Chlorine Dioxide: Friend or Foe for Cell Biomolecules? A Chemical Approach
Abstract
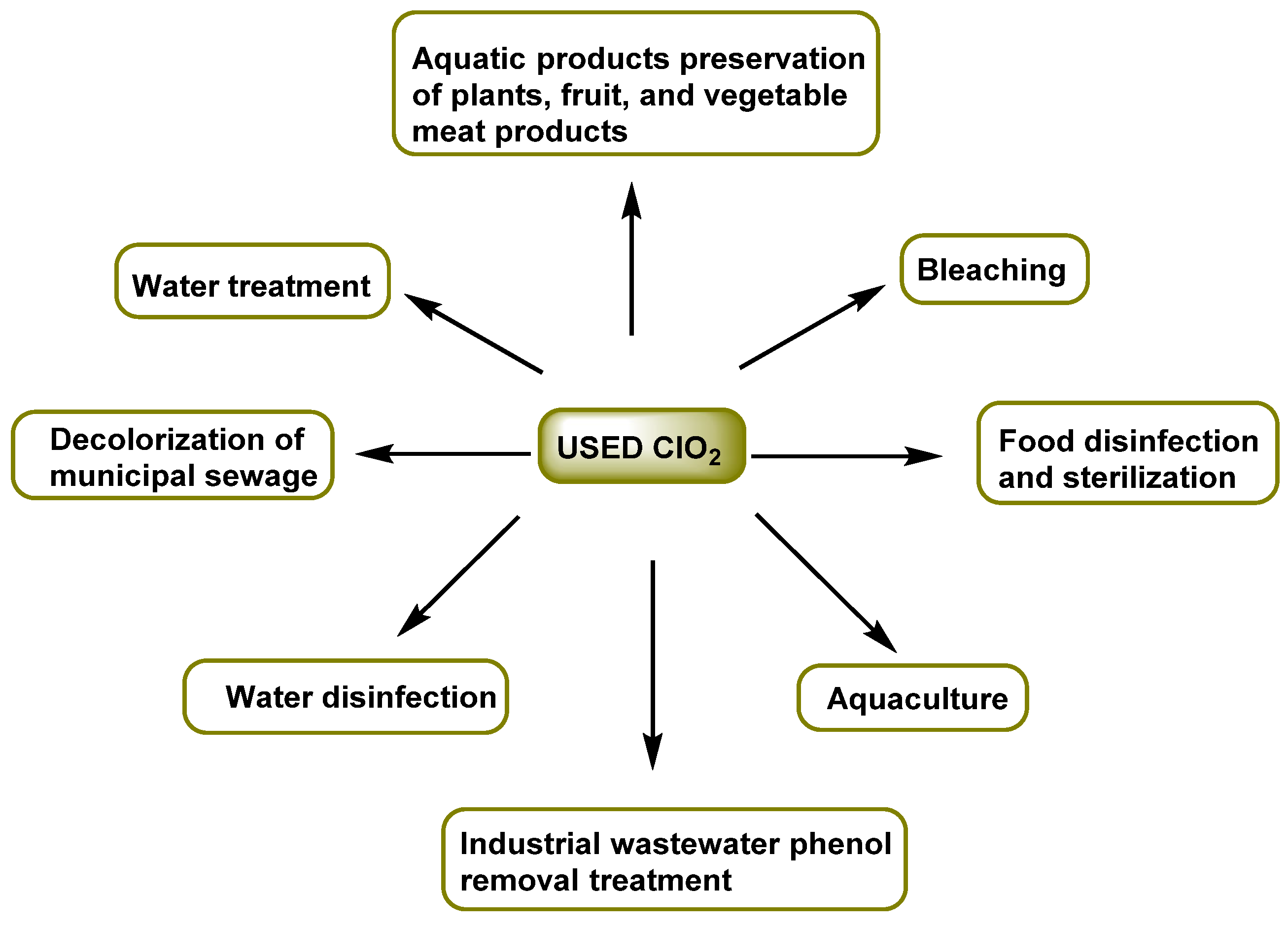
1. Introduction
2. Physicochemical Properties of ClO2
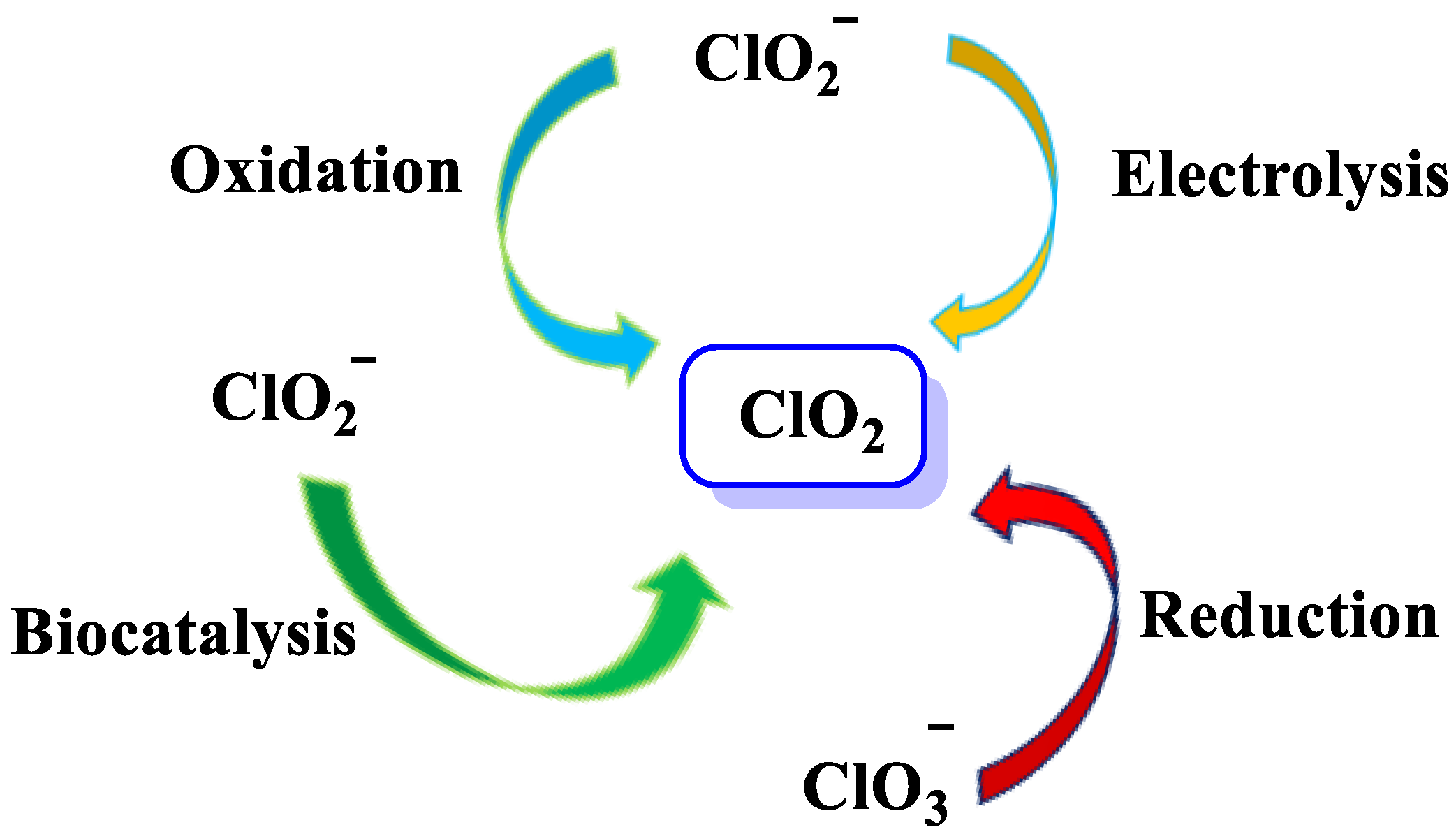
3. Generation of Chlorine Dioxide
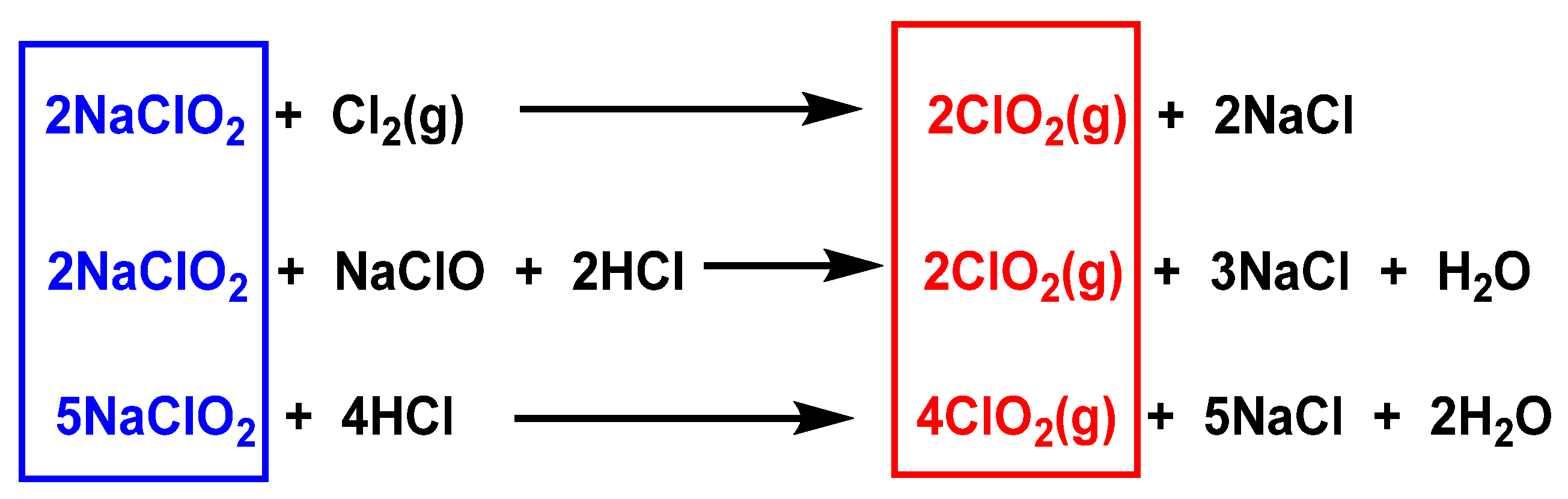
3.1. From Chlorite Ions
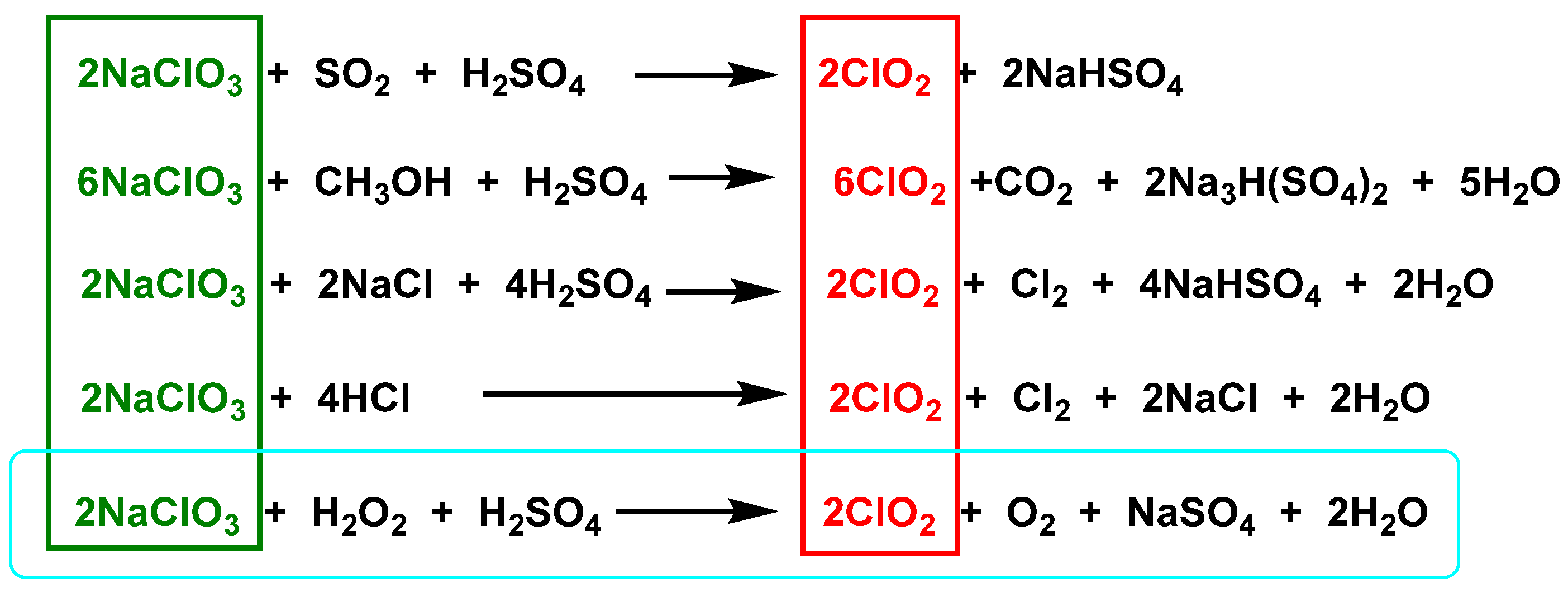
3.2. From Sodium Chlorate
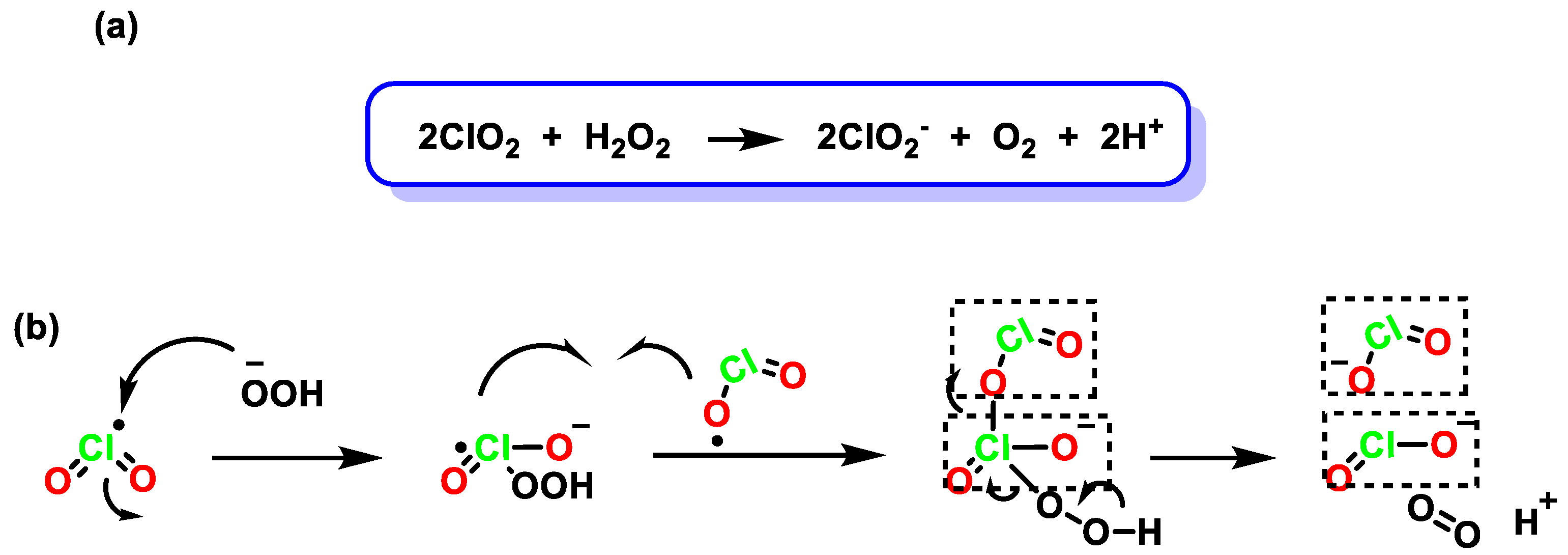
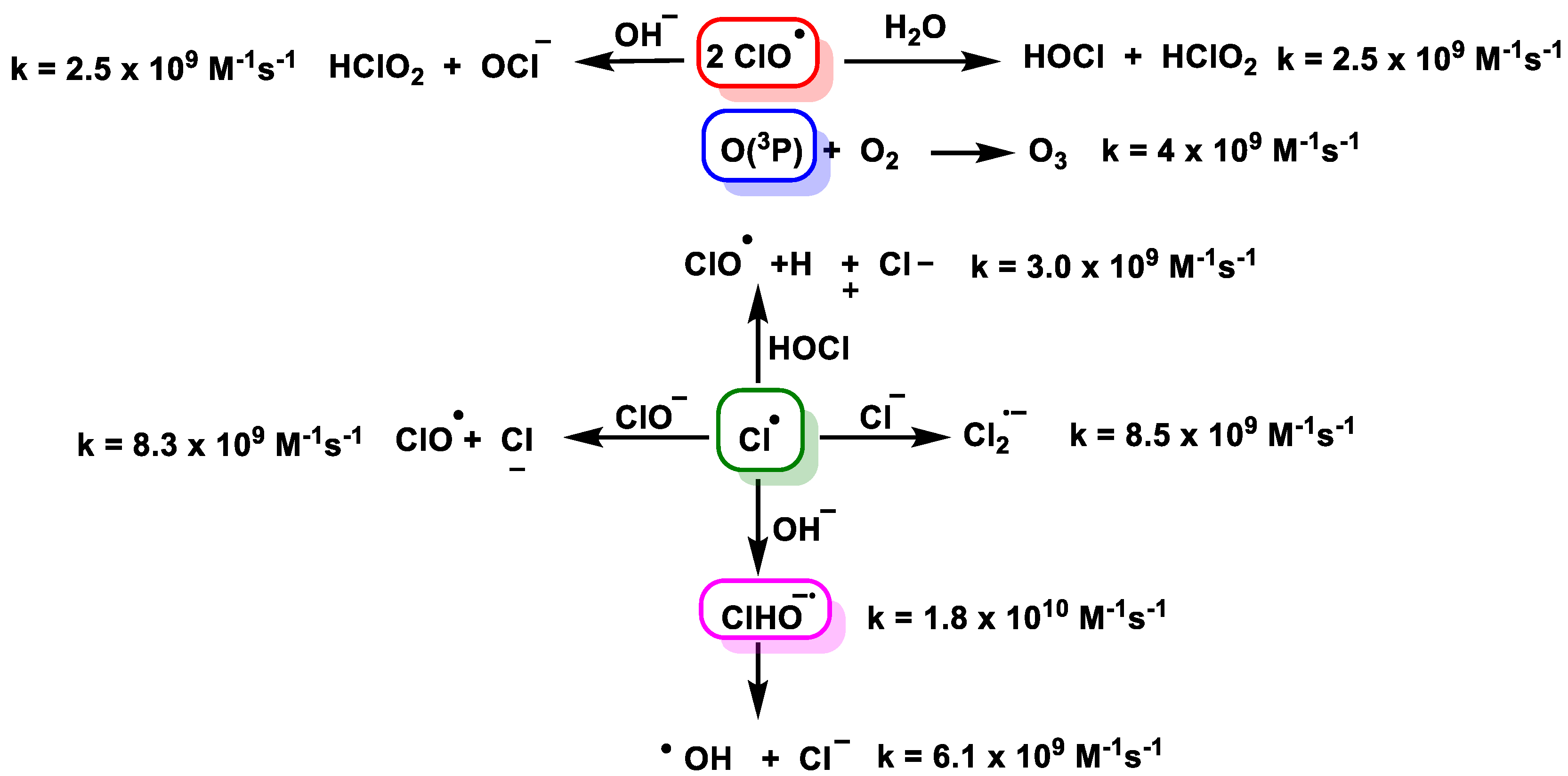
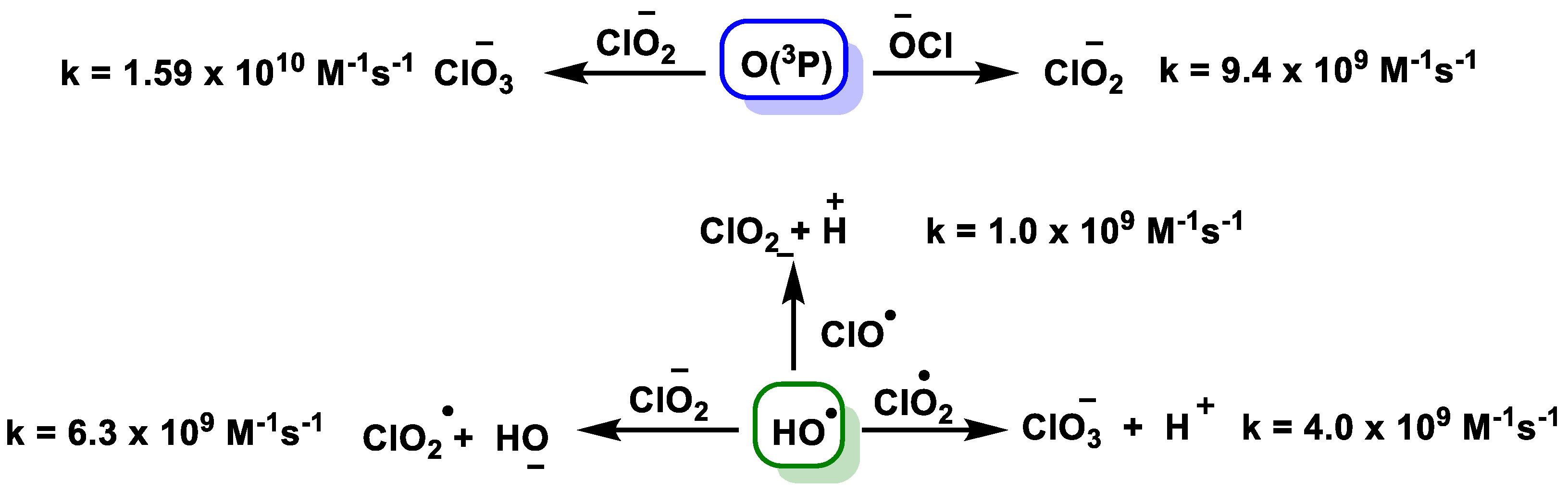
4. Decomposition of ClO2
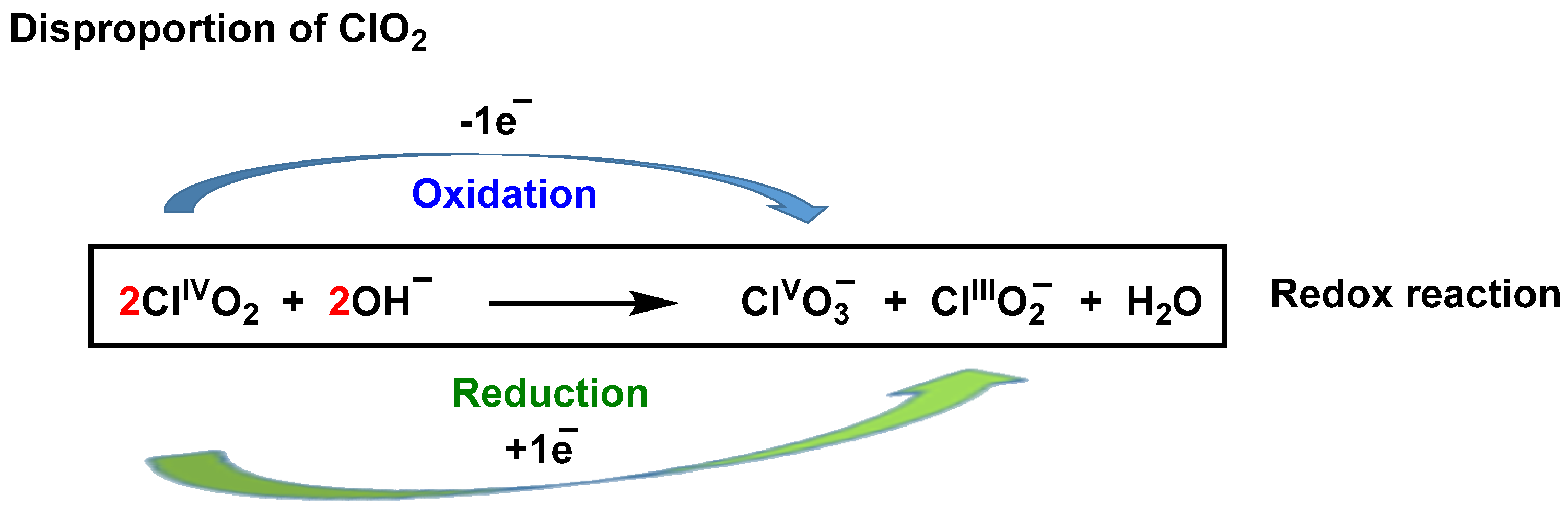
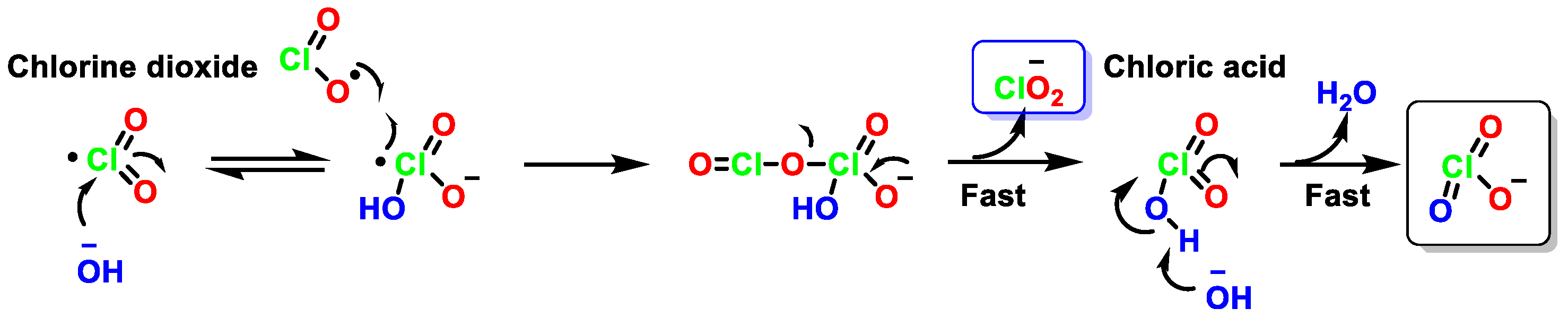
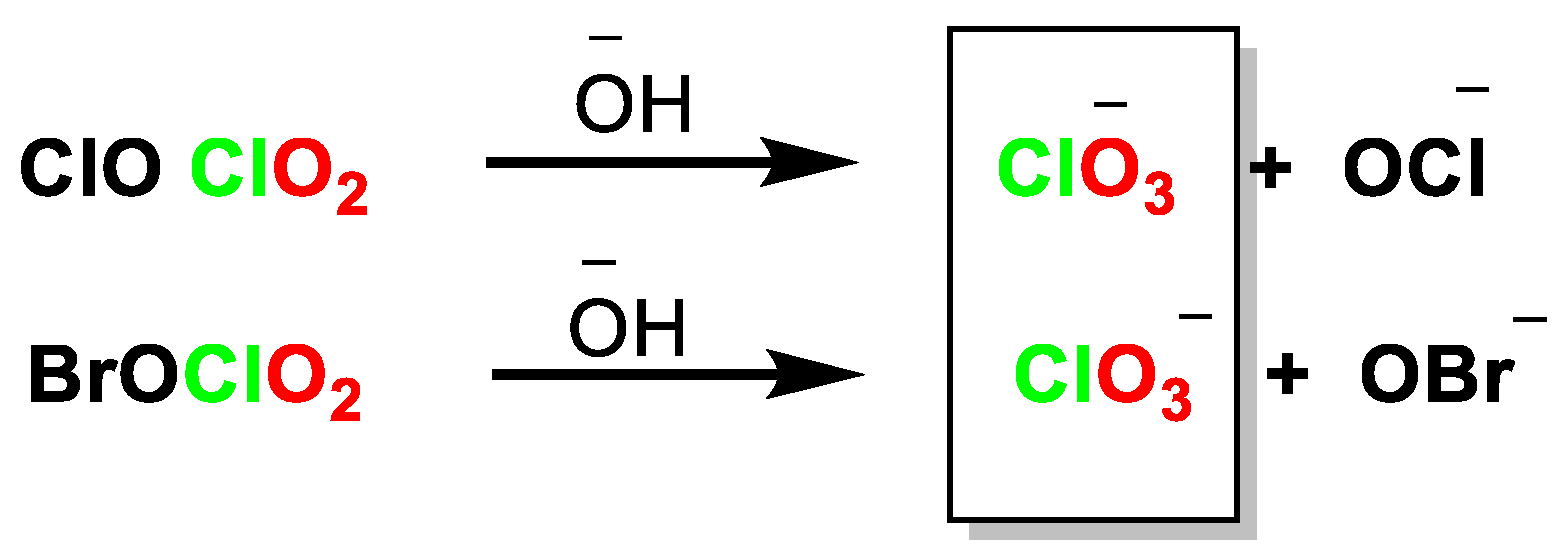
4.1. Disproportionation of Chlorine Dioxide with OH−
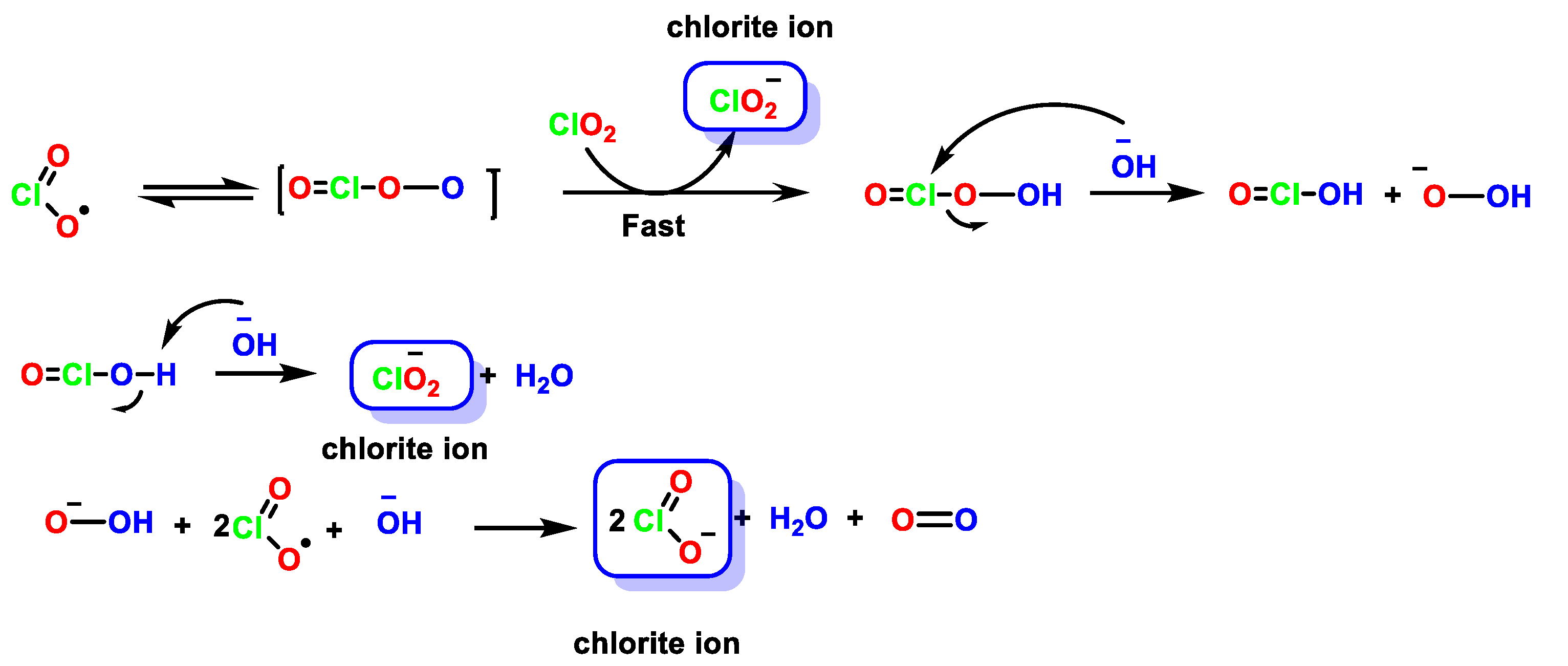
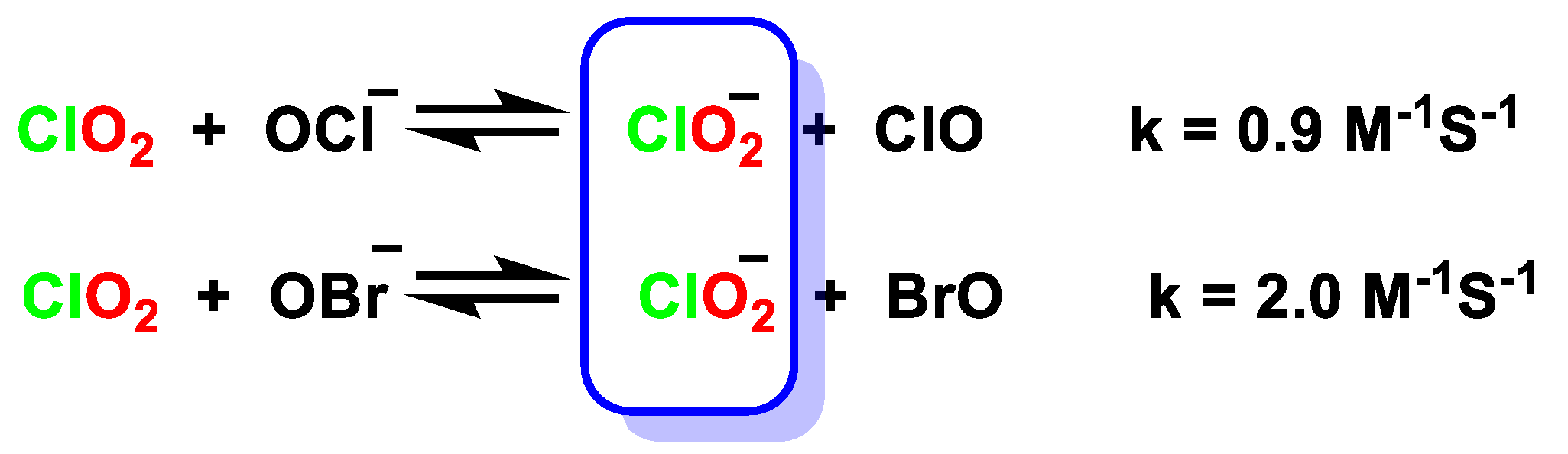
4.2. Disproportionation of Chlorine Dioxide with Nucleophile
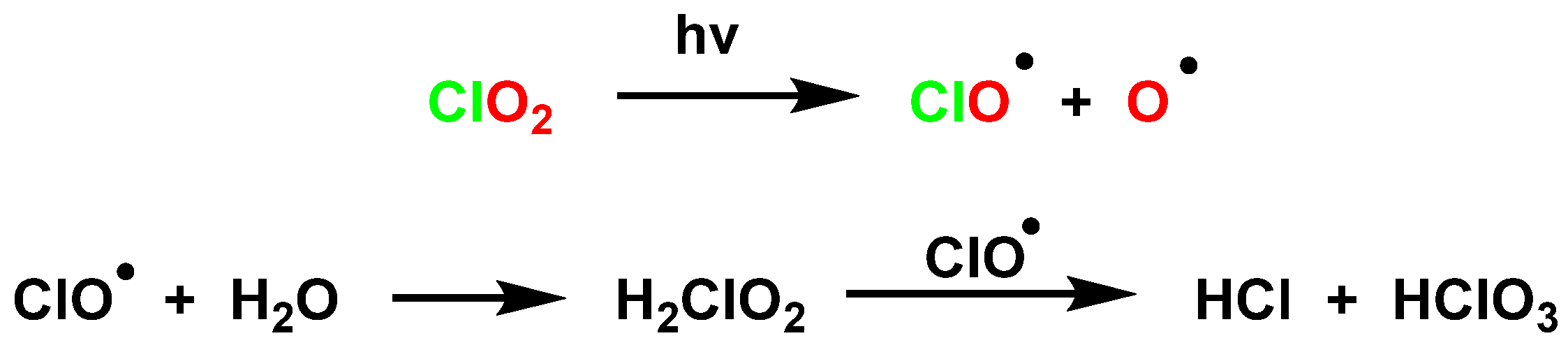
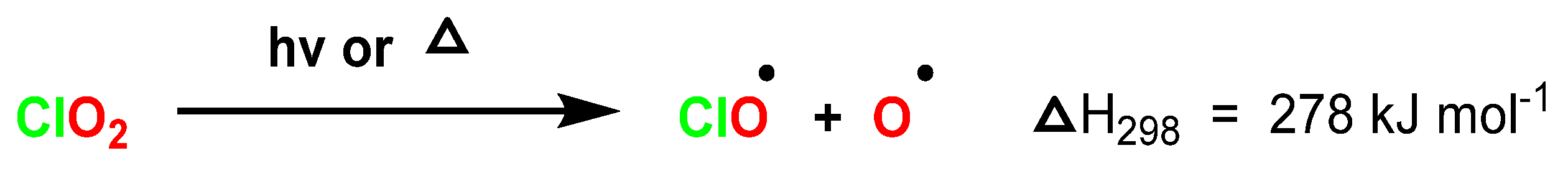
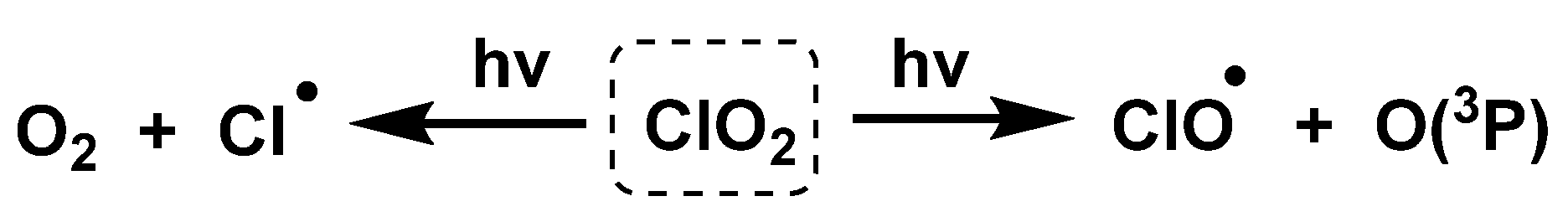
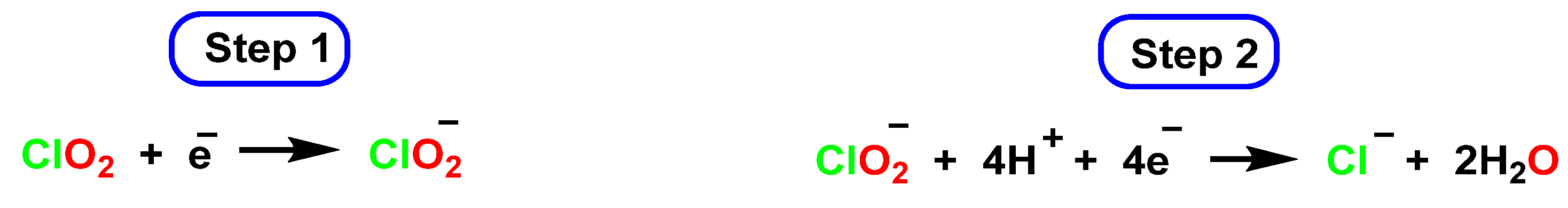
4.3. Photodissociation of ClO2
5. Reactivity of ClO2
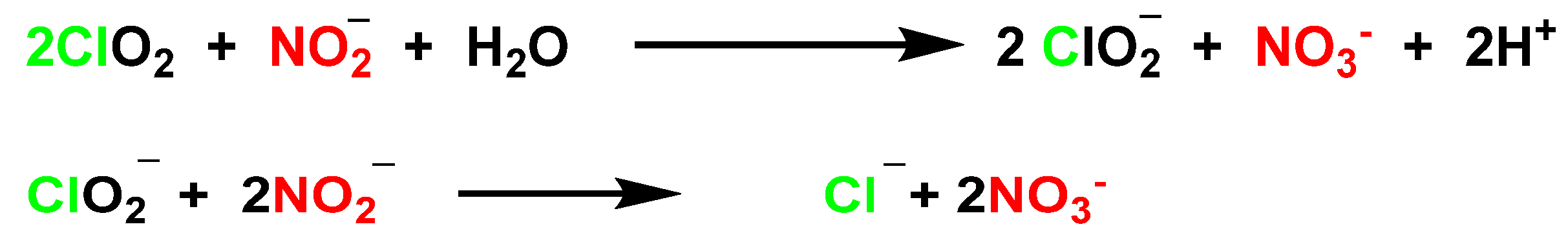
