Cyclic Adenosine Monophosphate: A Central Player in Gamete Development and Fertilization, and Possible Target for Infertility Therapies
Abstract
:1. Introduction
2. cAMP Function
3. cAMP Role in Spermatogenesis
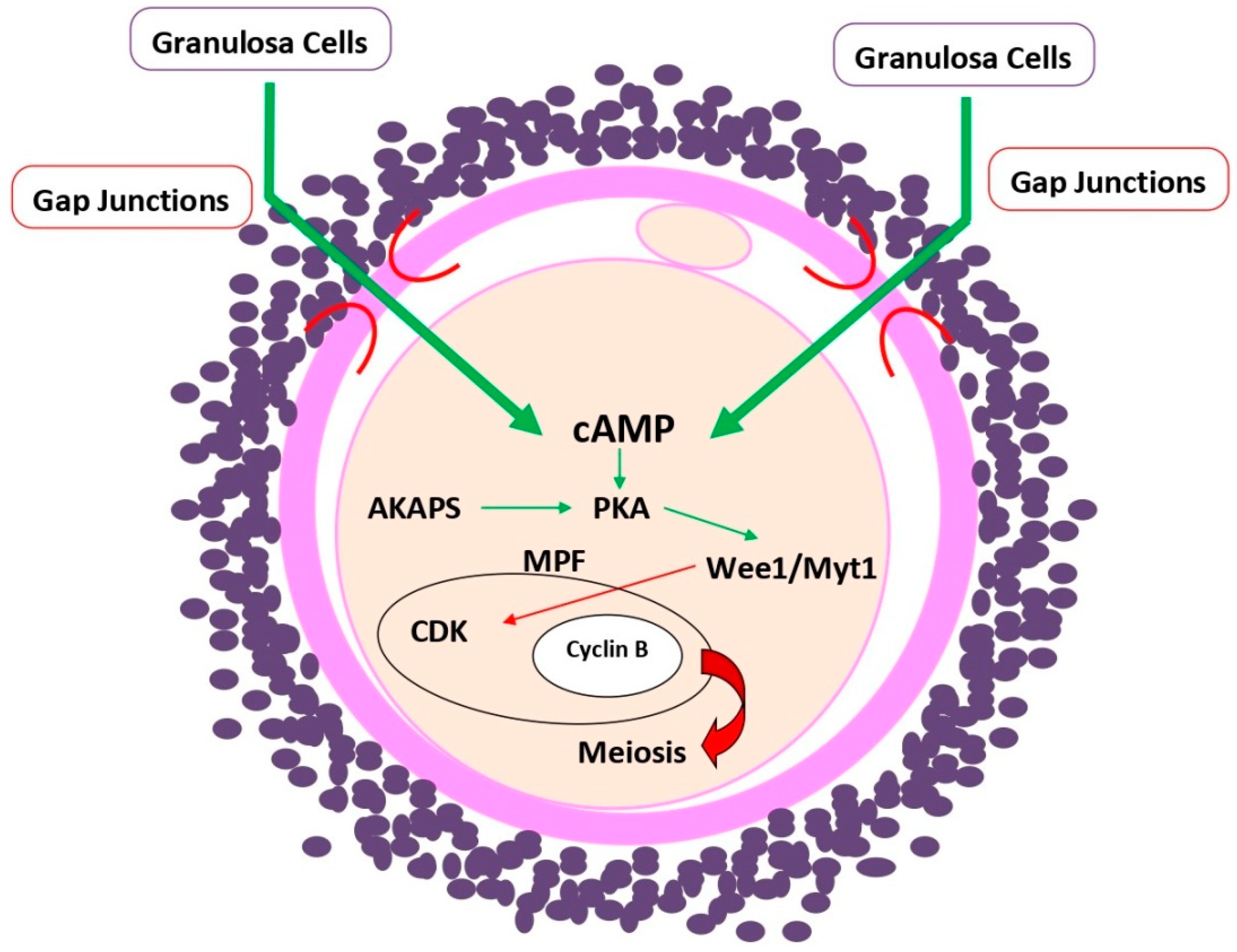
4. cAMP Role in Oogenesis
5. cAMP Role in Fertilization
6. cAMP as Target of Therapeutic Action in Human Infertility
6.1. Male Infertility
6.2. Female Infertility
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gleicher, N.; Kushnir, V.A.; Barad, D.H. Worldwide decline of IVF birth rates and its probable causes. Hum. Reprod. Open 2019, 2019, hoz017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, K.; Gao, L.N.; Cui, Y.L.; Zhang, Y.; Zhou, X. The cyclic AMP signaling pathway: Exploring targets for successful drug discovery (Review). Mol. Med. Rep. 2016, 13, 3715–3723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serezani, C.H.; Ballinger, M.N.; Aronoff, D.M.; Peters-Golden, M. Cyclic AMP: Master regulator of innate immune cell function. Am. J. Respir. Cell Mol. Biol. 2008, 39, 127–132. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Rasmussen, S.G.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamato, D.; Thach, L.; Bernard, R.; Chan, V.; Zheng, W.; Kaur, H.; Brimble, M.; Osman, N.; Little, P.J. Structure, Function, Pharmacology, and Therapeutic Potential of the G Protein, Gα/q,11. Front. Cardiovasc. Med. 2015, 24, 14. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.V.; Yang, W.L.; Ravatn, R.; Kita, T.; Reitman, E.; Vettori, D.; Cvijic, M.E.; Shin, M.; Iacono, L. Reinventing the wheel of cyclic AMP: Novel mechanisms of cAMP signaling. Ann. N. Y. Acad. Sci. 2002, 968, 49–64. [Google Scholar] [CrossRef]
- Dyson, H.J.; Wright, P.E. Role of intrinsic protein disorder in the function and interactions of the transcriptional coactivators CREB-binding protein (CBP) and p300. J. Biol. Chem. 2016, 291, 6714–6722. [Google Scholar] [CrossRef] [Green Version]
- Zaccolo, M.; Zerio, A.; Lobo, M.J. Subcellular organization of the cAMP signaling pathway. Pharmacol. Rev. 2021, 73, 278–309. [Google Scholar] [CrossRef]
- Oduwole, O.O.; Peltoketo, H.; Huhtaniemi, I.T. Role of follicle-stimulating hormone in spermatogenesis. Front. Endocrinol. 2018, 9, 763. [Google Scholar] [CrossRef] [Green Version]
- Welsh, M.; Saunders, P.T.; Atanassova, N.; Sharpe, R.M.; Smith, L.B. Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J. 2009, 23, 4218–4230. [Google Scholar] [CrossRef]
- Behr, R.; Weinbauer, G.F. cAMP response element modulator (CREM): An essential factor for spermatogenesis in primates? Int. J. Androl. 2001, 24, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Mendoza, C.; Greco, E. The effect of FSH on male germ cell survival and differentiation in vitro is mimicked by pentoxifylline but not insulin. Mol. Hum. Reprod. 2000, 6, 877–881. [Google Scholar] [CrossRef] [Green Version]
- Brindle, P.; Linke, S.; Montminy, M. Protein kinase-A-dependent activator in transcription factor CREB reveals new role for CREM repressors. Nature 1993, 364, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, N.S.; Mellstrom, B.; Benusiglio, E.; Sassone-Corsi, P. Developmental switch of CREM function during spermatogenesis: From antagonist to activator. Nature 1992, 355, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, N.S.; Schlotter, F.; Pévet, P.; Sassone-Corsi, P. Pituitary hormone FSH directs the CREM functional switch during spermatogenesis. Nature 1993, 362, 264–267. [Google Scholar] [CrossRef]
