Optogenetic Therapy for Visual Restoration
Abstract
:1. Introduction
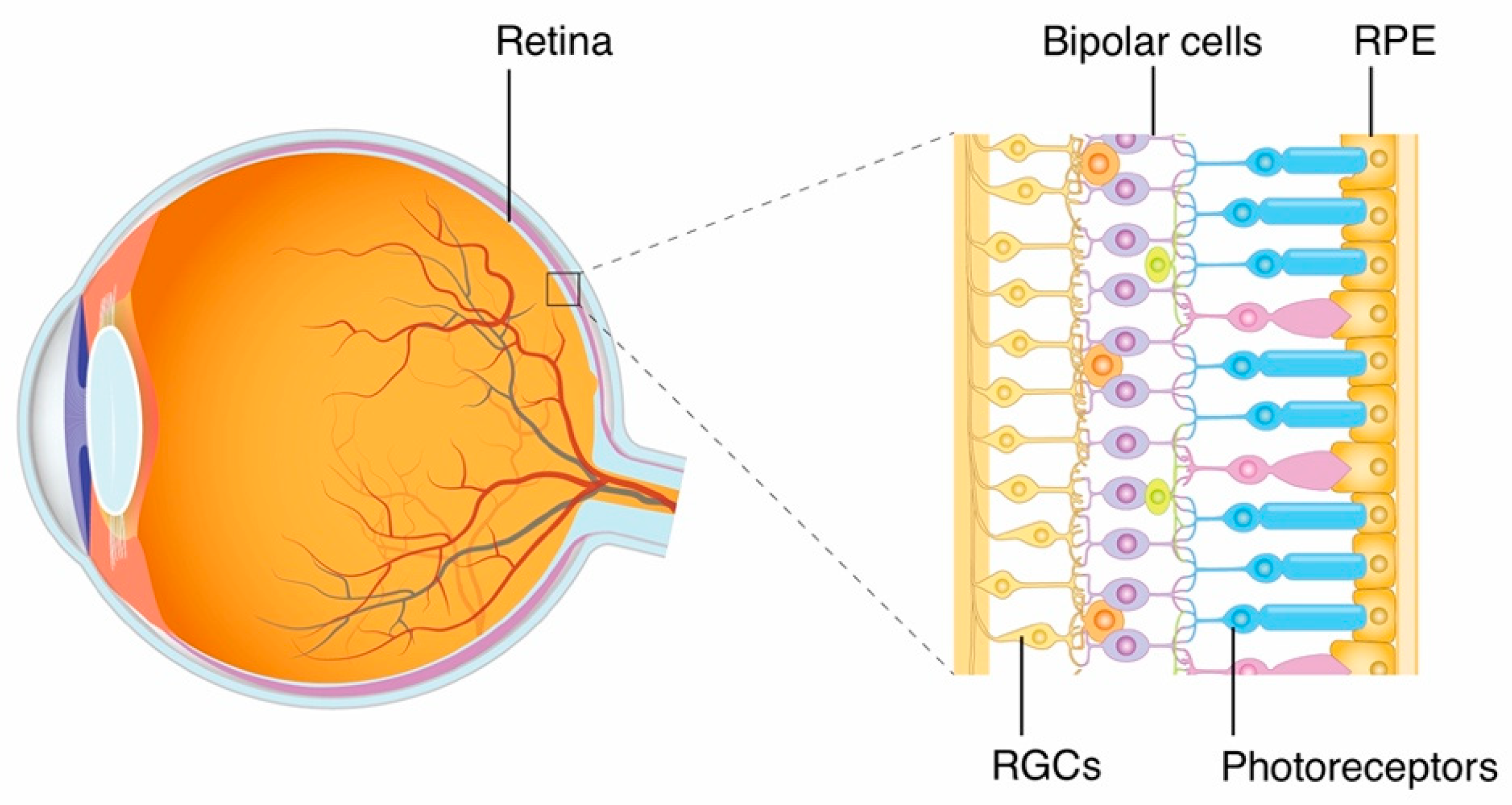
1.1. Retinal Structure and Visual Pathway
1.2. Optogenetics and Retinal Degenerative Diseases
2. Strategies and Target Cells
2.1. Dormant Cone
2.2. Bipolar Cell
2.3. RGC
2.4. Application for Photoreceptor Transplantation
3. Optogenetic Tools
3.1. Microbial (Type 1) Opsins
3.1.1. Depolarizing Opsin
3.1.2. Hyperpolarizing Opsin
3.2. Animal (Type 2) Opsins
3.2.1. Melanopsin
3.2.2. Rhodopsin
3.2.3. Cone Opsin
4. Gene Delivery
4.1. Adeno-Associated Viral Vectors
4.2. Intervention
5. Road to Clinical Application
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deisseroth, K.; Feng, G.; Majewska, A.K.; Miesenböck, G.; Ting, A.; Schnitzer, M.J. Next-generation optical technologies for illuminating genetically targeted brain circuits. J. Neurosci. 2006, 26, 10380–10386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.L.; Barlow, W.E.; Humayun, M.S.; de Juan, E.; Milam, A.H. Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch. Ophthalmol. 1992, 110, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Humayun, M.S.; de Juan, E.; Greenburg, R.J.; Marsh, M.J.; Klock, I.B.; Milam, A.H. Preservation of the inner retina in retinitis pigmentosa. A morphometric analysis. Arch. Ophthalmol. 1997, 115, 511–515. [Google Scholar] [CrossRef]
- Garafalo, A.V.; Cideciyan, A.V.; Héon, E.; Sheplock, R.; Pearson, A.; WeiYang Yu, C.; Sumaroka, A.; Aguirre, G.D.; Jacobson, S.G. Progress in treating inherited retinal diseases: Early subretinal gene therapy clinical trials and candidates for future initiatives. Prog. Retin. Eye Res. 2020, 77, 100827. [Google Scholar] [CrossRef]
- Ellingford, J.M.; Barton, S.; Bhaskar, S.; O’Sullivan, J.; Williams, S.G.; Lamb, J.A.; Panda, B.; Sergouniotis, P.I.; Gillespie, R.L.; Daiger, S.P.; et al. Molecular findings from 537 individuals with inherited retinal disease. J. Med. Genet. 2016, 53, 761–767. [Google Scholar] [CrossRef] [Green Version]
- Maeda, A.; Yoshida, A.; Kawai, K.; Arai, Y.; Akiba, R.; Inaba, A.; Takagi, S.; Fujiki, R.; Hirami, Y.; Kurimoto, Y.; et al. Development of a molecular diagnostic test for Retinitis pigmentosa in the Japanese population. Jpn. J. Ophthalmol. 2018, 62, 451–457. [Google Scholar] [CrossRef]
- Bi, A.; Cui, J.; Ma, Y.P.; Olshevskaya, E.; Pu, M.; Dizhoor, A.M.; Pan, Z.H. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 2006, 50, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Song, D.J.; Bao, X.L.; Fan, B.; Li, G.Y. Mechanism of cone degeneration in retinitis pigmentosa. Cell. Mol. Neurobiol. 2022. [Google Scholar] [CrossRef]
- Lin, B.; Masland, R.H.; Strettoi, E. Remodeling of cone photoreceptor cells after rod degeneration in rd mice. Exp. Eye Res. 2009, 88, 589–599. [Google Scholar] [CrossRef]
- Sahel, J.A.; Léveillard, T.; Picaud, S.; Dalkara, D.; Marazova, K.; Safran, A.; Paques, M.; Duebel, J.; Roska, B.; Mohand-Said, S. Functional rescue of cone photoreceptors in retinitis pigmentosa. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 1669–1677. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.P.; Brauner, M.; Liewald, J.F.; Kay, K.; Watzke, N.; Wood, P.G.; Bamberg, E.; Nagel, G.; Gottschalk, A.; et al. Multimodal fast optical interrogation of neural circuitry. Nature 2007, 446, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Busskamp, V.; Duebel, J.; Balya, D.; Fradot, M.; Viney, T.J.; Siegert, S.; Groner, A.C.; Cabuy, E.; Forster, V.; Seeliger, M.; et al. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science 2010, 329, 413–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagali, P.S.; Balya, D.; Awatramani, G.B.; Münch, T.A.; Kim, D.S.; Busskamp, V.; Cepko, C.L.; Roska, B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat. Neurosci. 2008, 11, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Doroudchi, M.M.; Greenberg, K.P.; Liu, J.; Silka, K.A.; Boyden, E.S.; Lockridge, J.A.; Arman, A.C.; Janani, R.; Boye, S.E.; Boye, S.L.; et al. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol. Ther. 2011, 19, 1220–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaub, B.M.; Berry, M.H.; Holt, A.E.; Reiner, A.; Kienzler, M.A.; Dolgova, N.; Nikonov, S.; Aguirre, G.D.; Beltran, W.A.; Flannery, J.G.; et al. Restoration of visual function by expression of a light-gated mammalian ion channel in retinal ganglion cells or ON-bipolar cells. Proc. Natl Acad. Sci. USA 2014, 111, E5574–E5583. [Google Scholar] [CrossRef] [Green Version]
