Construction of scFv Antibodies against the Outer Loops of the Microsporidium Nosema bombycis ATP/ADP-Transporters and Selection of the Fragment Efficiently Inhibiting Parasite Growth
Abstract
:1. Introduction
2. Results
2.1. Constructing of scFv Immune Library, Its Panning and Selection of NbAAC1-Specific Abs
2.2. Originality of Selected scFv Sequences
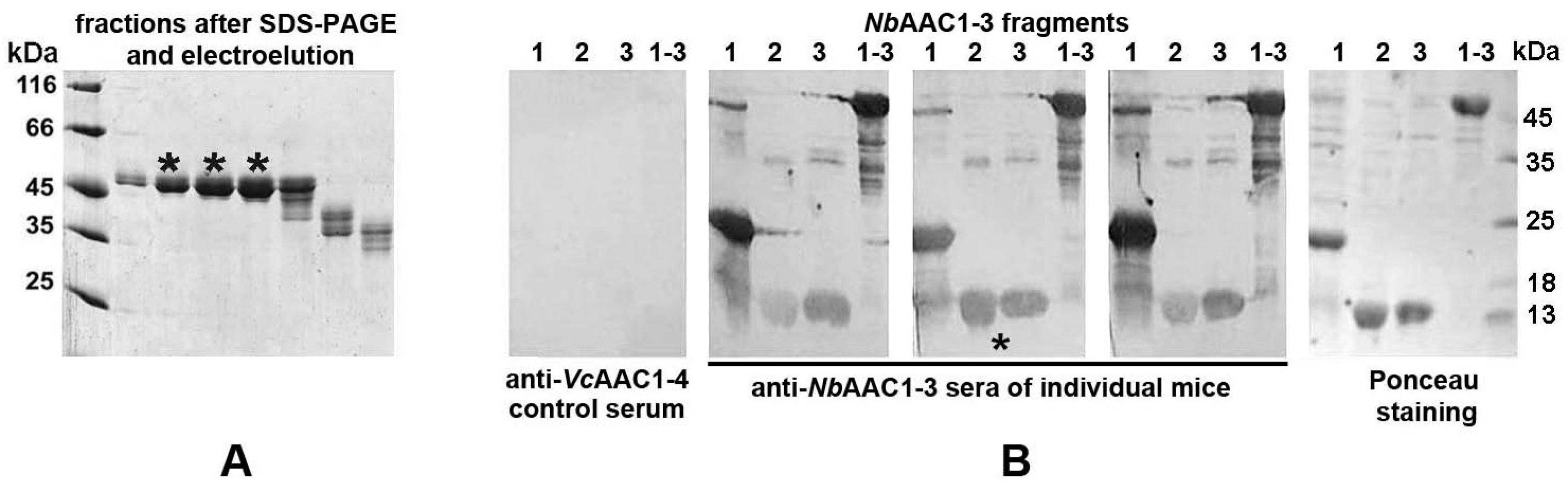
2.3. Heterologous Expression of Selected scFv Fragments in Sf9 Cells
2.4. Expression of NbAAC1-Specific scFv Abs Differently Affected the Parasite Intracellular Growth
2.5. The Effectiveness of scFv5 to Suppress Intracellular Growth of N. bombycis Was Confirmed by Additional Infections and qPCR Assays
2.6. Western Blot Analysis of Infected Transformants with Abs against N. bombycis β-Tubulin Confirmed the Inhibitory Activity of scFv5
2.7. Identification of Complementarity Determining Regions (CDRs) of the scFv5 Unique VH Domain
3. Discussion
4. Materials and Methods
4.1. Bacterial Overexpression of Chimeric Proteins and Development of Immune scFv Library
4.2. Antigen Preparation and Immunization of Mice
4.3. Construction of the scFv Immune Library
4.4. Library Panning
4.5. Bacterial Expression and Analysis of Selected scFv Fragments
4.6. Heterologous Expression of scFv Abs in Sf9 Cells and Their Infection with N. bombycis Spores
4.7. qPCR Analysis of N. bombycis Growth in Infected Cultures
4.8. Analysis of N. bombycis β-Tubulin Accumulation in Infected Transformants
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Didier, E.S.; Weiss, L.M. Overview of microsporidia and microsporidiosis. Protistology 2008, 5, 243–255. [Google Scholar]
- Solter, L.F.; Becnel, J.J.; Oi, D.H. Microsporidian entomopathogens. In Insect Pathology, 2nd ed.; Vega, F.E., Kaya, H.K., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 221–263. [Google Scholar]
- Becnel, J.J.; Andreadis, T.G. Microsporidia in insects. In Microsporidia: Pathogens of Opportunity, 1st ed.; Weiss, L.M., Becnel, J.J., Eds.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2014; pp. 521–570. [Google Scholar]
- James, R.R.; Li, Z. From silkworms to bees: Diseases of beneficial Insects. In Insect Pathology, 2nd ed.; Vega, F.E., Kaya, H.K., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 429–459. [Google Scholar]
- Bjørnson, S.; Oi, D. Microsporidia biological control agents and pathogens of beneficial insects. In Microsporidia: Pathogens of Opportunity, 1st ed.; Weiss, L.M., Becnel, J.J., Eds.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2014; pp. 635–670. [Google Scholar]