5.1. Reactivity of ClO2 with Inorganic Compounds
5.2. Reactivity with Organic Compounds
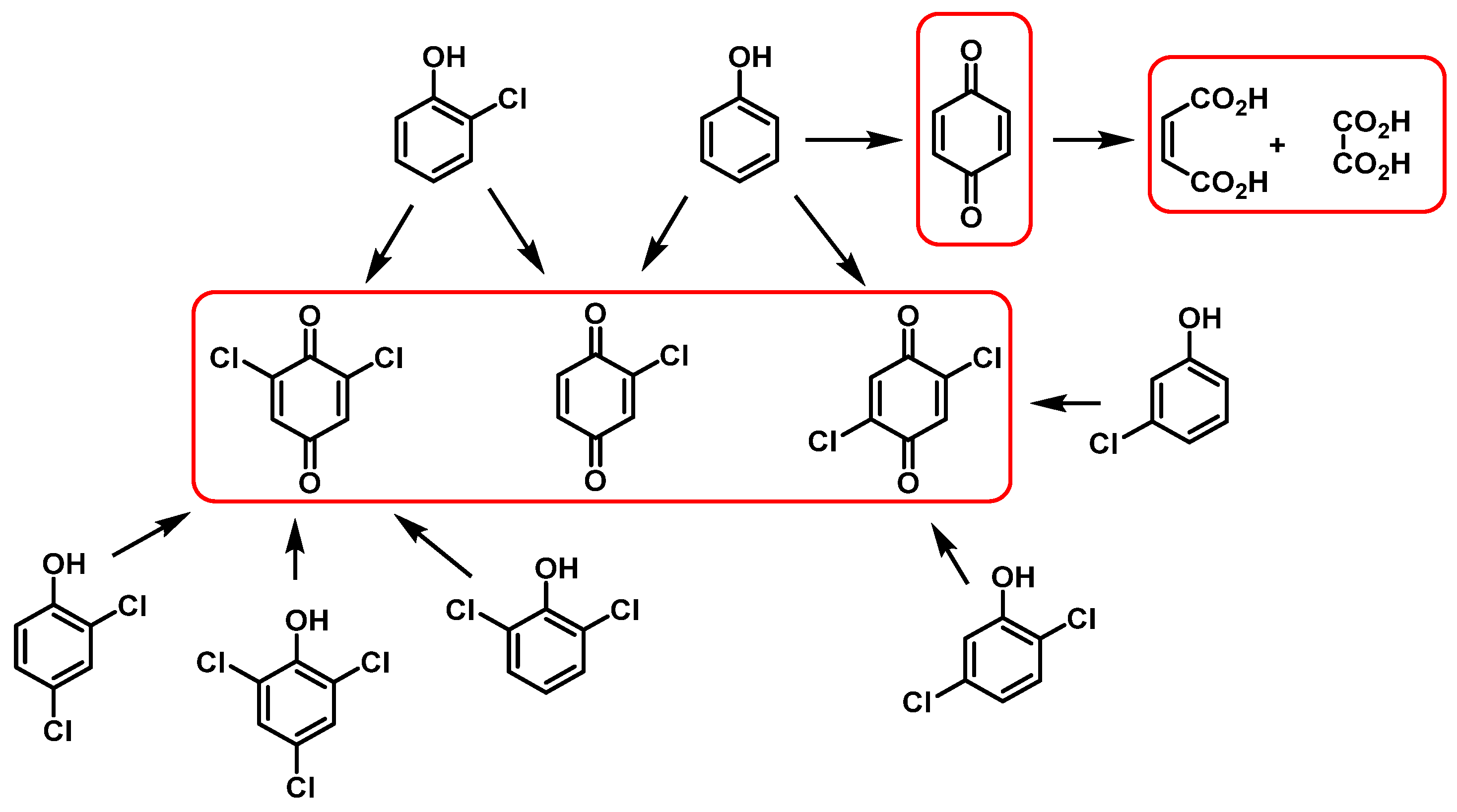
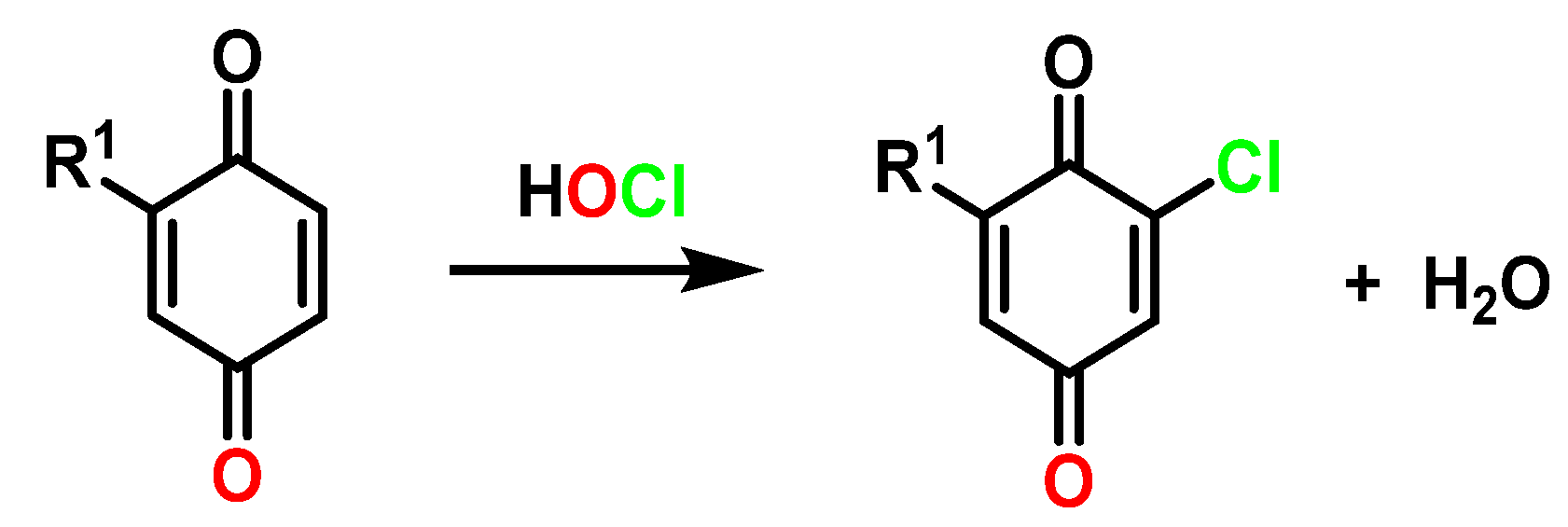
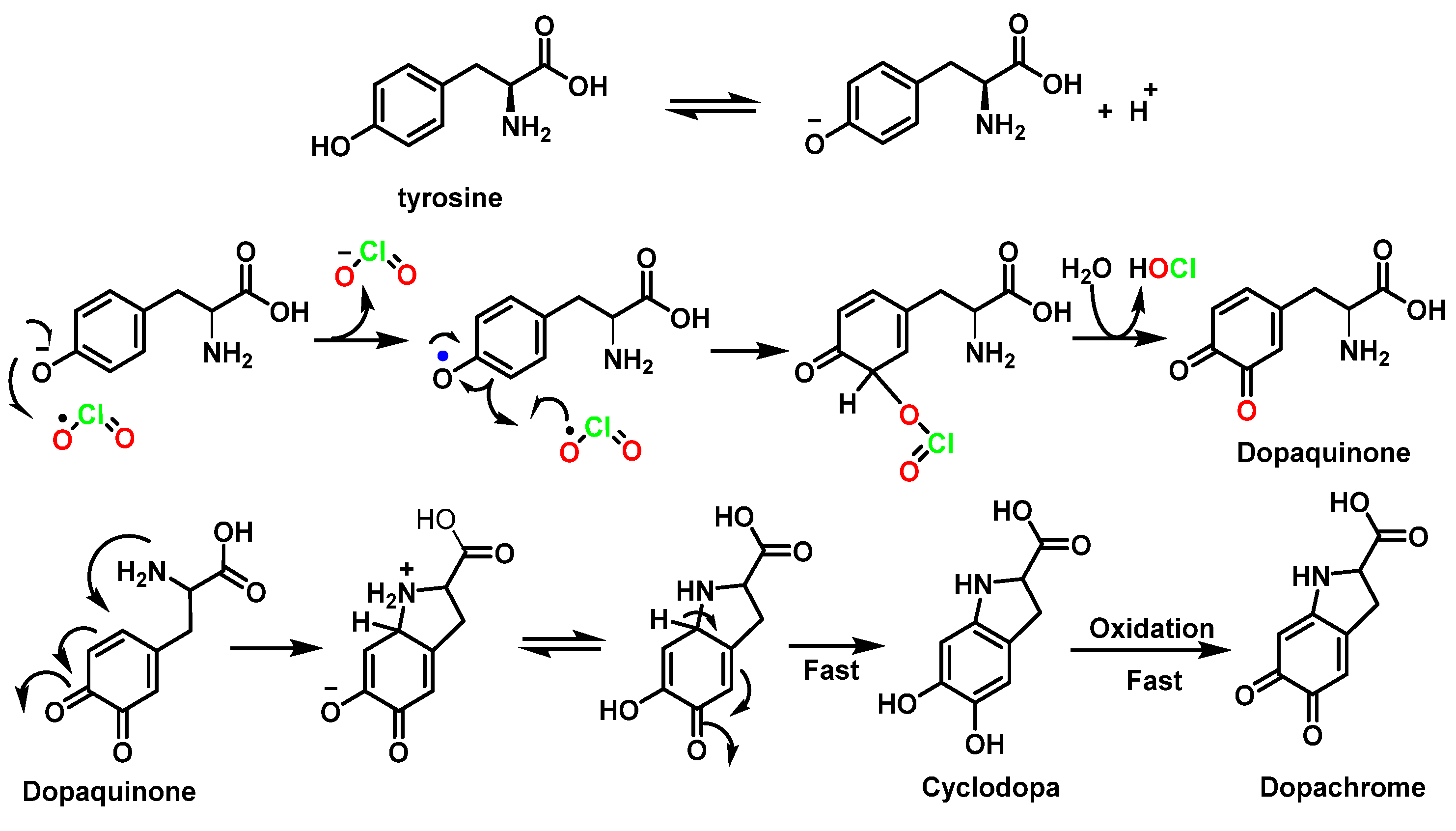
5.3. Reactivity with Phenolic Compounds
5.4. Reactivity with Amines
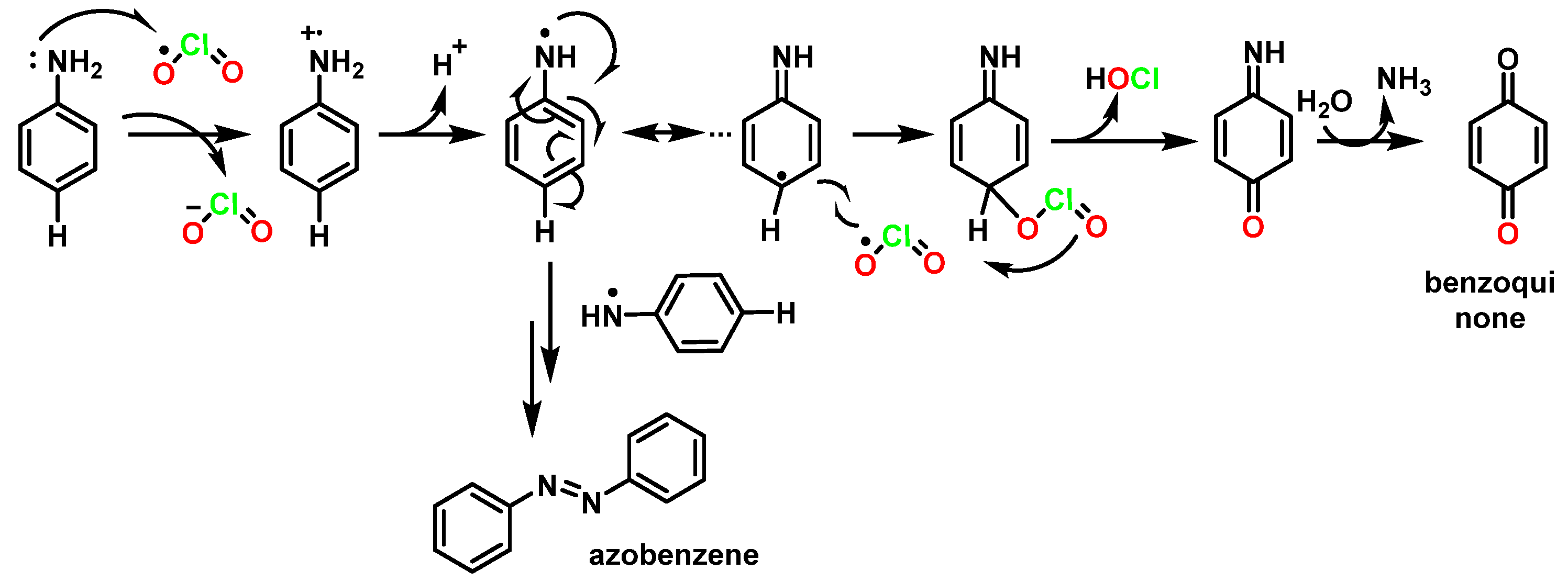
5.4.1. Reactivity with Aromatic Amines
5.4.2. Reactivity with Aliphatic Amines
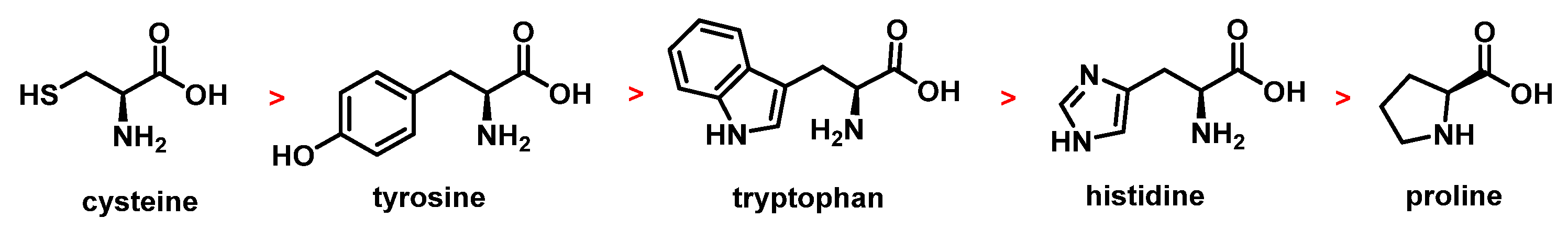
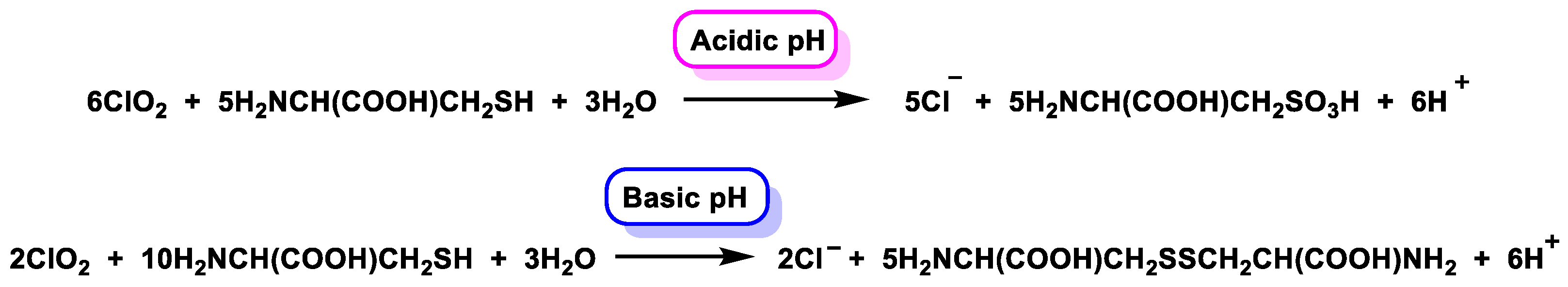
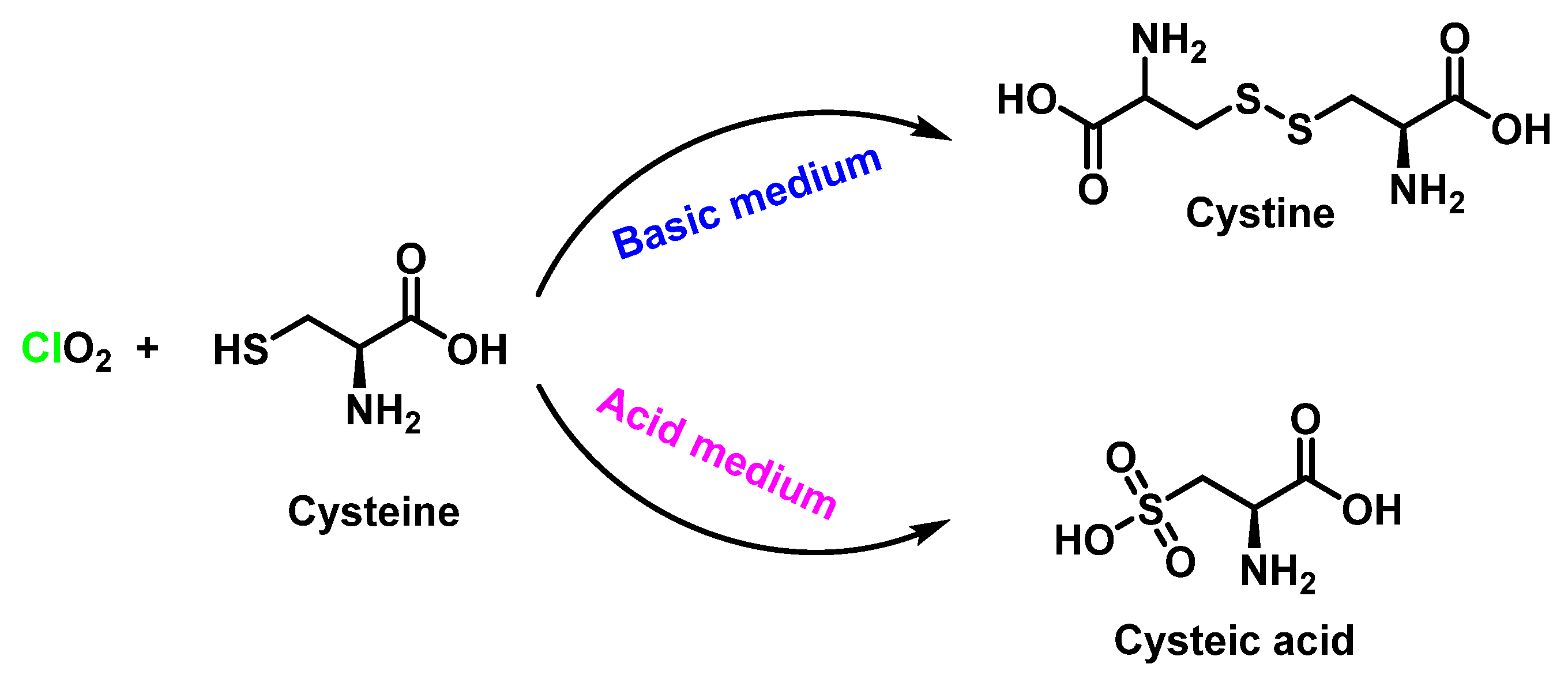
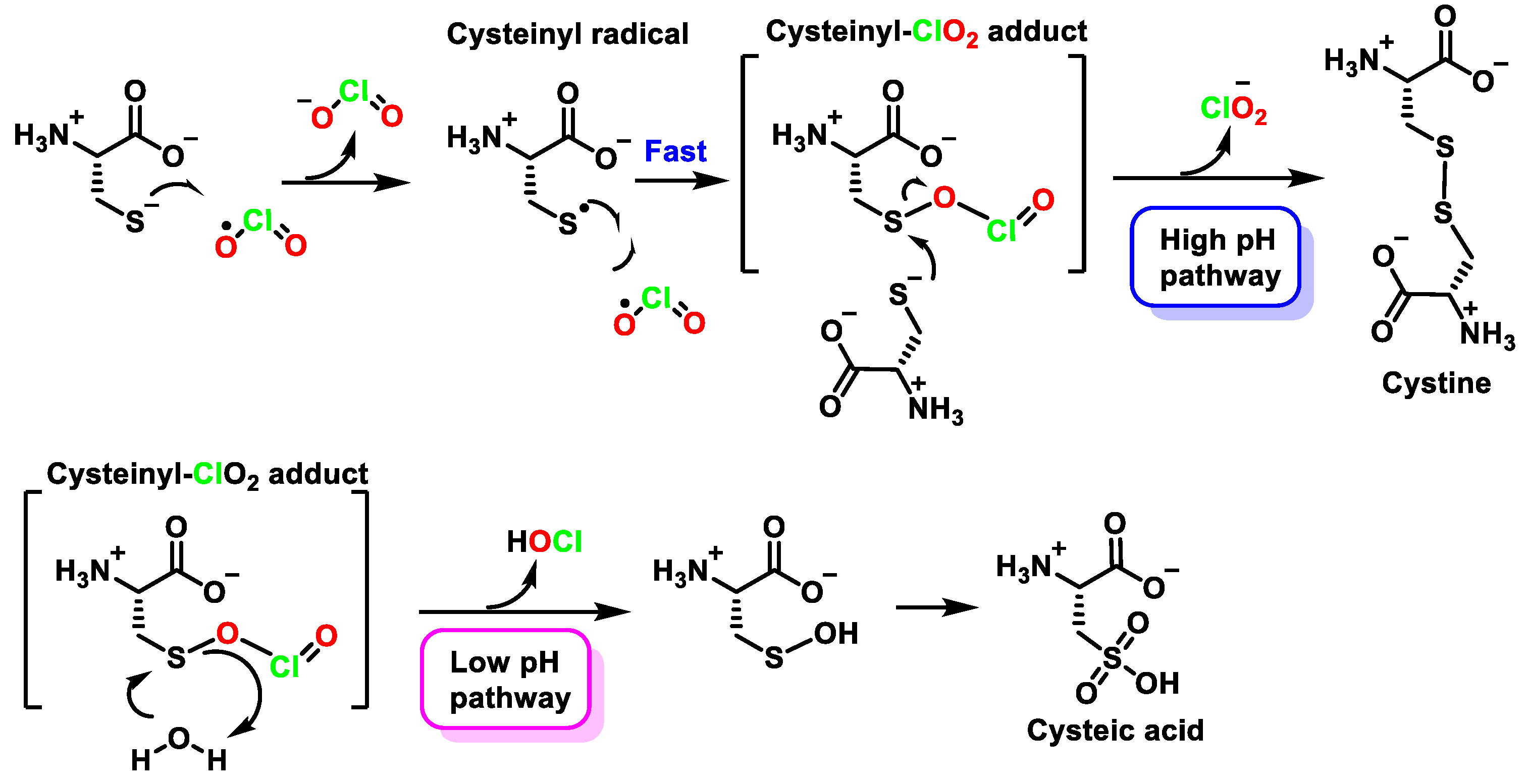
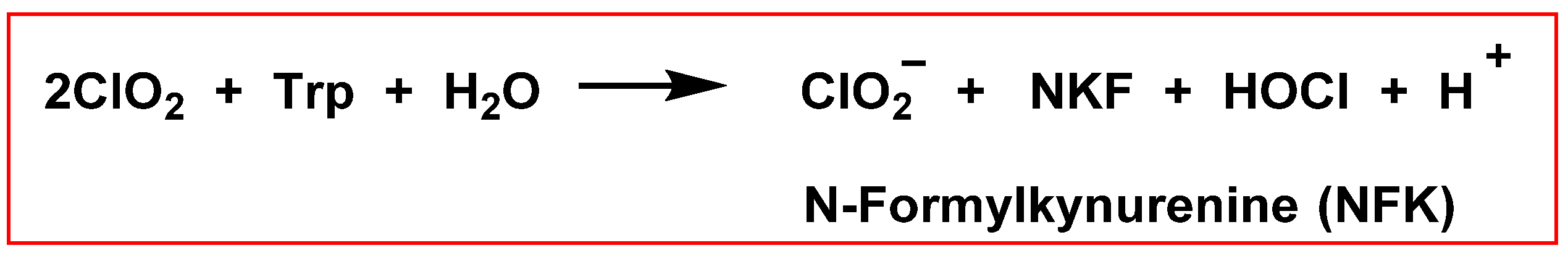
5.5. Reactivity with Amino Acids, Peptides, and Proteins
5.6. Oxidation of Peptides and Proteins by ClO2
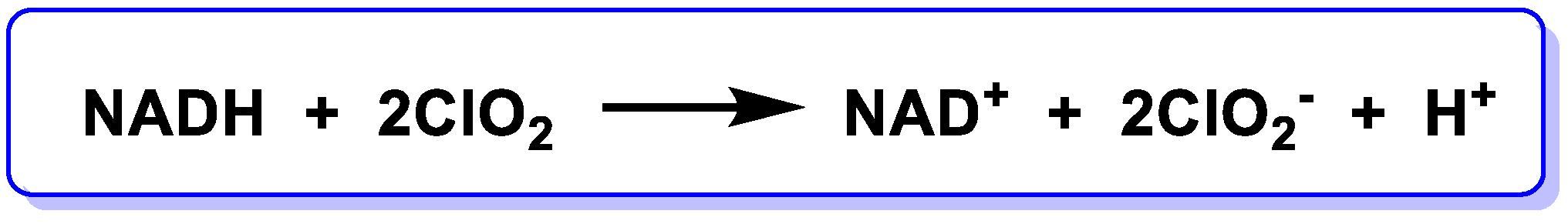
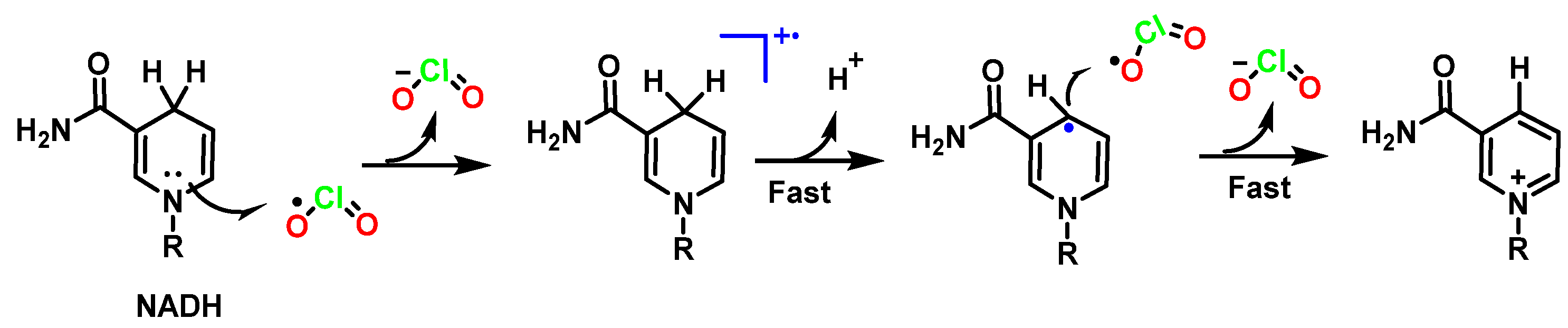
5.7. Oxidation of NADH by Chlorine Dioxide
6. Oxidation of Hemoglobin by ClO2
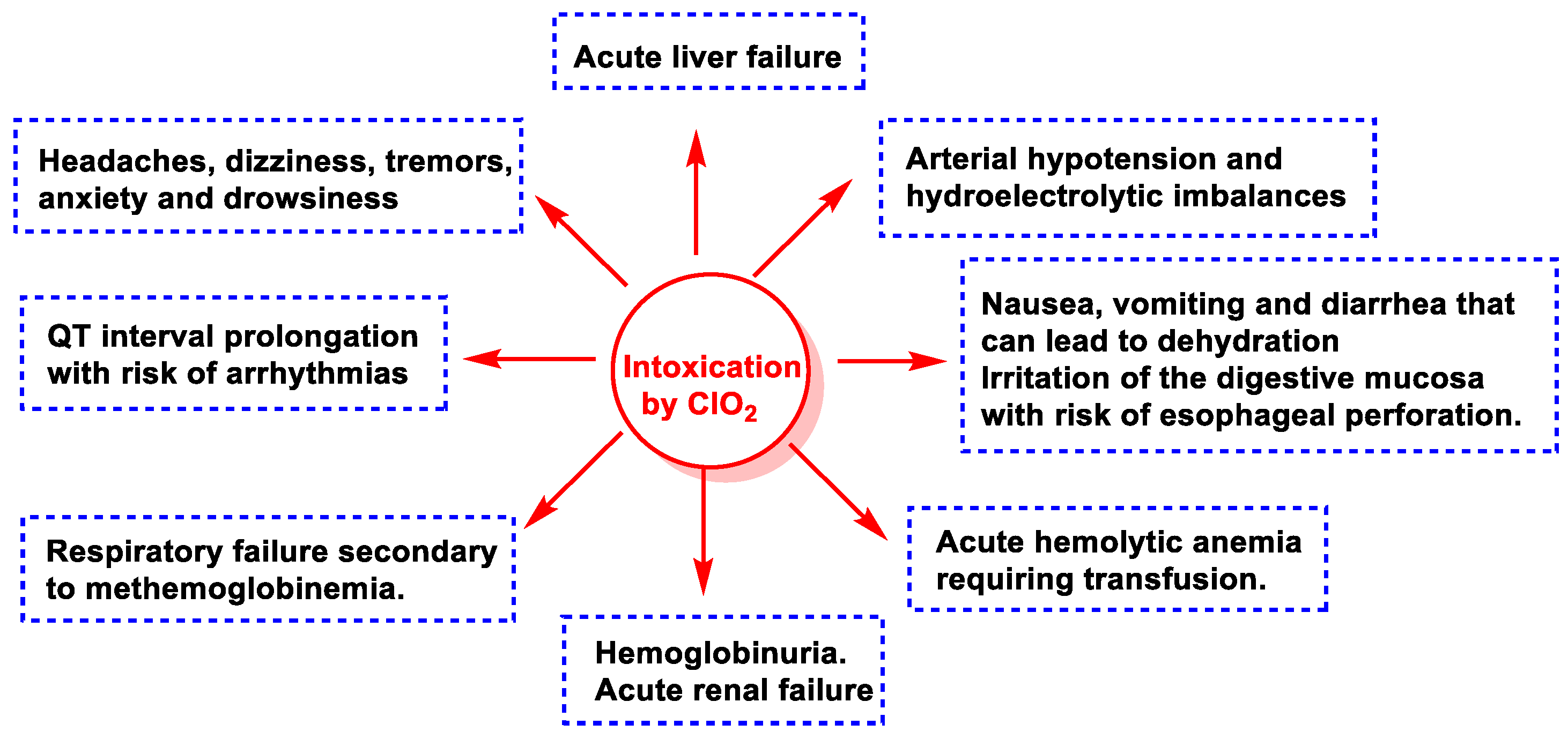
7. Toxicity of ClO2
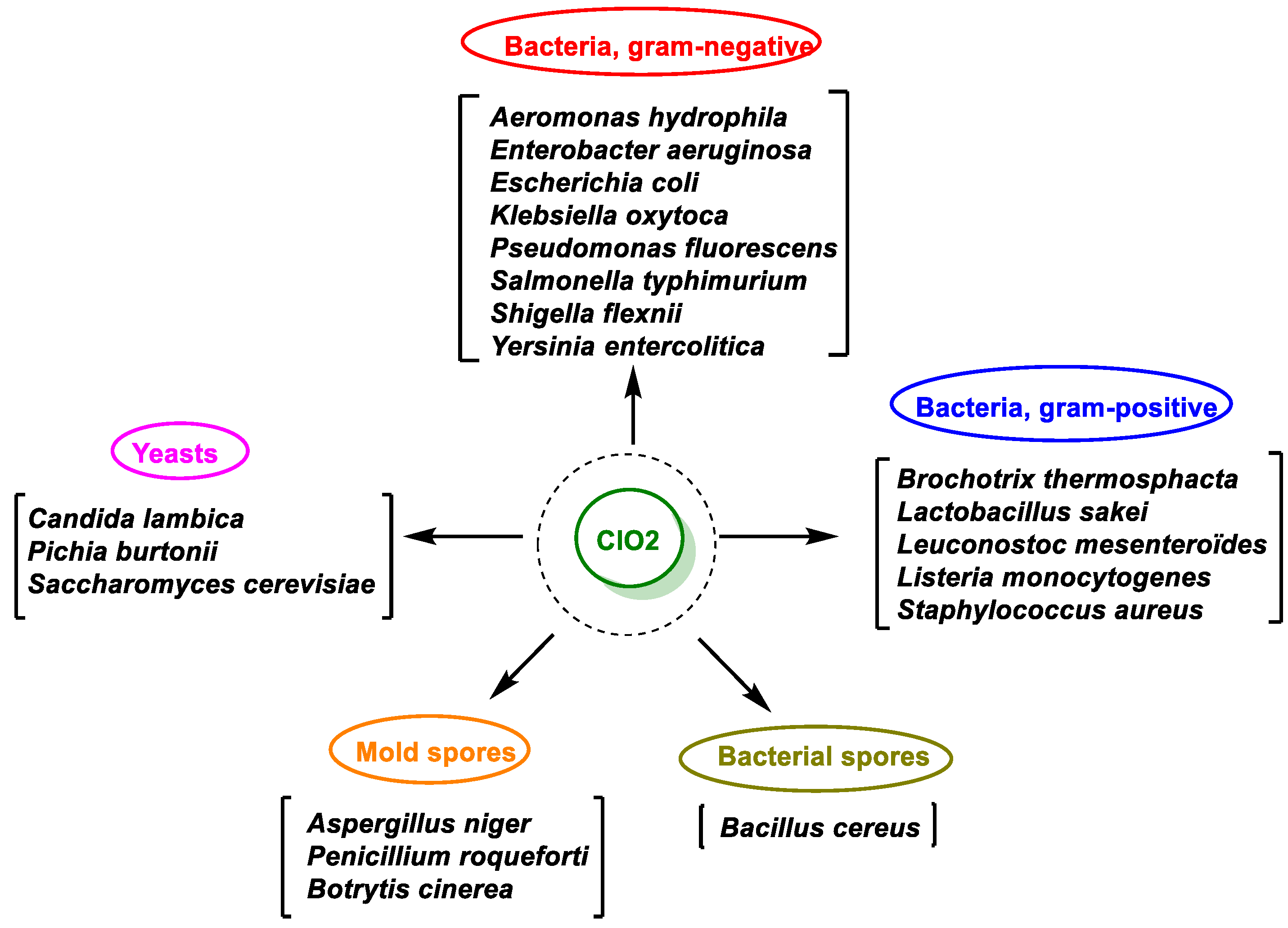
8. Antimicrobial Activity of ClO2
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aieta, E.M.; Berg, J.D. A Review of Chlorine Dioxide in Drinking Water Treatment. J. (Am. Water Work. Assoc.) 1986, 78, 62–72. [Google Scholar] [CrossRef]