- Macé, E.; Caplette, R.; Marre, O.; Sengupta, A.; Chaffiol, A.; Barbe, P.; Desrosiers, M.; Bamberg, E.; Sahel, J.A.; Picaud, S.; et al. Targeting channelrhodopsin-2 to ON-bipolar cells with vitreally administered AAV Restores ON and OFF visual responses in blind mice. Mol. Ther. 2015, 23, 7–16. [Google Scholar] [CrossRef] [Green Version]
- van Wyk, M.; Pielecka-Fortuna, J.; Löwel, S.; Kleinlogel, S. Restoring the ON switch in blind retinas: Opto-mGluR6, a next-generation, cell-tailored optogenetic tool. PLoS Biol. 2015, 13, e1002143. [Google Scholar] [CrossRef] [Green Version]
- Eleftheriou, C.G.; Cehajic-Kapetanovic, J.; Martial, F.P.; Milosavljevic, N.; Bedford, R.A.; Lucas, R.J. Meclofenamic acid improves the signal to noise ratio for visual responses produced by ectopic expression of human rod opsin. Mol. Vis. 2017, 23, 334–345. [Google Scholar]
- McClements, M.E.; Staurenghi, F.; Visel, M.; Flannery, J.G.; MacLaren, R.E.; Cehajic-Kapetanovic, J. AAV induced expression of human rod and cone opsin in bipolar cells of a mouse model of retinal degeneration. BioMed Res. Int. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Wright, P.; Rodgers, J.; Wynne, J.; Bishop, P.N.; Lucas, R.J.; Milosavljevic, N. Viral transduction of human rod opsin or channelrhodopsin variants to mouse ON bipolar cells does not impact retinal anatomy or cause measurable death in the targeted cells. Int. J. Mol. Sci. 2021, 22, 13111. [Google Scholar] [CrossRef]
- Jones, B.W.; Pfeiffer, R.L.; Ferrell, W.D.; Watt, C.B.; Marmor, M.; Marc, R.E. Retinal remodeling in human retinitis pigmentosa. Exp. Eye Res. 2016, 150, 149–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cehajic-Kapetanovic, J.; Eleftheriou, C.; Allen, A.E.; Milosavljevic, N.; Pienaar, A.; Bedford, R.; Davis, K.E.; Bishop, P.N.; Lucas, R.J. Restoration of vision with ectopic expression of human rod opsin. Curr. Biol. 2015, 25, 2111–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilhooley, M.J.; Lindner, M.; Palumaa, T.; Hughes, S.; Peirson, S.N.; Hankins, M.W. A systematic comparison of optogenetic approaches to visual restoration. Mol. Ther. Methods Clin. Dev. 2022, 25, 111–123. [Google Scholar] [CrossRef]
- Lu, Q.; Ganjawala, T.H.; Krstevski, A.; Abrams, G.W.; Pan, Z.H. Comparison of AAV-mediated optogenetic vision restoration between retinal ganglion cell expression and ON bipolar cell targeting. Mol. Ther. Methods Clin. Dev. 2020, 18, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.S.; Lee, V.; Wei, Z.; Song, J.Y.; Casal, G.; Cronin, T.; Willett, K.; Huckfeldt, R.; Morgan, J.I.; Aleman, T.S.; et al. Evaluation of dose and safety of AAV7m8 and AAV8BP2 in the non-human primate retina. Hum. Gene Ther. 2017, 28, 154–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahel, J.A.; Boulanger-Scemama, E.; Pagot, C.; Arleo, A.; Galluppi, F.; Martel, J.N.; Esposti, S.D.; Delaux, A.; de Saint Aubert, J.B.; de Montleau, C.; et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat. Med. 2021, 27, 1223–1229. [Google Scholar] [CrossRef]
- Yamada, E.S.; Bordt, A.S.; Marshak, D.W. Wide-field ganglion cells in macaque retinas. Vis. Neurosci. 2005, 22, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Masri, R.A.; Percival, K.A.; Koizumi, A.; Martin, P.R.; Grünert, U. Survey of retinal ganglion cell morphology in marmoset. J. Comp. Neurol. 2019, 527, 236–258. [Google Scholar] [CrossRef] [Green Version]
