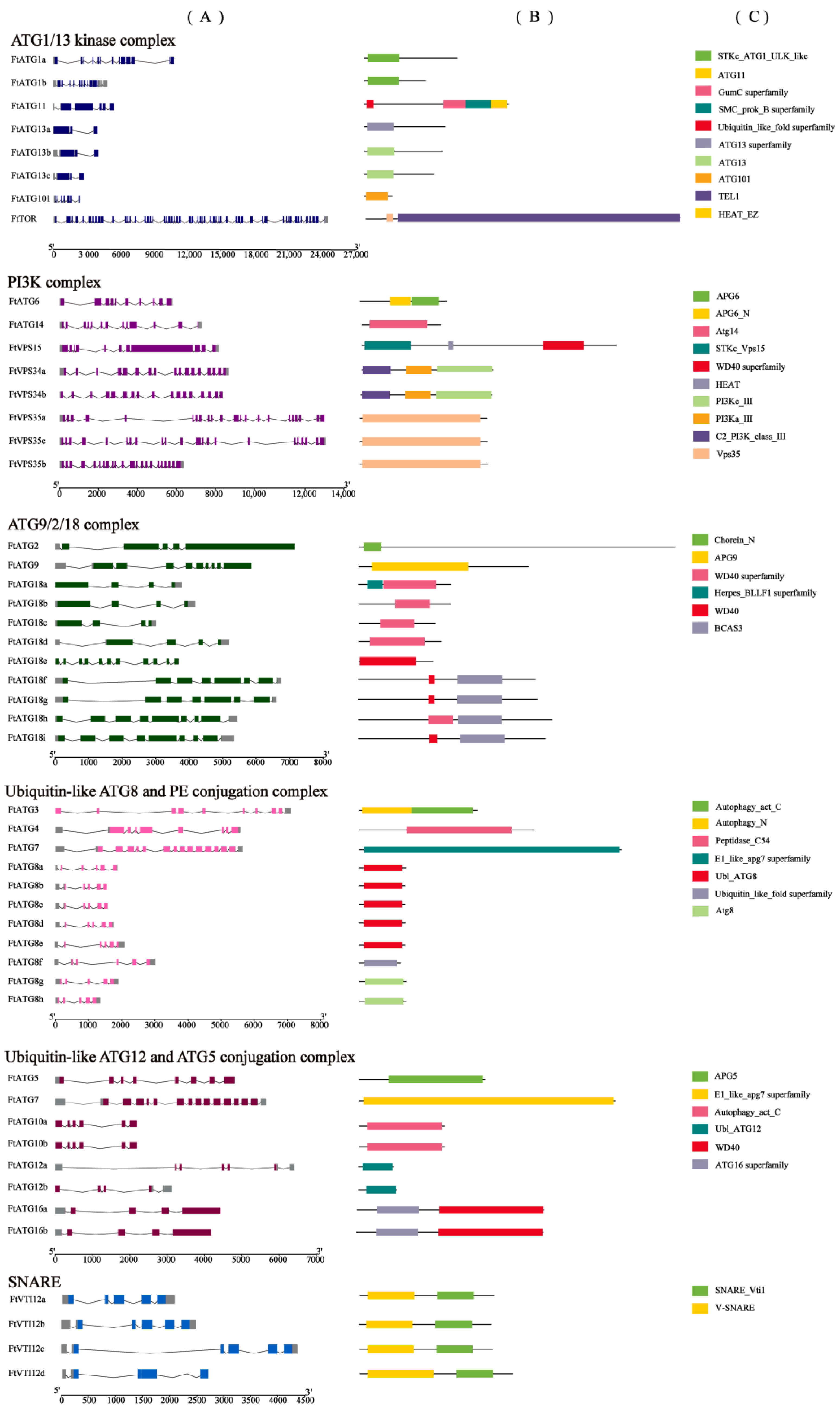
2.2. Genome-Wide Identification of FtATGs in TB
2.3. Phylogenetic Tree Construction and Conserved Motifs of FtATGs
2.4. Analysis of FtATGs Expression Pattern under Stress
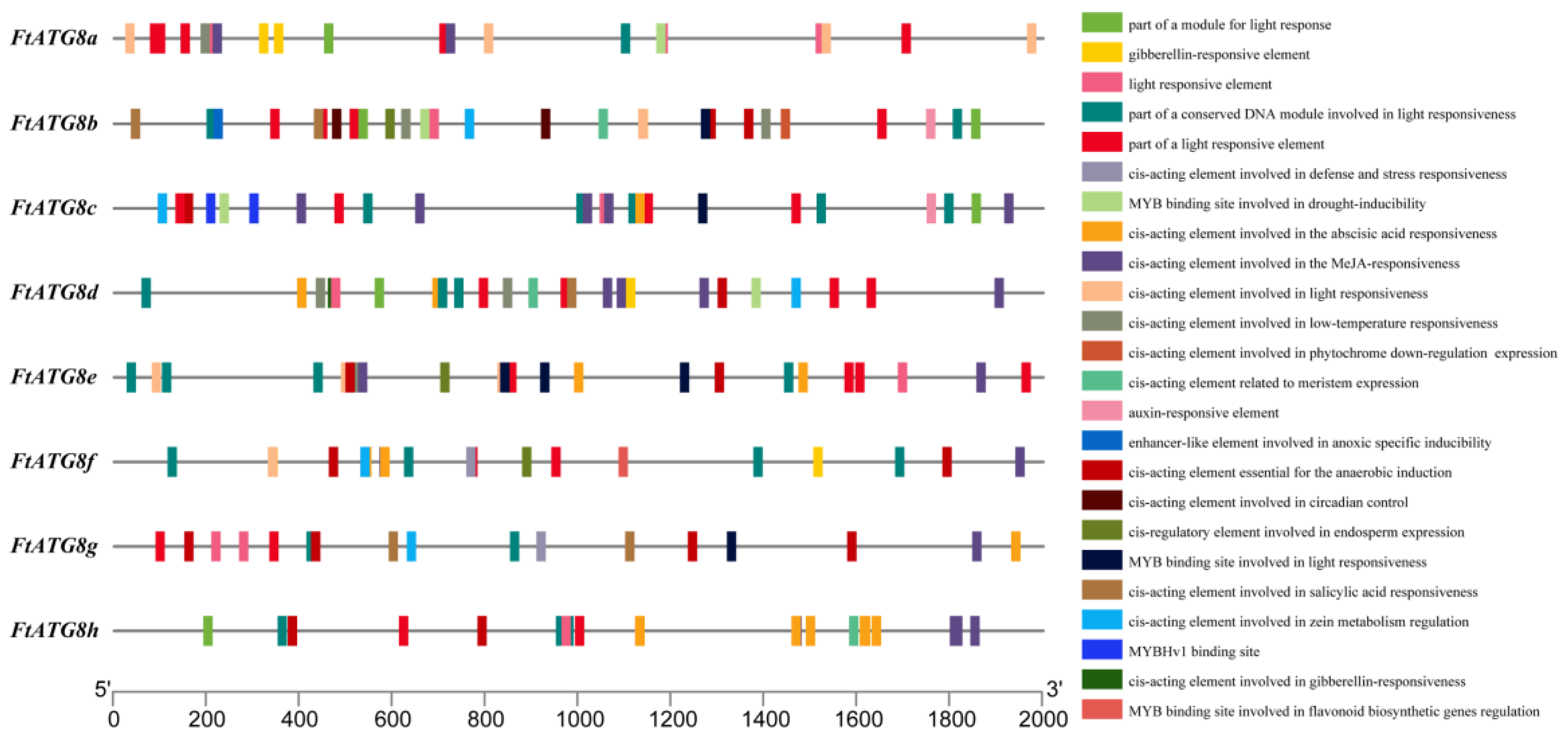
2.5. Cis-Acting Elements in FtATG8s Promoter
2.6. Prediction of FtATGs Interacting Proteins
2.7. Correlation Analysis between FtATG8s and Stress Resistance-Related Transcription Factors
2.8. Expression Analysis of FtATG8s in Different TB Tissues
2.9. Expression Modes of FtATG8s under Abiotic Stresses
2.10. Overexpression of FtATG8a and FtATG8f May Enhance Drought Resistance in TB
3. Discussion
4. Materials and Methods
4.1. Identification of FtATG Genes
4.2. Autophagosome Monitoring of TB Roots
4.3. Analysis of Main Characteristics of ATG Family Members in TB
4.4. Heat Map of the FtATG Expression Patterns under Stresses
4.5. Plant Materials and Treatments
4.6. Construction of Protein-Protein Interaction Network
4.7. Correlation Analysis between FtATG8s and Transcription Factors
4.8. Transient Expression of FtATG8a and FtATG8f in TB Cotyledons
4.9. Quantitative Real-Time PCR Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, J.-K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miryeganeh, M. Plants’ epigenetic mechanisms and abiotic stress. Genes 2021, 12, 1106. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Masanori, I. Chlorophagy is ATG gene-dependent microautophagy process. Plant Signal. Behav. 2019, 14, 1554469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumi, M.; Ishida, H.; Nakamura, S.; Hidema, J. Entire photodamaged chloroplasts are transported to the central vacuole by autophagy. Plant Cell 2017, 29, 377–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, P.; Zhou, X.-M.; Zhao, L.-L.; Cheung, A.Y.; Sun, M.-X. Autophagy-mediated compartmental cytoplasmic deletion is essential for tobacco pollen germination and male fertility. Autophagy 2020, 16, 2180–2192. [Google Scholar] [CrossRef] [PubMed]
- Yoshitake, Y.; Ohta, H.; Shimojima, M. Autophagy-mediated regulation of lipid metabolism and its impact on the growth in algae and seed plants. Front. Plant Sci. 2019, 10, 709. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Dong, J.; Wang, T. Autophagy in plant abiotic stress management. Int. J. Mol. Sci. 2021, 22, 4075. [Google Scholar] [CrossRef]