- Pan, G.; Xu, J.; Li, T.; Xia, Q.; Liu, S.L.; Zhang, G.; Li, S.; Li, C.; Liu, H.; Yang, L.; et al. Comparative genomics of parasitic silkworm microsporidia reveal an association between genome expansion and host adaptation. BMC Genom. 2013, 14, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hukuhara, T. The epizootiology of pebrine, one of the great scourges of sericulture. J. Biochem. Biotech. 2017, 1, 1–3. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, S.; Mei, X.; Yu, B.; Sun, B.; Li, B.; Wei, J.; Chen, J.; Li, T.; Pan, G.; et al. A secretory hexokinase plays an active role in the proliferation of Nosema bombycis. PeerJ 2018, 6, e5658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.; Hang, Y.; Huang, H.; Yu, B.; Zhou, N.; Wei, J.; Pan, G.; Li, C.; Zhou, Z. The role of NbTMP1, a surface protein of sporoplasm, in Nosema bombycis infection. Parasit. Vectors 2021, 14, 81. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, E.; Chen, Y.; He, P.; Wang, R.; Wang, Q.; Tang, X.; Zhang, Y.; Zhu, F.; Shen, Z. Identification and subcellular localization analysis of membrane protein Ycf 1 in the microsporidian Nosema bombycis. PeerJ 2022, 10, e13530. [Google Scholar] [CrossRef]
- Yao, M.; Wang, R.; Chen, Y.; He, P.; Wei, E.; Zhu, F.; Wang, Q.; Zhang, Y.; Tang, X.; Shen, Z. Identification and subcellular localization analysis of CCTα in microsporidian Nosema bombycis. Infect. Genet. Evol. 2022, 102, 105309. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, Q.; Long, J.; Lu, B.; Zheng, N.; Hu, C.; Chen, P.; Hu, N.; Lu, C.; Pan, M. Silver nanoparticles are effective in controlling microsporidia. Mater. Sci. Eng. C 2021, 125, 112106. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, J.; Sun, B.; Zheng, R.; Li, B.; Li, Z.; Tan, Y.; Wei, J.; Pan, G.; Li, C.; et al. Engineered resistance to Nosema bombycis by in vitro expression of a single-chain antibody in Sf9-III cells. PLoS ONE 2018, 13, e0193065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Zheng, S.; Huang, H.; Sun, X.; Huang, Y.; Wei, J.; Pan, G.; Li, C.; Zhou, Z. Expression of anti-NbHK single-chain antibody in fusion with NSlmb enhances the resistance to Nosema bombycis in Sf9-III cells. Bull. Entomol. Res. 2022, 112, 502–508. [Google Scholar] [CrossRef]
- Tsaousis, A.D.; Kunji, E.R.; Goldberg, A.V.; Lucocq, J.M.; Hirt, R.P.; Embley, T.M. A novel route for ATP acquisition by the remnant mitochondria of Encephalitozoon cuniculi. Nature 2008, 453, 553–556. [Google Scholar] [CrossRef]
- Heinz, E.; Hacker, C.; Dean, P.; Mifsud, J.; Goldberg, A.V.; Williams, T.A.; Nakjang, S.; Gregory, A.; Hirt, R.P.; Lucocq, J.M. Plasma membrane-located purine nucleotide transport proteins are key components for host exploitation by microsporidian intracellular parasites. PLoS Pathog. 2014, 10, e1004547. [Google Scholar] [CrossRef] [Green Version]
- Dean, P.; Sendra, K.M.; Williams, T.A.; Watson, A.K.; Major, P.; Nakjang, S.; Kozhevnikova, E.; Goldberg, A.V.; Kunji, E.R.S.; Hirt, R.P.; et al. Transporter gene acquisition and innovation in the evolution of microsporidia intracellular parasites. Nat. Commun. 2018, 9, 1709. [Google Scholar] [CrossRef] [Green Version]
- Plano, G.V.; Winkler, H.H. Identification and initial topological analysis of the Rickettsia prowazekii ATP/ADP translocase. J. Bacteriol. 1991, 173, 3389–3396. [Google Scholar] [CrossRef] [Green Version]