- Deshwal, B.R.; Lee, H.-K. Manufacture of Chlorine Dioxide from Sodium Chlorate: State of the Art. J. Ind. Eng. Chem. 2005, 11, 330–346. [Google Scholar]
- Mei, L.; Shilong, T.; Jin, S.; Xizhuo, W.; Jianxin, C.; Shouqiang, L.; Xia, G.; Jiachun, T. Effects of chlorine dioxide on morphology and ultrastructure of Fusarium sulphureum and its virulence to potato tubers. Int. J. Agric. Biol. Eng. 2017, 10, 242–250. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Xie, Y.; Ni, T.; Liu, G. Oxidative removal of diclofenac by chlorine dioxide: Reaction kinetics and mechanism. Chem. Eng. J. 2015, 279, 409–415. [Google Scholar] [CrossRef]
- Friedline, A.W.; Zachariah, M.M.; Middaugh, A.N.; Heiser, M.J.; Khanna, N.; Vaishampayan, P.; Rice, C.V. Sterilization of hydrogen peroxide resistant bacterial spores with stabilized chlorine dioxide. AMB Express 2015, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamzah, A.; Rahman, M.M.; Kurup, P.K.; Barnawi, A.; Ghannam, B.; Musharraf, I.; Najjar, F.A.; Obeidallah, A.; Palmer, N. Use of chlorine dioxide as alternative to chlorination in reverse osmosis product water. Desalinat. Water Treat. 