- Garita-Hernandez, M.; Lampič, M.; Chaffiol, A.; Guibbal, L.; Routet, F.; Santos-Ferreira, T.; Gasparini, S.; Borsch, O.; Gagliardi, G.; Reichman, S.; et al. Restoration of visual function by transplantation of optogenetically engineered photoreceptors. Nat. Commun. 2019, 10, 4524. [Google Scholar] [CrossRef] [Green Version]
- Garita-Hernandez, M.; Chaffiol, A.; Guibbal, L.; Routet, F.; Khabou, H.; Riancho, L.; Toualbi, L.; Picaud, S.; Sahel, J.A.; Goureau, O.; et al. Control of Microbial Opsin Expression in Stem Cell Derived Cones for Improved Outcomes in Cell Therapy. Front. Cell. Neurosci. 2021, 15, 648219. [Google Scholar] [CrossRef]
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, H.; Sugano, E.; Yawo, H.; Ishizuka, T.; Isago, H.; Narikawa, S.; Kügler, S.; Tamai, M. Restoration of visual response in aged dystrophic RCS rats using AAV-mediated Channelopsin-2 gene transfer. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3821–3826. [Google Scholar] [CrossRef] [PubMed]
- Farah, N.; Reutsky, I.; Shoham, S. Patterned optical activation of retinal ganglion cells. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; Volume 2007, pp. 6368–6370. [Google Scholar] [CrossRef]
- Tomita, H.; Sugano, E.; Fukazawa, Y.; Isago, H.; Sugiyama, Y.; Hiroi, T.; Ishizuka, T.; Mushiake, H.; Kato, M.; Hirabayashi, M.; et al. Visual properties of transgenic rats harboring the channelrhodopsin-2 gene regulated by the thy-1.2 promoter. PLoS ONE 2009, 4, e7679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, E.; Pan, Z.H. Evaluation of the adeno-associated virus mediated long-term expression of channelrhodopsin-2 in the mouse retina. Mol. Vis. 2009, 15, 1680–1689. [Google Scholar]
- Zhang, Y.; Ivanova, E.; Bi, A.; Pan, Z.H. Ectopic expression of multiple microbial rhodopsins restores ON and OFF light responses in retinas with photoreceptor degeneration. J. Neurosci. 2009, 29, 9186–9196. [Google Scholar] [CrossRef] [Green Version]
- Tomita, H.; Sugano, E.; Isago, H.; Hiroi, T.; Wang, Z.; Ohta, E.; Tamai, M. Channelrhodopsin-2 gene transduced into retinal ganglion cells restores functional vision in genetically blind rats. Exp. Eye Res. 2010, 90, 429–436. [Google Scholar] [CrossRef]
- Thyagarajan, S.; van Wyk, M.; Lehmann, K.; Löwel, S.; Feng, G.; Wässle, H. Visual function in mice with photoreceptor degeneration and transgenic expression of channelrhodopsin 2 in ganglion cells. J. Neurosci. 2010, 30, 8745–8758. [Google Scholar] [CrossRef]
- Ivanova, E.; Roberts, R.; Bissig, D.; Pan, Z.H.; Berkowitz, B.A. Retinal channelrhodopsin-2-mediated activity in vivo evaluated with manganese-enhanced magnetic resonance imaging. Mol. Vis. 2010, 16, 1059–1067. [Google Scholar]
- Sugano, E.; Isago, H.; Wang, Z.; Murayama, N.; Tamai, M.; Tomita, H. Immune responses to adeno-associated virus type 2 encoding channelrhodopsin-2 in a genetically blind rat model for gene therapy. Gene Ther. 2011, 18, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Isago, H.; Sugano, E.; Wang, Z.; Murayama, N.; Koyanagi, E.; Tamai, M.; Tomita, H. Age-dependent differences in recovered visual responses in Royal College of Surgeons rats transduced with the Channelrhodopsin-2 gene. J. Mol. Neurosci. 2012, 46, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ivanova, E.; Zhang, Y.; Pan, Z.H. rAAV-mediated subcellular targeting of optogenetic tools in retinal ganglion cells in vivo. PLoS ONE 2013, 8, e66332. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.; Hwang, G.S.; Pan, Z.H.; Troilo, D. Evaluation of AAV-mediated expression of Chop2-GFP in the marmoset retina. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5288–5296. [Google Scholar] [CrossRef] [Green Version]
- Chaffiol, A.; Caplette, R.; Jaillard, C.; Brazhnikova, E.; Desrosiers, M.; Dubus, E.; Duhamel, L.; Macé, E.; Marre, O.; Benoit, P.; et al. A new promoter allows optogenetic vision restoration with enhanced sensitivity in macaque retina. Mol. Ther. 2017, 25, 2546–2560. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.H.; Ganjawala, T.H.; Lu, Q.; Ivanova, E.; Zhang, Z. ChR2 mutants at L132 and T159 with improved operational light sensitivity for vision restoration. PLoS ONE 2014, 9, e98924. [Google Scholar] [CrossRef]