- Tjaden, J.; Winkler, H.H.; Schwöppe, C.; Van Der Laan, M.; Möhlmann, T.; Neuhaus, H.E. Two nucleotide transport proteins in Chlamydia trachomatis, one for net nucleoside triphosphate uptake and the other for transport of energy. J. Bacteriol. 1999, 181, 1196–1202. [Google Scholar] [CrossRef] [Green Version]
- Kampfenkel, K.; Mohlmann, T.; Batz, O.; Van Montagu, M.; Inze, D.; Neuhaus, H.E. Molecular characterization of an Arabidopsis thaliana cDNA encoding a novel putative adenylate translocator of higher plants. FEBS Lett. 1995, 374, 351–355. [Google Scholar] [PubMed]
- Dolgikh, V.V.; Timofeev, S.A.; Zhuravlyov, V.S.; Senderskiy, I.V. Construction and heterologous overexpression of two chimeric proteins carrying outer hydrophilic loops of Vairimorpha ceranae and Nosema bombycis ATP/ADP carriers. J. Invertebr. Pathol. 2020, 171, 107337. [Google Scholar] [CrossRef] [PubMed]
- Dolgikh, V.V.; Senderskiy, I.V.; Zhuravlyov, V.S.; Ignatieva, A.N.; Timofeev, S.A.; Ismatullaeva, D.A.; Mirzakhodjaev, B.A. Molecular analysis of the microsporidia Vairimorpha ceranae and Nosema bombycis growth in the lepidoptera Sf9 cell culture. Protistology 2022, 16, 21–29. [Google Scholar] [CrossRef]
- Dolgikh, V.V.; Zhuravlyov, V.S.; Senderskiy, I.V.; Ignatieva, A.N.; Timofeev, S.A.; Seliverstova, E.V. Heterologous expression of scFv fragment against Vairimorpha (Nosema) ceranae hexokinase in Sf9 cell culture inhibits microsporidia intracellular growth. J. Invertebr. Pathol. 2022, 191, 107755. [Google Scholar] [CrossRef]
- He, Q.; Luo, J.; Xu, J.; Wang, C.; Meng, X.; Pan, G.; Li, T.; Zhou, Z. Morphology and transcriptome analysis of Nosema bombycis sporoplasm and insights into the initial infection of microsporidia. mSphere 2020, 5, e00958-19. [Google Scholar] [CrossRef] [Green Version]
- Gisder, S.; Genersch, E. Identification of candidate agents active against N. ceranae infection in honey bees: Establishment of a medium throughput screening assay based on N. ceranae infected cultured cells. PLoS ONE 2015, 10, e0117200. [Google Scholar] [CrossRef] [PubMed]
- Bratic, A.; Clemente, P.; Calvo-Garrido, J.; Maffezzini, C.; Felser, A.; Wibom, R.; Wedell, A.; Freyer, C.; Wredenberg, A. Mitochondrial Polyadenylation Is a One-Step Process Required for mRNA Integrity and tRNA Maturation. PLoS Genet. 2016, 12, e1006028. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Ren, Y.; Sun, Y.; Wu, Z.; Ruan, J.; He, B.; Zhang, T.; Yu, X.; Tian, X.; Bu, W. PacBio full-length transcriptome profiling of insect mitochondrial gene expression. RNA Biol. 2016, 13, 820–825. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, R. The life cycle of Nosema bombycis as revealed in tissue culture cells of Bombyx mori. J. Invertebr. Pathol. 1969, 14, 316–320. [Google Scholar] [CrossRef]
- Iwano, H.; Ishihara, R. Intracellular germination of spores of a Nosema sp. immediately after their formation in cultured cell. J. Invertebr. Pathol. 1989, 54, 125–127. [Google Scholar] [CrossRef]