- Strączyńska, P.; Papis, K.; Morawiec, E.; Czerwiński, M.; Gajewski, Z.; Olejek, A.; Bednarska-Czerwińska, A. Signaling mechanisms and their regulation during in vivo or in vitro maturation of mammalian oocytes. Reprod. Biol. Endocrinol. 2022, 20, 37. [Google Scholar] [CrossRef]
- Tesarik, J.; Kopecny, V.; Plachot, M.; Mandelbaum, J. Activation of nucleolar and extranucleolar RNA synthesis and changes in the ribosomal content of human embryos developing in vitro. J. Reprod. Fertil. 1986, 78, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Braude, P.; Bolton, V.; Moore, S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature 1988, 332, 459–461. [Google Scholar] [CrossRef]
- Tesarik, J.; Kopecny, V.; Plachot, M.; Mandelbaum, J. Early morphological signs of embryonic genome expression in human preimplantation development as revealed by quantitative electron microscopy. Dev. Biol. 1988, 128, 15–20. [Google Scholar] [CrossRef]
- Jukam, D.; Shariati, S.A.M.; Skotheim, J.M. Zygotic Genome Activation in Vertebrates. Dev. Cell 2017, 42, 316–332. [Google Scholar] [CrossRef]
- Tesarik, J. Control of maternal-to-zygotic transition in human embryos and other animal species (especially mouse): Similarities and differences. Int. J. Mol. Sci. 2022, 23, 8562. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.; Agrawal, H.; Saha, S.; Straughn, A.R.; Roy, P.; Kakar, S.S. Overview of follicle stimulating hormone and its receptors in reproduction and in stem cells and cancer stem cells. Int. J. Biol. Sci. 2022, 18, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Timossi, C.M.; Barrios de Tomasi, J.; Zambrano, E.; González, R.; Ulloa-Aguirre, A. A naturally occurring basically charged human follicle-stimulating hormone (FSH) variant inhibits FSH-induced androgen aromatization and tissue-type plasminogen activator enzyme activity in vitro. Neuroendocrinology 1998, 67, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, D.A.; Paul, D.L. Gap junctions. Cold Spring Harb. Perspect. Biol. 2009, 1, a002576. [Google Scholar] [CrossRef] [Green Version]
- Méduri, G.; Charnaux, N.; Driancourt, M.A.; Combettes, L.; Granet, P.; Vannier, B.; Loosfelt, H.; Milgrom, E. Follicle-stimulating hormone receptors in oocytes? J. Clin. Endocrinol. Metab. 2002, 87, 2266–2276. [Google Scholar] [CrossRef] [Green Version]
- Yung, Y.; Aviel-Ronen, S.; Maman, E.; Rubinstein, N.; Avivi, C.; Orvieto, R.; Hourvitz, A. Localization of luteinizing hormone receptor protein in the human ovary. Mol. Hum. Reprod. 2014, 20, 844–849. [Google Scholar] [CrossRef] [Green Version]
- Tesarik, J.; Dvorak, M. Human cumulus oophorus preovulatory development. J. Ultrastruct. Res. 1982, 78, 60–72. [Google Scholar] [CrossRef]
- Oh, J.S.; Han, S.J.; Conti, M. Wee1B, Myt1, and Cdc25 function in distinct compartments of the mouse oocyte to control meiotic resumption. J. Cell Biol. 2010, 188, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Abe, S.; Nagasaka, K.; Hirayama, Y.; Kozuka-Hata, H.; Oyama, M.; Aoyagi, Y.; Obuse, C.; Hirota, T. The initial phase of chromosome condensation requires Cdk1-mediated phosphorylation of the CAP-D3 subunit of condensin II. Genes Dev. 2011, 25, 863–874. [Google Scholar] [CrossRef] [Green Version]