- Lu, Q.; Ganjawala, T.H.; Hattar, S.; Abrams, G.W.; Pan, Z.H. A robust optomotor assay for assessing the efficacy of optogenetic tools for vision restoration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1288–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganjawala, T.H.; Lu, Q.; Fenner, M.D.; Abrams, G.W.; Pan, Z.H. Improved CoChR variants restore visual acuity and contrast sensitivity in a mouse model of blindness under ambient light conditions. Mol. Ther. 2019, 27, 1195–1205. [Google Scholar] [CrossRef]
- Klapoetke, N.C.; Murata, Y.; Kim, S.S.; Pulver, S.R.; Birdsey-Benson, A.; Cho, Y.K.; Morimoto, T.K.; Chuong, A.S.; Carpenter, E.J.; Tian, Z.; et al. Independent optical excitation of distinct neural populations. Nat. Methods 2014, 11, 338–346. [Google Scholar] [CrossRef] [Green Version]
- SenGupta, A.; Chaffiol, A.; Macé, E.; Caplette, R.; Desrosiers, M.; Lampič, M.; Forster, V.; Marre, O.; Lin, J.Y.; Sahel, J.A.; et al. Red-shifted channelrhodopsin stimulation restores light responses in blind mice, macaque retina, and human retina. EMBO Mol. Med. 2016, 8, 1248–1264. [Google Scholar] [CrossRef]
- Cheong, S.K.; Strazzeri, J.M.; Williams, D.R.; Merigan, W.H. All-optical recording and stimulation of retinal neurons in vivo in retinal degeneration mice. PLoS ONE 2018, 13, e0194947. [Google Scholar] [CrossRef] [Green Version]
- McGregor, J.E.; Godat, T.; Dhakal, K.R.; Parkins, K.; Strazzeri, J.M.; Bateman, B.A.; Fischer, W.S.; Williams, D.R.; Merigan, W.H. Optogenetic restoration of retinal ganglion cell activity in the living primate. Nat. Commun. 2020, 11, 1703. [Google Scholar] [CrossRef] [Green Version]
- Gauvain, G.; Akolkar, H.; Chaffiol, A.; Arcizet, F.; Khoei, M.A.; Desrosiers, M.; Jaillard, C.; Caplette, R.; Marre, O.; Bertin, S.; et al. Optogenetic therapy: High spatiotemporal resolution and pattern discrimination compatible with vision restoration in non-human primates. Commun. Biol. 2021, 4, 125. [Google Scholar] [CrossRef]
- McGregor, J.E.; Kunala, K.; Xu, Z.; Murphy, P.J.; Godat, T.; Strazzeri, J.M.; Bateman, B.A.; Fischer, W.S.; Parkins, K.; Chu, C.J.; et al. Optogenetic therapy restores retinal activity in primate for at least a year following photoreceptor ablation. Mol. Ther. 2022, 30, 1315–1328. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Sugano, E.; Murayama, N.; Ozaki, T.; Nishiyama, F.; Tabata, K.; Takahashi, M.; Saito, T.; Tamai, M. Restoration of the majority of the visual spectrum by using modified Volvox channelrhodopsin-1. Mol. Ther. 2014, 22, 1434–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugano, E.; Tabata, K.; Takahashi, M.; Nishiyama, F.; Shimizu, H.; Sato, M.; Tamai, M.; Tomita, H. Local and systemic responses following intravitreous injection of AAV2-encoded modified Volvox channelrhodopsin-1 in a genetically blind rat model. Gene Ther. 2016, 23, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Sugano, E.; Tabata, K.; Sannohe, K.; Watanabe, Y.; Ozaki, T.; Tamai, M.; Tomita, H. Visual responses of photoreceptor-degenerated rats expressing two different types of channelrhodopsin genes. Sci. Rep. 2017, 7, 41210. [Google Scholar] [CrossRef] [Green Version]
- Tabata, K.; Sugano, E.; Hatakeyama, A.; Watanabe, Y.; Suzuki, T.; Ozaki, T.; Fukuda, T.; Tomita, H. Phototoxicities caused by continuous light exposure were not induced in retinal ganglion cells transduced by an optogenetic gene. Int. J. Mol. Sci. 2021, 22, 6732. [Google Scholar] [CrossRef]
- Watanabe, Y.; Sugano, E.; Tabata, K.; Hatakeyama, A.; Sakajiri, T.; Fukuda, T.; Ozaki, T.; Suzuki, T.; Sayama, T.; Tomita, H. Development of an optogenetic gene sensitive to daylight and its implications in vision restoration. NPJ Regen. Med. 2021, 6, 64. [Google Scholar] [CrossRef]
- Wright, W.W.; Gajjeraman, S.; Batabyal, S.; Pradhan, S.; Bhattacharya, S.; Mahapatra, V.; Tripathy, A.; Mohanty, S.K. Restoring vision in mice with retinal degeneration using multicharacteristic opsin. Neurophotonics 2017, 4, 41505. [Google Scholar] [CrossRef]