- Iwano, H.; Ishihara, R. Dimorphism of spores of Nosema spp. in cultured cell. J. Invertebr. Pathol. 1991, 57, 211–219. [Google Scholar] [CrossRef]
- Iwano, H.; Kurtti, T.J. Identification and isolation of dimorphic spores from Nosema furnacalis (Microspora: Nosematidae). J. Invertebr. Pathol. 1995, 65, 230–236. [Google Scholar] [CrossRef]
- Tsarev, A.A.; Senderskiy, I.V.; Timofeev, S.A.; Zhuravlyov, V.S.; Dolgikh, V.V. Recombinant single chain antibodies as an instrument to search proteins involved in interaction of microsporidia and other intracellular parasites with infected host cell. Protistology 2019, 13, 5–13. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Muyldermans, S.; Atarhouch, T.; Saldanha, J.; Barbosa, J.A.; Hamers, R. Sequence and structure of VH domain from naturally occurring camel heavy chain immunoglobulins lacking light chains. Protein Eng. 1994, 7, 1129–1135. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Avila, D.; Hughes, M.; Hughes, A.; McKinney, E.C.; Flajnik, M.F. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature 1995, 374, 168–173. [Google Scholar] [CrossRef]
- Zielonka, S.; Empting, M.; Grzeschik, J.; Könning, D.; Barelle, C.J.; Kolmar, H. Structural insights and biomedical potential of IgNAR scaffolds from sharks. MAbs 2015, 7, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Riechmann, L.; Muyldermans, S. Single domain antibodies: Comparison of camel VH and camelised human VH domains. J. Immunol. Methods 1999, 231, 25–38. [Google Scholar] [CrossRef]
- Muyldermans, S.; Cambillau, C.; Wyns, L. Recognition of antigens by single-domain antibody fragments: The superfluous luxury of paired domains. Trends. Biochem. Sci. 2001, 26, 230–235. [Google Scholar] [CrossRef]
- Xu, J.L.; Davis, M.M. Diversity in the CDR3 region of VH is sufficient for most antibody specificities. Immunity 2000, 13, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Rouet, R.; Dudgeon, K.; Christie, M.; Langley, D.; Christ, D. Fully human VH single domains that rival the stability and cleft recognition of camelid antibodies. J. Biol. Chem. 2015, 290, 11905–11917. [Google Scholar] [CrossRef] [Green Version]
- Shinozaki, N.; Hashimoto, R.; Fukui, K.; Uchiyama, S. Efficient generation of single domain antibodies with high affinities and enhanced thermal stabilities. Sci. Rep. 2017, 7, 5794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekker, G.J.; Ma, B.; Kamiya, N. Thermal stability of single-domain antibodies estimated by molecular dynamics simulations. Protein Sci. 2019, 28, 429–438. [Google Scholar] [CrossRef] [Green Version]