2019, 163, 57–66. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Zhang, X.; Chen, J.-H.; Deng, C.-H.; Xu, N.; Shi, W.; Cheng, P. Highly selective luminescent sensing of xylene isomers by a water stable Zn-organic framework. Inorg. Chem. Commun. 2016, 69, 1–3. [Google Scholar] [CrossRef]
- Gan, W.; Ge, Y.; Zhong, Y.-H.; Yang, X. The reactions of chlorine dioxide with inorganic and organic compounds in water treatment: Kinetics and mechanisms. Environ. Sci. Water Res. Technol. 2020, 6, 2287–2312. [Google Scholar] [CrossRef]
- Jeong, C.H.; Wagner, E.D.; Siebert, V.R.; Anduri, S.; Richardson, S.D.; Daiber, E.J.; Mckague, A.B.; Kogevinas, M.; Villanueva, C.M.; Goslan, E.H.; et al. Occurrence and toxicity of disinfection byproducts in European drinking waters in relation with the HIWATE epidemiology study. Environ. Sci. Technol. 2012, 46, 12120–12128. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sohn, M. Oxidation of Amino Acids, Peptides, and Proteins by Chlorine Dioxide. Implications for Water Treatment. In Environmental chemistry for a sustainable world; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Tsai, L.S.; Higby, R.; Schade, J.E. Disinfection of Poultry Chiller Water with Chlorine Dioxide: Consumption and Byproduct Formation. J. Agric. Food Chem. 1995, 43, 2768–2773. [Google Scholar] [CrossRef]
- Kim, J.; Huang, T.-S.; Marshall, M.R.; Wei, C.I. Chlorine Dioxide Treatment of Seafoods to Reduce Bacterial Loads. J. Food Sci. 1999, 64, 1089–1093. [Google Scholar] [CrossRef]
- Kim, H.; Kang, Y.; Beuchat, L.R.; Ryu, J.-H. Production and stability of chlorine dioxide in organic acid solutions as affected by pH, type of acid, and concentration of sodium chlorite, and its effectiveness in inactivating Bacillus cereus spores. Food Microbiol. 2008, 25, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Kull, T.P.J.; Backlund, P.H.; Karlsson, K.M.; Meriluoto, J. Oxidation of the cyanobacterial hepatotoxin microcystin-LR by chlorine dioxide: Reaction kinetics, characterization, and toxicity of reaction products. Environ. Sci. Technol. 2004, 38, 6025–6031. [Google Scholar] [CrossRef] [PubMed]