- Tchedre, K.T.; Batabyal, S.; Galicia, M.; Narcisse, D.; Mustafi, S.M.; Ayyagari, A.; Chavala, S.; Mohanty, S.K. Biodistribution of adeno-associated virus type 2 carrying multi-characteristic opsin in dogs following intravitreal injection. J. Cell. Mol. Med. 2021, 25, 8676–8686. [Google Scholar] [CrossRef]
- Nikonov, S.; Aravand, P.; Lyubarsky, A.; Nikonov, R.; Luo, A.J.; Wei, Z.; Maguire, A.M.; Phelps, N.T.; Shpylchak, I.; Willett, K.; et al. Restoration of vision and retinal responses After adeno-associated virus-mediated optogenetic therapy in blind dogs. Transl. Vis. Sci. Technol. 2022, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Chuong, A.S.; Miri, M.L.; Busskamp, V.; Matthews, G.A.; Acker, L.C.; Sørensen, A.T.; Young, A.; Klapoetke, N.C.; Henninger, M.A.; Kodandaramaiah, S.B.; et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 2014, 17, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Khabou, H.; Garita-Hernandez, M.; Chaffiol, A.; Reichman, S.; Jaillard, C.; Brazhnikova, E.; Bertin, S.; Forster, V.; Desrosiers, M.; Winckler, C.; et al. Noninvasive gene delivery to foveal cones for vision restoration. JCI Insight 2018, 3, 96029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, B.; Koizumi, A.; Tanaka, N.; Panda, S.; Masland, R.H. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc. Natl. Acad. Sci. USA 2008, 105, 16009–16014. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.M.; Dai, J.M.; Liu, W.Y.; Zhao, C.J.; Lin, B.; Yin, Z.Q. Human melanopsin-AAV2/8 transfection to retina transiently restores visual function in rd1 mice. Int. J. Ophthalmol. 2016, 9, 655–661. [Google Scholar] [CrossRef]
- Ameline, B.; Tshilenge, K.T.; Weber, M.; Biget, M.; Libeau, L.; Caplette, R.; Mendes-Madeira, A.; Provost, N.; Guihal, C.; Picaud, S.; et al. Long-term expression of melanopsin and channelrhodopsin causes no gross alterations in the dystrophic dog retina. Gene Ther. 2017, 24, 735–741. [Google Scholar] [CrossRef]
- De Silva, S.R.; Barnard, A.R.; Hughes, S.; Tam, S.K.E.; Martin, C.; Singh, M.S.; Barnea-Cramer, A.O.; McClements, M.E.; During, M.J.; Peirson, S.N.; et al. Long-term restoration of visual function in end-stage retinal degeneration using subretinal human melanopsin gene therapy. Proc. Natl. Acad. Sci. USA 2017, 114, 11211–11216. [Google Scholar] [CrossRef] [Green Version]
- Gaub, B.M.; Berry, M.H.; Holt, A.E.; Isacoff, E.Y.; Flannery, J.G. Optogenetic vision restoration using rhodopsin for enhanced sensitivity. Mol. Ther. 2015, 23, 1562–1571. [Google Scholar] [CrossRef] [Green Version]
- Berry, M.H.; Holt, A.; Salari, A.; Veit, J.; Visel, M.; Levitz, J.; Aghi, K.; Gaub, B.M.; Sivyer, B.; Flannery, J.G.; et al. Restoration of high-sensitivity and adapting vision with a cone opsin. Nat. Commun. 2019, 10, 1221. [Google Scholar] [CrossRef] [Green Version]
- Schilardi, G.; Kleinlogel, S. Two functional classes of rod bipolar cells in the healthy and degenerated optogenetically treated murine retina. Front. Cell. Neurosci. 2021, 15, 809531. [Google Scholar] [CrossRef]
- Katada, Y.; Yoshida, K.; Kobayashi, K.; Neghisi, K.; Okano, H.; Kandori, H.; Tsubota, K.; Kurihara, T. High-Sensitivity Vision Restoration via Ectopic Expression of Chimeric Rhodopsin in Mice. biorXiv Prepr. 2020. [Google Scholar] [CrossRef]
- Kleinlogel, S.; Feldbauer, K.; Dempski, R.E.; Fotis, H.; Wood, P.G.; Bamann, C.; Bamberg, E. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat. Neurosci. 2011, 14, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Mattis, J.; Tye, K.M.; Ferenczi, E.A.; Ramakrishnan, C.; O’Shea, D.J.; Prakash, R.; Gunaydin, L.A.; Hyun, M.; Fenno, L.E.; Gradinaru, V.; et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 2011, 9, 159–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). ICNIRP Guidelines on Limits of Exposure to Laser Radiation of Wavelengths between 180 nm and 1,000 μm. Health Phys. 2013, 105, 271–295. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Prigge, M.; Beyrière, F.; Tsunoda, S.P.; Mattis, J.; Yizhar, O.; Hegemann, P.; Deisseroth, K. Red-shifted optogenetic excitation: A tool for fast neural control derived from Volvox carteri. Nat. Neurosci. 2008, 11, 631–633. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.Y.; Knutsen, P.M.; Muller, A.; Kleinfeld, D.; Tsien, R.Y. ReaChR: A red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 2013, 16, 1499–1508. [Google Scholar] [CrossRef] [Green Version]