- McAuley, A.; Jacob, J.; Kolvenbach, C.G.; Westland, K.; Lee, H.J.; Brych, S.R.; Rehder, D.; Kleemann, G.R.; Brems, D.N.; Matsumura, M. Contributions of a disulfide bond to the structure, stability, and dimerization of human IgG1 antibody CH3 domain. Protein Sci. 2008, 17, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolgikh, V.V.; Seliverstova, E.V.; Naumov, A.M.; Senderskiy, I.V.; Pavlova, O.A.; Beznoussenko, G.V. Heterologous expression of pyruvate dehydrogenase E1 subunits of the microsporidium Paranosema (Antonospora) locustae and immunolocalization of the mitochondrial protein in amitochondrial cells. FEMS Microbiol. Lett. 2009, 293, 285–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burland, T.G. DNASTAR’s Lasergene sequence analysis software. Methods Mol. Biol. 2000, 132, 71–91. [Google Scholar] [PubMed]
- Tetz, G.V.; Artemenko, N.K.; Yankovskii, G.M.; Kever, L.V.; Komissarchik, Y.Y.; Tetz, V.V. Effects of multicide, antibacterial drug, on Staphylococcus biomembranes. Bull. Exp. Biol. Med. 2017, 163, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, K. On the function of the polar filament of Nosema bombycis. Parasitology 1937, 29, 220–224. [Google Scholar] [CrossRef]



| VH | 9 * | 8 | 7 | 6 | 5 | 4 | 3 | 2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 86.2 | 89.7 | 89.7 | 87.6 | 45.6 | 86.2 | 48.3 | 84.5 | ||
| 2 | 76.3 | 83.6 | 83.1 | 85 | 54.4 | 82.8 | 50.4 | 36.1 | 2 | |
| 3 | 50 | 49.1 | 50.8 | 52.2 | 84.2 | 49.1 | 88.6 | 37 | 3 | |
| 4 | 81.9 | 93.1 | 90.5 | 85 | 46.5 | 36 | 36.9 | 100 | 5 | |
| 5 | 47.4 | 46.5 | 48.2 | 48.7 | 37.8 | 94.8 | 87.7 | 37 | 6 | |
| 6 | 85.8 | 86.7 | 86.7 | 82.8 | 36.9 | 83.5 | 83.3 | 38 | 7 | |
| 7 | 87.3 | 92.2 | 81 | 94.8 | 36.9 | 93 | 86.8 | 38 | 8 | |
| 8 | 85.3 | 35.3 | 34.5 | 36.2 | 42.3 | 35.7 | 36 | 40.7 | 9 | |
| 8 | 7 | 6 | 5 | 3 | 2 | 1 | VL |
| scFv | Cq Values * and Number of Transcripts in 1 μL of cDNA Samples (in Brackets) | ΔCq ** and Number of NbPTP2 Transcripts per 103 SfCOXI Ones (in Brackets) | N Samples and Variants with ΔCq ≥ 7 (in Brackets) | |
|---|---|---|---|---|
| SfCOXI | NbPTP2 | |||
| 1 | 13.8 ± 0.2 (7.1 × 106) | 20.9 ± 0.6 (20.7 × 103) | 7.1 ± 0.6 a (2.9) | 9 (6) |
| 2 | 14.2 ± 0.3 (5.4 × 106) | 21.6 ± 0.5 (12.3 × 103) | 7.4 ± 0.6 a (2.3) | 9 (4) |
| 3 | 15.3 ± 0.5 (2.7 × 106) | 22.2 ± 0.4 (7.9 × 103) | 6.9 ± 0.2 a (3) | 6 (3) |
| 4 | 14.7 ± 0.2 (3.9 × 106) | 19.9 ± 0.7 (43.3 × 103) | 5.2 ± 0.6 b (11) | 5 (1) |
| 5 | 13.5 ± 0.3 (8.6 × 106) | 22 ± 0.3 (9.2 × 103) | 8.5 ± 0.5 a (1.1) | 8 (8) |
| 6 | 14.3 ± 0.2 (5.1 × 106) | 20.1 ± 0.4 (37. 4 × 103) | 5.8 ± 0.4 b (7.3) | 9 (2) |
| 7 | 14 ± 0.3 (6.2 × 106) | 21.8 ± 0.3 (10.6 × 103) | 7.8 ± 0.5 a (1.7) | 4 (4) |
| 8 | 14.2 ± 0.5 (5.4 × 106) | 20.2 ± 0.4 (34.7 × 103) | 6 ± 0.6 b (6.4) | 8 (1) |
| 9 | 14.7 ± 0.3 (3.9 × 106) | 22.7 ± 0.5 (5.4 × 103) | 8 ± 0.3 a (1.4) | 6 (6) |
| scFv | Spores per Cell | Cq Values * and Number of Transcripts in 1 μL of cDNA Samples (in Brackets) | ΔCq ** and Number of NbPTP2 Transcripts per 106 SfCOXI Ones (in Brackets) | |
|---|---|---|---|---|
| SfCOXI | NbPTP2 | |||
| 1 | 10 | 15.5 ± 0.4 (2.3 × 106) | 23.2 ± 0.2 (3.8 × 103) | 7.7 ± 0.5 a (1.7 × 103) |
| 5 | 10 | 14.4 ± 0.2 (5.1 × 106) | 25.1 ± 0.3 (1.2 × 103) | 10.7 ± 0.4 b (2.4 × 102) |
| 7 | 10 | 14.8 ± 0.2 (3.7 × 106) | 23.7 ± 0.2 (2.6 × 103) | 8.9 ± 0.2 a (7 × 102) |