- Kull, T.P.J.; Sjövall, O.; Tammenkoski, M.; Backlund, P.H.; Meriluoto, J. Oxidation of the cyanobacterial hepatotoxin microcystin-LR by chlorine dioxide: Influence of natural organic matter. Environ. Sci. Technol. 2006, 40, 1504–1510. [Google Scholar] [CrossRef]
- Gordon, G.; Rosenblatt, A.A. Chlorine Dioxide: The Current State of the Art. Ozone Sci. Eng. 2005, 27, 203–207. [Google Scholar] [CrossRef]
- Vandekinderen, I.; Devlieghere, F.; Van Camp, J.; Kerkaert, B.; Cucu, T.; Ragaert, P.; De Bruyne, J.; De Meulenaer, B. Effects of food composition on the inactivation of foodborne microorganisms by chlorine dioxide. Int. J. Food Microbiol. 2009, 131, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Wohlers, D.W.; Amata, R. Toxicological Profile for Chlorine Dioxide and Chlorite. In Draft for Public Comment; Agency for Toxic Substances and Disease Registry (ATSDR): Atlanta, GA, USA, 2004. Available online: https://www.atsdr.cdc.gov (accessed on 25 October 2022).
- Vogt, H.; Balej, J.; Bennett, J.E.; Wintzer, P.; Sheikh, S.A.; Gallone, P.; Vasudevan, S.; Pelin, K. Chlorine Oxides and Chlorine Oxygen Acids. 2010. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a06_483 (accessed on 25 October 2022).
- Bastidas-Ortiz. Chlorine Dioxide: Does It Contribute to Human Health? A Brief Review. 2021. Available online: https://www.truthforhealth.org/2022/06/chlorine-dioxide-does-it-contribute-to-human-health-a-brief-review/ (accessed on 25 October 2022).
- Jin, R.; Hu, S.-Q.; Zhang, Y.-G.; Bo, T. Concentration-dependence of the explosion characteristics of chlorine dioxide gas. J. Hazard. Mater. 2009, 166, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tanaka, T. CO2 and N2O laser Stark spectroscopy of the ν1 band of the ClO2 radical. J. Mol. Spectrosc. 1983, 98, 425–452. [Google Scholar] [CrossRef]
- Brockway, L.O. The Three-Electron Bond in Chlorine Dioxide. Proc. Natl. Acad. Sci. USA 1933, 19, 303–307. [Google Scholar] [CrossRef]
- Jost, W. Linus Pauling: “General Chemistry”, 2nd ed.; W.H. Freeman and Company: San Francisco, CA, USA, 1954. [Google Scholar]