- Posch, C.; Matolin, D.; Wohlgenannt, R. A QVGA 143 dB dynamic range Frame-Free PWM image sensor with lossless pixel-level video compression and time-domain CDS. IEEE J. Solid-State Circuits 2011, 46, 259–275. [Google Scholar] [CrossRef]
- Batabyal, S.; Gajjeraman, S.; Pradhan, S.; Bhattacharya, S.; Wright, W.; Mohanty, S. Sensitization of ON-bipolar cells with ambient light activatable multi-characteristic opsin rescues vision in mice. Gene Ther. 2021, 28, 162–176. [Google Scholar] [CrossRef]
- Gradinaru, V.; Thompson, K.R.; Deisseroth, K. eNpHR: A Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 2008, 36, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Hattar, S.; Lucas, R.J.; Mrosovsky, N.; Thompson, S.; Douglas, R.H.; Hankins, M.W.; Lem, J.; Biel, M.; Hofmann, F.; Foster, R.G.; et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 2003, 424, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Provencio, I.; Tu, D.C.; Pires, S.S.; Rollag, M.D.; Castrucci, A.M.; Pletcher, M.T.; Sato, T.K.; Wiltshire, T.; Andahazy, M.; et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science 2003, 301, 525–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melyan, Z.; Tarttelin, E.E.; Bellingham, J.; Lucas, R.J.; Hankins, M.W. Addition of human melanopsin renders mammalian cells photoresponsive. Nature 2005, 433, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yau, K.W. Phototransduction in mouse rods and cones. Pflug. Arch. 2007, 454, 805–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiser, P.D. Retinal pigment epithelium 65 kDa protein (RPE65): An update. Prog. Retin. Eye Res. 2022, 88, 101013. [Google Scholar] [CrossRef]
- Sasaki, K.; Yamashita, T.; Yoshida, K.; Inoue, K.; Shichida, Y.; Kandori, H. Chimeric proton-pumping rhodopsins containing the cytoplasmic loop of bovine rhodopsin. PLoS ONE 2014, 9, e91323. [Google Scholar] [CrossRef] [Green Version]
- Hickey, D.G.; Davies, W.I.L.; Hughes, S.; Rodgers, J.; Thavanesan, N.; MacLaren, R.E.; Hankins, M.W. Chimeric human opsins as optogenetic light sensitisers. J. Exp. Biol. 2021, 224, jeb240580. [Google Scholar] [CrossRef]
- Berns, K.I.; Giraud, C. Biology of adeno-associated virus. Curr. Top. Microbiol. Immunol. 1996, 218, 1–23. [Google Scholar] [CrossRef]
- Bainbridge, J.W.B.; Smith, A.J.; Barker, S.S.; Robbie, S.; Henderson, R.; Balaggan, K.; Viswanathan, A.; Holder, G.E.; Stockman, A.; Tyler, N.; et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008, 358, 2231–2239. [Google Scholar] [CrossRef]
- Cideciyan, A.V.; Aleman, T.S.; Boye, S.L.; Schwartz, S.B.; Kaushal, S.; Roman, A.J.; Pang, J.J.; Sumaroka, A.; Windsor, E.A.; Wilson, J.M.; et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. USA 2008, 105, 15112–15117. [Google Scholar] [CrossRef] [Green Version]
- Maguire, A.M.; Simonelli, F.; Pierce, E.A.; Pugh, E.N.; Mingozzi, F.; Bennicelli, J.; Banfi, S.; Marshall, K.A.; Testa, F.; Surace, E.M.; et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008, 358, 2240–2248. [Google Scholar] [CrossRef] [Green Version]
- MacLaren, R.E.; Groppe, M.; Barnard, A.R.; Cottriall, C.L.; Tolmachova, T.; Seymour, L.; Clark, K.R.; During, M.J.; Cremers, F.P.; Black, G.C.; et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet 2014, 383, 1129–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalkara, D.; Byrne, L.C.; Klimczak, R.R.; Visel, M.; Yin, L.; Merigan, W.H.; Flannery, J.G.; Schaffer, D.V. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 2013, 5, 189ra76. [Google Scholar] [CrossRef]