| 9 | 10 | 13.8 ± 0.3 (7.1 × 106) | 22.1 ± 0.3 (8.5 × 103) | 8.3 ± 0.9 a (1.2 × 103) |
| 1 | 3 | 14.7 ± 0.1 (3.9 × 106) | 22.4 ± 0.1 (6.8 × 103) | 7.7 ± 0.2 a (1.7 × 103) |
| 5 | 3 | 15.5 ± 0.3 (2.3 × 106) | 26.1 ± 0.2 (4.4 × 102) | 10.6 ± 0.2 b (1.9 × 102) |
| 7 | 3 | 15.3 ± 0.6 (2.7 × 106) | 24.1 ± 0.9 (1.9 × 103) | 8.8 ± 0.4 a (7 × 102) |
| 9 | 3 | 14.9 ± 0.2 (3.4 × 106) | 23.9 ± 0.2 (2.2 × 103) | 9 ± 0.3 a (6.5 × 102) |
| scFv | Cq * | ΔCq ** | |
|---|---|---|---|
| SfCOXI-2 | NbPTP2-2 | ||
| 2 | 19.4 ± 0.1 | 21.7 ± 0.1 | 2.3 ± 0.1 a |
| 3 | 19.7 ± 0.8 | 22.1 ± 0.4 | 2.4 ± 0.5 a |
| 5 | 20.6 ± 0.4 | 26.4 ± 1 | 5.8 ± 0.6 b |
| 7 | 18.1 ± 0.3 | 20.4 ± 0.4 | 2.3 ± 0.4 a |
| 8 | 19.2 ± 0.1 | 19.6 ± 0.1 | 0.4 ± 0.1 a |
| eGFP | 18.5 ± 0.2 | 19.1 ± 0.2 | 0.6 ± 0.2 a |
| Primer | Sequence (5′-3′) |
|---|---|
| NbAAC3 rev a | TGCAAGCTTACGGTGCAGCACGACGAATGTTGTC |
| pOPE101seq for | AAGAGGAGAAATTAACCATGA |
| pOPE101seq rev | TCATTAGCACAGGCCTCTAGA |
| pOPEpIB for b | ACGGAGCTCGGTACCTGCTGCTGGCAGCTCAGCCGGCCATG |
| pOPEpIB rev c | GCTGAATTCTCGAGTTAATGATGATGGTGATGATGGGATAG |
| GFP for d | CATGAATTCATGGTGAGCAAGGGCGAGGAGCTG |
| GFP rev e | TCACTCGAGTTACTTGTACAGCTCGTCCATGCCGA |
| pIB seq for | CGCAACGATCTGGTAAACAC |
| pIB seq rev | GACAATACAAACTAAGATTTAGTCAG |
| SfCOXI for | TTTGAGCAGGAATAGTAGGT |
| SfCOXI rev | TAAAGATGGGGGTAAAAGT |
| SfCOXI-2 for | TACCGCATTTTTATTATTATTATC |
| SfCOXI-2 rev | GTTTCCTTTTTACCTCTTTCTTGA |
| NbPTP2 for | TGGCATCAGTAGCTCCTCCTCAAG |
| NbPTP2 rev | ACGGCCCTAGCTGCTGTTTCAA |
| NbPTP2-2 for | CAATAATCCAGCCGAGTGTCAA |
| NbPTP2-2 rev | AGTGGGGTACCTTCAGCAGTTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolgikh, V.V.; Senderskiy, I.V.; Timofeev, S.A.; Zhuravlyov, V.S.; Dolgikh, A.V.; Seliverstova, E.V.; Ismatullaeva, D.A.; Mirzakhodjaev, B.A. Construction of scFv Antibodies against the Outer Loops of the Microsporidium Nosema bombycis ATP/ADP-Transporters and Selection of the Fragment Efficiently Inhibiting Parasite Growth. Int. J. Mol. Sci. 2022, 23, 15307. https://doi.org/10.3390/ijms232315307
Dolgikh VV, Senderskiy IV, Timofeev SA, Zhuravlyov VS, Dolgikh AV, Seliverstova EV, Ismatullaeva DA, Mirzakhodjaev BA. Construction of scFv Antibodies against the Outer Loops of the Microsporidium Nosema bombycis ATP/ADP-Transporters and Selection of the Fragment Efficiently Inhibiting Parasite Growth. International Journal of Molecular Sciences. 2022; 23(23):15307. https://doi.org/10.3390/ijms232315307
Chicago/Turabian StyleDolgikh, Viacheslav V., Igor V. Senderskiy, Sergej A. Timofeev, Vladimir S. Zhuravlyov, Alexandra V. Dolgikh, Elena V. Seliverstova, Diloram A. Ismatullaeva, and Bakhtiyar A. Mirzakhodjaev. 2022. "Construction of scFv Antibodies against the Outer Loops of the Microsporidium Nosema bombycis ATP/ADP-Transporters and Selection of the Fragment Efficiently Inhibiting Parasite Growth" International Journal of Molecular Sciences 23, no. 23: 15307. https://doi.org/10.3390/ijms232315307