- Yue, J.; Wang, Y.; Jiao, J.; Wang, H. Silencing of ATG2 and ATG7 promotes programmed cell death in wheat via inhibition of autophagy under salt stress. Ecotoxicol. Environ. Saf. 2021, 225, 112761. [Google Scholar] [CrossRef]
- Sun, X.; Wang, P.; Jia, X.; Huo, L.; Che, R.; Ma, F. Improvement of drought tolerance by overexpressing MdATG18a is mediated by modified antioxidant system and activated autophagy in transgenic apple. Plant Biotechnol. J. 2018, 16, 545–557. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.N.; Padman, B.S.; Usher, J.; Oorschot, V.; Ramm, G.; Lazarou, M. ATG8 family LC3/GABARAP proteins are crucial for autophagosome–lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J. Cell Biol. 2016, 215, 857–874. [Google Scholar] [CrossRef]
- Lystad, A.H.; Simonsen, A. Mechanisms and pathophysiological roles of the ATG8 conjugation machinery. Cells 2019, 8, 973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Liu, G.; Wang, Y.; Wei, Y.; Shi, H. Overexpression of banana ATG8f modulates drought stress resistance in Arabidopsis. Biomolecules 2019, 9, 814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, L.; Sun, X.; Guo, Z.; Jia, X.; Che, R.; Sun, Y.; Zhu, Y.; Wang, P.; Gong, X.; Ma, F. MdATG18a overexpression improves basal thermotolerance in transgenic apple by decreasing damage to chloroplasts. Hortic. Res. 2020, 7, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, L.; Guo, Z.; Wang, P.; Zhang, Z.; Jia, X.; Sun, Y.; Sun, X.; Gong, X.; Ma, F. MdATG8i functions positively in apple salt tolerance by maintaining photosynthetic ability and increasing the accumulation of arginine and polyamines. Environ. Exp. Bot. 2020, 172, 103989. [Google Scholar] [CrossRef]
- Li, H.; Lv, Q.; Liu, A.; Wang, J.; Sun, X.; Deng, J.; Chen, Q.; Wu, Q. Comparative metabolomics study of Tartary (Fagopyrum tataricum (L.) Gaertn) and common (Fagopyrum esculentum Moench) buckwheat seeds. Food Chem. 2022, 371, 131125. [Google Scholar] [CrossRef]
- Jacques, C.; Salon, C.; Barnard, R.L.; Vernoud, V.; Prudent, M. Drought stress memory at the plant cycle level: A review. Plants 2021, 10, 1873. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Zeng, X.; Zeng, Z.; Liu, C.; Yuan, W.; Hou, N.; Bian, H.; Zhu, M.; Han, N. A barley homolog of yeast ATG6 is involved in multiple abiotic stress responses and stress resistance regulation. Plant Physiol. Biochem. 2017, 115, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Xia, K.; Liu, T.; Ouyang, J.; Wang, R.; Fan, T.; Zhang, M. Genome-wide identification, classification, and expression analysis of autophagy-associated gene homologues in rice (Oryza sativa L.). DNA Res. 2011, 18, 363–377. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.-Z.; Zhou, X.; Xu, Y.-Y.; Hui, Q.-L.; Chun, C.-P.; Ling, L.-L.; Peng, L.-Z. Comprehensive analysis of autophagy-related genes in sweet orange (Citrus sinensis) highlights their roles in response to abiotic stresses. Int. J. Mol. Sci. 2020, 21, 2699. [Google Scholar] [CrossRef]
- Huang, W.; Ma, D.-N.; Liu, H.-L.; Luo, J.; Wang, P.; Wang, M.-L.; Guo, F.; Wang, Y.; Zhao, H.; Ni, D.-J. Genome-wide identification of CsATGs in tea plant and the involvement of CsATG8e in nitrogen utilization. Int. J. Mol. Sci. 2020, 21, 7043. [Google Scholar] [CrossRef] [PubMed]
- Faraji, S.; Heidari, P.; Amouei, H.; Filiz, E.; Abdullah; Poczai, P. Investigation and computational analysis of the sulfotransferase (SOT) gene family in Potato (Solanum tuberosum): Insights into sulfur adjustment for proper development and stimuli responses. Plants 2021, 10, 2597. [Google Scholar] [CrossRef]
- Heidari, P.; Abdullah; Faraji, S.; Poczai, P. Magnesium transporter gene family: Genome-wide Identification and characterization in Theobroma cacao, Corchorus capsularis, and Gossypium hirsutum of family malvaceae. Agronomy 2021, 11, 1651. [Google Scholar] [CrossRef]
- Sheshadri, S.A.; Nishanth, M.J.; Simon, B. Stress-mediated cis-element transcription factor interactions interconnecting primary and specialized metabolism in planta. Front. Plant Sci 2016, 7, 1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Y.; Guo, M.; Wang, H.; Lu, J.; Liu, J.; Zhang, C.; Gong, Z.; Lu, M. Autophagy, a conserved mechanism for protein degradation, responds to heat, and other abiotic stresses in Capsicum annuum L. Front. Plant Sci. 2016, 7, 131. [Google Scholar] [CrossRef] [Green Version]
- Yan, B. Links between drought stress and autophagy in plants. Plant Signal. Behav. 2020, 15, 87. [Google Scholar]
- Lv, X.; Pu, X.; Qin, G.; Zhu, T.; Lin, H. The roles of autophagy in development and stress responses in Arabidopsis thaliana. Apoptosis 2014, 19, 905–921. [Google Scholar] [CrossRef]
- Shangguan, L.; Fang, X.; Chen, L.; Cui, L.; Fang, J. Genome-wide analysis of autophagy-related genes (ARGs) in grapevine and plant tolerance to copper stress. Planta 2018, 247, 1449–1463. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, H.; Sun, L.; Wu, W.; Li, C.; Wu, Q. Genome-wide identification of MAPK gene family members in Fagopyrum tataricum and their expression during development and stress responses. BMC Genom. 2022, 23, 96. [Google Scholar] [CrossRef]