- Reid, C.A.; Ertel, K.J.; Lipinski, D.M. Improvement of photoreceptor targeting via intravitreal delivery in mouse and human retina using combinatory rAAV2 capsid mutant vectors. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6429–6439. [Google Scholar] [CrossRef]
- Kotterman, M.; Beliakoff, G.; Croze, R.; Vazin, T.; Schmitt, C.; Szymanski, P.; Leong, M.; Quezada, M.; Holt, J.; Barglow, K.; et al. Directed Evolution of AAV Targeting Primate Retina by Intravitreal Injection Identifies R100, a Variant Demonstrating Robust Gene Delivery and Therapeutic Efficacy in Non-human Primates. biorXiv Prepr. 2021. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Cideciyan, A.V.; Ratnakaram, R.; Heon, E.; Schwartz, S.B.; Roman, A.J.; Peden, M.C.; Aleman, T.S.; Boye, S.L.; Sumaroka, A.; et al. Gene therapy for Leber congenital amaurosis caused by RPE65 mutations: Safety and efficacy in 15 children and adults followed up to 3 years. Arch. Ophthalmol. 2012, 130, 9–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalkara, D.; Kolstad, K.D.; Caporale, N.; Visel, M.; Klimczak, R.R.; Schaffer, D.V.; Flannery, J.G. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol. Ther. 2009, 17, 2096–2102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cehajic-Kapetanovic, J.; Le Goff, M.M.; Allen, A.; Lucas, R.J.; Bishop, P.N. Glycosidic enzymes enhance retinal transduction following intravitreal delivery of AAV2. Mol. Vis. 2011, 17, 1771–1783. [Google Scholar]
- Cehajic-Kapetanovic, J.; Milosavljevic, N.; Bedford, R.A.; Lucas, R.J.; Bishop, P.N. Efficacy and Safety of Glycosidic Enzymes for Improved Gene Delivery to the retina following intravitreal Injection in Mice. Mol. Ther. Methods Clin. Dev. 2018, 9, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Igarashi, T.; Miyake, K.; Kobayashi, M.; Yaguchi, C.; Iijima, O.; Yamazaki, Y.; Katakai, Y.; Miyake, N.; Kameya, S.; et al. Improved intravitreal AAV-mediated inner retinal gene transduction after surgical internal limiting membrane peeling in cynomolgus monkeys. Mol. Ther. 2017, 25, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.K.; Wang, S.K.; Chu, C.J.; Copland, D.A.; Letizia, A.J.; Costa Verdera, H.; Chiang, J.J.; Sethi, M.; Wang, M.K.; Neidermyer, W.J.; et al. Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci. Transl. Med. 2021, 13, eabd3438. [Google Scholar] [CrossRef]
- Yin, L.; Greenberg, K.; Hunter, J.J.; Dalkara, D.; Kolstad, K.D.; Masella, B.D.; Wolfe, R.; Visel, M.; Stone, D.; Libby, R.T.; et al. Intravitreal injection of AAV2 transduces macaque inner retina. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2775–2783. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.J.; Cideciyan, A.V.; Wu, V.; Garafalo, A.V.; Jacobson, S.G. Full-field stimulus testing: Role in the clinic and as an outcome measure in clinical trials of severe childhood retinal disease. Prog. Retin. Eye Res. 2022, 87, 101000. [Google Scholar] [CrossRef] [PubMed]
- Gekeler, F.; Messias, A.; Ottinger, M.; Bartz-Schmidt, K.U.; Zrenner, E. Phosphenes electrically evoked with DTL electrodes: A study in patients with retinitis pigmentosa, glaucoma, and homonymous visual field loss and normal subjects. Invest. Ophthalmol. Vis. Sci. 2006, 47, 4966–4974. [Google Scholar] [CrossRef] [PubMed]
- Kelbsch, C.; Maeda, F.; Lisowska, J.; Lisowski, L.; Strasser, T.; Stingl, K.; Wilhelm, B.; Wilhelm, H.; Peters, T. Analysis of retinal function using chromatic pupillography in retinitis pigmentosa and the relationship to electrically evoked phosphene thresholds. Acta Ophthalmol. 2017, 95, e261–e269. [Google Scholar] [CrossRef] [PubMed]

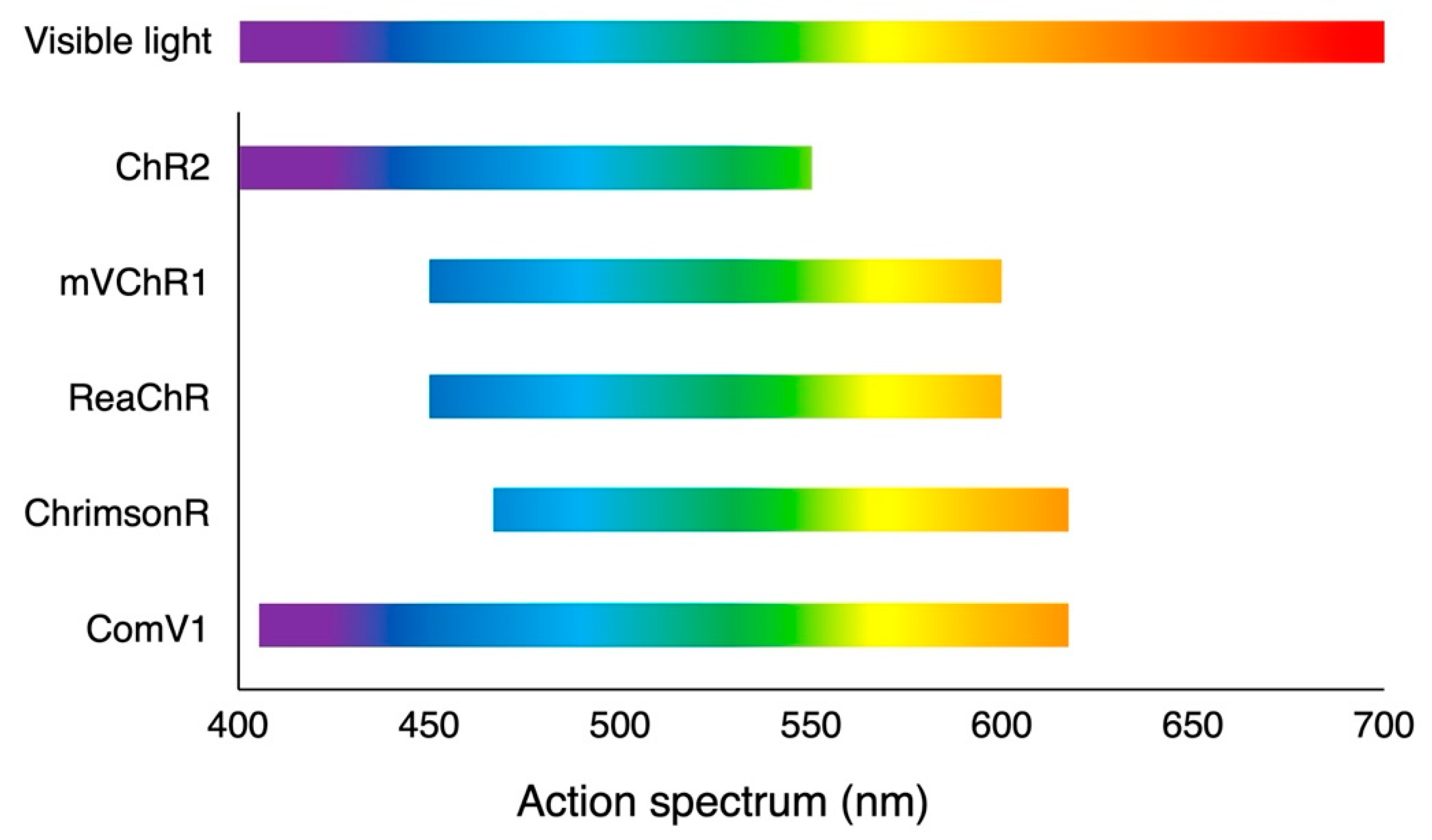
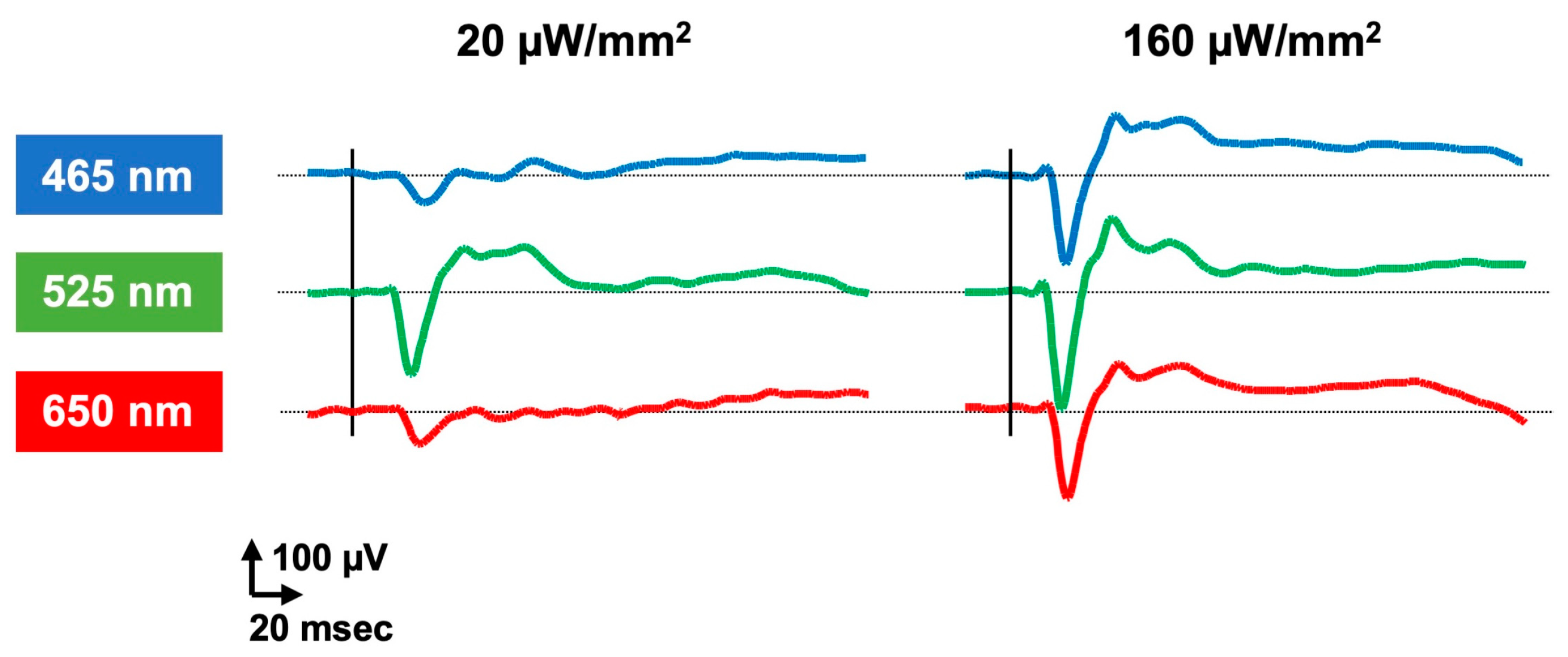
| Rodents | Dogs | Primates | Clinical Trials | |
|---|---|---|---|---|
| Microbial Opsins | ||||
| Depolarizing opsins | ||||
| ChR2 | Bi et al. [8]; Tomita et al. [33]; Farah et al. [34]; Lagali et al. [14]; Tomita et al. [35]; Ivanova and Pan [36]; Zhang et al. [37]; Tomita et al. [38]; Thyagarajan et al. [39]; Ivanova et al. [40]; Doroudchi et al. [15]; Sugano et al. [41]; Isago et al. [42]; Wu et al. [43] | - | Ivanova et al. [44] | NCT02556736 |