- Sela-Abramovich, S.; Edry, I.; Galiani, D.; Nevo, N.; Dekel, N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology 2006, 147, 2280–2286. [Google Scholar] [CrossRef]
- Shuhaibar, L.C.; Egbert, J.R.; Norris, R.P.; Lampe, P.D.; Nikolaev, V.O.; Thunemann, M.; Wen, L.; Feil, R.; Jaffe, L.A. Intercellular signaling via cyclic GMP diffusion through gap junctions restarts meiosis in mouse ovarian follicles. Proc. Natl. Acad. Sci. USA 2015, 112, 5527–5532. [Google Scholar] [CrossRef] [Green Version]
- Tesarik, J.; Mendoza, C. Nongenomic effects of 17 beta-estradiol on maturing human oocytes: Relationship to oocyte developmental potential. J. Clin. Endocrinol. Metab. 1995, 80, 1438–1443. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.; Brothag, C.; Vijayaraghavan, S. Signaling enzymes required for sperm maturation and fertilization in mammals. Front. Cell Dev. Biol. 2019, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Garcia, M.A.; Carlson, A.E.; Schuh, S.M.; Babcock, D.F.; Jaiswal, B.S.; Gossen, J.A.; Esposito, G.; van Duin, M.; Conti, M. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev. Biol. 2006, 296, 353–362. [Google Scholar] [CrossRef]
- Lin, R.Y.; Moss, S.B.; Rubin, C.S. Characterization of S-AKAP84, a novel developmentally regulated A kinase anchor protein of male germ cells. J. Biol. Chem. 1995, 270, 27804–27811. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.M.O.; Eriksson, R.L.M.; Gerton, G.L.; Moss, S.B. Relationship between sperm motility and the processing and tyrosine phosphorylation of two human sperm fibrous sheath proteins, pro-hAKAP82 and hAKAP82. Mol. Hum. Reprod. 1999, 5, 816–824. [Google Scholar] [CrossRef]
- Sosa, C.M.; Zanetti, M.N.; Pocognoni, C.A.; Mayorga, L.S. Acrosomal swelling is triggered by cAMP downstream of the opening of store-operated calcium channels during acrosomal exocytosis in human sperm. Biol. Reprod. 2016, 94, 57. [Google Scholar] [CrossRef]
- Lefièvre, L.; Jha, K.N.; de Lamirande, E.; Visconti, P.E.; Gagnon, C. Activation of protein kinase A during human sperm capacitation and acrosome reaction. J. Androl. 2002, 23, 709–716. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza, C.; Carreras, A. Effects of phosphodiesterase inhibitors caffeine and pentoxifylline on spontaneous and stimulus-induced acrosome reactions in human sperm. Fertil. Steril. 1992, 58, 1185–1190. [Google Scholar] [CrossRef]
- Yovich, J.L. Pentoxifylline: Actions and applications in assisted reproduction. Hum. Reprod. 1993, 8, 1786–1791. [Google Scholar] [CrossRef]
- Shen, M.R.; Chiang, P.H.; Yang, R.C.; Hong, C.Y.; Chen, S.S. Pentoxifylline stimulates human sperm motility both in vitro and after oral therapy. Br. J. Clin. Pharmacol. 1991, 31, 711–7144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satish, M.; Kumari, S.; Deeksha, W.; Abhishek, S.; Nitin, K.; Adiga, S.K.; Hegde, P.; Dasappa, J.P.; Kalthur, G.; Rajakumara, E. Structure-based redesigning of pentoxifylline analogs against selective phosphodiesterases to modulate sperm functional competence for assisted reproductive technologies. Sci. Rep. 2021, 11, 12293. [Google Scholar] [CrossRef] [PubMed]