- Bernat-Silvestre, C.; De Sousa Vieira, V.; Sánchez-Simarro, J.; Aniento, F.; Marcote, M.J. Transient Transformation of A. thaliana Seedlings by Vacuum Infiltration; Springer: New York, NY, USA, 2021; pp. 147–155. [Google Scholar]
- Hu, D.; Bent, A.F.; Hou, X.; Li, Y. Agrobacterium-mediated vacuum infiltration and floral dip transformation of rapid-cycling. Brassica rapa. BMC Plant Biol. 2019, 19, 246. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Caselles, C.; Faize, L.; Burgos, L.; Alburquerque, N. Improving adventitious shoot regeneration and transient Agrobacterium-mediated transformation of apricot (Prunus armeniaca L.) hypocotyl sections. Agronomy 2021, 11, 1338. [Google Scholar] [CrossRef]
- Wang, P.; Nolan, T.M.; Yin, Y.; Bassham, D.C. Identification of transcription factors that regulate ATG8 expression and autophagy in Arabidopsis. Autophagy 2020, 16, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Katarzyna, S.; Andisheh, P.; Kenji, Y.; Shino, G. Microautophagy in plants: Consideration of its molecular mechanism. Cells 2020, 9, 887. [Google Scholar]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Y.; Wang, S.; Wu, H.; Li, C.; Zhao, H.; Chen, H.; Wang, X.; Wu, Q. Genome-Wide Identification of ATG Gene Family Members in Fagopyrum tataricum and Their Expression during Stress Responses. Int. J. Mol. Sci. 2022, 23, 14845. https://doi.org/10.3390/ijms232314845
Fang Y, Wang S, Wu H, Li C, Zhao H, Chen H, Wang X, Wu Q. Genome-Wide Identification of ATG Gene Family Members in Fagopyrum tataricum and Their Expression during Stress Responses. International Journal of Molecular Sciences. 2022; 23(23):14845. https://doi.org/10.3390/ijms232314845
Chicago/Turabian StyleFang, Yue, Shuang Wang, Hula Wu, Chenglei Li, Haixia Zhao, Hui Chen, Xiaoli Wang, and Qi Wu. 2022. "Genome-Wide Identification of ATG Gene Family Members in Fagopyrum tataricum and Their Expression during Stress Responses" International Journal of Molecular Sciences 23, no. 23: 14845. https://doi.org/10.3390/ijms232314845
APA StyleFang, Y., Wang, S., Wu, H., Li, C., Zhao, H., Chen, H., Wang, X., & Wu, Q. (2022). Genome-Wide Identification of ATG Gene Family Members in Fagopyrum tataricum and Their Expression during Stress Responses. International Journal of Molecular Sciences, 23(23), 14845. https://doi.org/10.3390/ijms232314845