| Enhanced light sensitivity variants | ||||
| CatCh | Chaffiol et al. [45] | - | Chaffiol et al. [45] | - |
| ChR2(L132C/T159S) | Pan et al. [46]; Lu et al. [47] | - | - | - |
| CoChR-3M | Ganjawala et al. [48]; Wright et al. [21] | - | - | - |
| ChronosFP | Klapoetke et al. [49] | - | - | NCT04278131 |
| Red-shifted variants | ||||
| ReaChR | Sengupta et al. [50]; Wright et al. [21]; Gilhooley et al. [24] | - | Sengupta et al. [50] (ex vivo) | - |
| ChrimsonR | Cheong et al. [51] | - | McGregor et al. [52]; Gauvain et al. [53]; McGregor et al. [54] | NCT03326336 (PIONEER) |
| mVChR1 | Tomita et al. [55]; Sugano et al. [56]; Sato et al. [57]; Tabata et al. [58] | - | - | - |
| ComV1 | Watanabe et al. [59] | - | - | - |
| MCO1 | Wright et al. [60] | Tchedre et al. [61] | - | NCT04919473, NCT04945772 (RESTORE), NCT05417126 (STARLIGHT) |
| Hyperpolarizing opsins | ||||
| eNpHR | Busskamp et al. [13] | Nikonov et al. [62] | - | - |
| Jaws | Chuong et al. [63]; Khabou et al. [64] | - | Khabou et al. [64] | - |
| Animal opsins | ||||
| Melanopsin | Lin et al. [65]; Liu et al. [66]; Ameline et al. [67]; De Silva et al. [68]; Gilhooley et al. [24] | - | - | - |
| Rhodopsin | Cehajic-Kapetanovic et al. [23]; Gaub et al. [69]; Eleftheriou et al. [19]; Berry et al. [70]; McClements et al. [20]; Wright et al. [21] | - | - | - |
| Cone opsin | Berry et al. [70]; McClements et al. [20] | - | - | - |
| Chimeras | ||||
| Opto-mGluR | van Wyk et al. [18]; Schilardi et al. [71] | - | - | - |
| GHCR | Katada et al. [72] | - | - | - |
| Phase | Patients | Actual Study Start Date | Drug | Tool | Vector | Intervention | Sponsor | Reported results | |
|---|---|---|---|---|---|---|---|---|---|
| NCT02556736 | I/IIa | retinitis pigmentosa | 14 December 2015 | RST-001 | ChR2 | AAV2 | Intravitreal injection | AbbVie, Chicago, IL, USA (Allergan Inc., Irvine, CA, USA) | Clinical trials.gov. (https://clinicaltrials.gov/ct2/show/results/NCT02556736 accessed on 29 September 2022.) |
| NCT03326336 (PIONEER) | I/IIa | retinitis pigmentosa | 26 September 2018 | GS030-DP/ GS030-MD | ChrimsonR | AAV2(7m8) | Intravitreal injection | GenSight Biologics, Paris, France | Sahel et al. [27] |
| NCT04919473, NCT04945772 (RESTORE) | I/IIa, b | retinitis pigmentosa | 23 October 2019, 13 July 2021 | vMCO-010 | MCO | AAV2 | Intravitreal injection | Nanoscope Therapeutics Inc., Dallas, TX, USA | Press release (https://www.ophthalmologytimes.com/view/optogenetic-gene-therapy-restores-vision-in-11-rp-patients accessed on 29 September 2022.) |
| NCT04278131 | I/II | retinitis pigmentosa | 6 February 2020 | BS01 | ChronosFP | AAV (serotype is undisclosed) | Intravitreal injection | BionicSight LLC, New York, NY, USA | Press release (https://www.globenewswire.com/news-release/2021/03/30/2201412/0/en/First-Four-Patients-In-Bionic-Sight-s-Optogenetic-Gene-Therapy-Trial-Are-Able-To-Detect-Light-And-Motion.html accessed on 29 September 2022.) |
| NCT05417126 (STARLIGHT) | IIa | Stargardt disease | 5 July 2022 | vMCO-010 | MCO | AAV2 | Intravitreal injection | Nanoscope Therapeutics Inc., Dallas, TX, USA | Not reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakai, D.; Tomita, H.; Maeda, A. Optogenetic Therapy for Visual Restoration. Int. J. Mol. Sci. 2022, 23, 15041. https://doi.org/10.3390/ijms232315041
Sakai D, Tomita H, Maeda A. Optogenetic Therapy for Visual Restoration. International Journal of Molecular Sciences. 2022; 23(23):15041. https://doi.org/10.3390/ijms232315041
Chicago/Turabian StyleSakai, Daiki, Hiroshi Tomita, and Akiko Maeda. 2022. "Optogenetic Therapy for Visual Restoration" International Journal of Molecular Sciences 23, no. 23: 15041. https://doi.org/10.3390/ijms232315041
APA StyleSakai, D., Tomita, H., & Maeda, A. (2022). Optogenetic Therapy for Visual Restoration. International Journal of Molecular Sciences, 23(23), 15041. https://doi.org/10.3390/ijms232315041