- Lefièvre, L.; De Lamirande, E.; Gagnon, C. The cyclic GMP-specific phosphodiesterase inhibitor, sildenafil, stimulates human sperm motility and capacitation but not acrosome reaction. J. Androl. 2000, 21, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Su, H.; Zhang, J.; Wang, Y.; Li, H. Treatment of poor sperm quality and erectile dysfunction with oral pentoxifylline: A systematic review. Front. Pharmacol. 2022, 12, 789787. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Yasmin, E.; Balen, A.H. The use of a combination of pentoxifylline and tocopherol in women with a thin endometrium undergoing assisted conception therapies—A report of 20 cases. Hum. Fertil. 2009, 12, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Lédée-Bataille, N.; Olivennes, F.; Lefaix, J.L.; Chaouat, G.; Frydman, R.; Delanian, S. Combined treatment by pentoxifylline and tocopherol for recipient women with a thin endometrium enrolled in an oocyte donation programme. Hum. Reprod. 2002, 17, 1249–1253. [Google Scholar] [CrossRef] [Green Version]
- Letur-Konirsch, H.; Delanian, S. Successful pregnancies after combined pentoxifylline-tocopherol treatment in women with premature ovarian failure who are resistant to hormone replacement therapy. Fertil. Steril. 2003, 79, 439–441. [Google Scholar] [CrossRef]
- Krief, F.; Simon, C.; Goldstein, R.; Ellenberg, L.P.; Ledee, N. Efficacy of tocopherol and pentoxifylline combined therapy for women undergoing assisted reproductive treatment with poor endometrial development: A retrospective cohort study on 143 patients. Hum. Fertil. 2021, 24, 367–375. [Google Scholar] [CrossRef]
- Vitale, S.G.; Palumbo, M.; Rapisarda, A.M.C.; Carugno, J.; Conde-López, C.; Mendoza, N.; Mendoza-Tesarik, R.; Tesarik, J. Use of pentoxifylline during ovarian stimulation to improve oocyte and embryo quality: A retrospective study. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102398. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesarik, J.; Mendoza-Tesarik, R. Cyclic Adenosine Monophosphate: A Central Player in Gamete Development and Fertilization, and Possible Target for Infertility Therapies. Int. J. Mol. Sci. 2022, 23, 15068. https://doi.org/10.3390/ijms232315068
Tesarik J, Mendoza-Tesarik R. Cyclic Adenosine Monophosphate: A Central Player in Gamete Development and Fertilization, and Possible Target for Infertility Therapies. International Journal of Molecular Sciences. 2022; 23(23):15068. https://doi.org/10.3390/ijms232315068
Chicago/Turabian StyleTesarik, Jan, and Raquel Mendoza-Tesarik. 2022. "Cyclic Adenosine Monophosphate: A Central Player in Gamete Development and Fertilization, and Possible Target for Infertility Therapies" International Journal of Molecular Sciences 23, no. 23: 15068. https://doi.org/10.3390/ijms232315068
APA StyleTesarik, J., & Mendoza-Tesarik, R. (2022). Cyclic Adenosine Monophosphate: A Central Player in Gamete Development and Fertilization, and Possible Target for Infertility Therapies. International Journal of Molecular Sciences, 23(23), 15068. https://doi.org/10.3390/ijms232315068