- Marcon, J.; Mortha, G.; Marlin, N.; Molton, F.; Duboc, C.; Burnet, A. New insights into the decomposition mechanism of chlorine dioxide at alkaline pH. Holzforschung 2017, 71, 599–610. [Google Scholar] [CrossRef]
- Svenson, D.R.; Kadla, J.F.; Chang, H.m.; Jameel, H. Effect of pH on the Inorganic Species Involved in a Chlorine Dioxide Reaction System. Ind. Eng. Chem. Res. 2002, 41, 5927–5933. [Google Scholar] [CrossRef]
- White, G.C. The Handbook of Chlorination and Alternative Disinfectants, 5th ed. Wiley: Hoboken, NJ, USA, 1992.
- Tsai, Y.-T.; Chang, C.Y.; Hsieh, Y.H. The Generation of Chlorine Dioxide by Electrochemistry Technology. Adv. Sci. Lett. 2013, 19, 3285–3288. [Google Scholar] [CrossRef]
- Pillai, K.C.; Kwon, T.O.; Park, B.B.; Moon, I.S. Studies on process parameters for chlorine dioxide production using IrO2 anode in an un-divided electrochemical cell. J. Hazard. Mater. 2009, 164, 812–819. [Google Scholar] [CrossRef]
- Brito, C.d.N.; Araújo, D.M.d.; Martínez-Huitle, C.A.A.; Rodrigo, M.A. Understanding active chlorine species production using boron doped diamond films with lower and higher sp3/sp2 ratio. Electrochem. Commun. 2015, 55, 34–38. [Google Scholar] [CrossRef]
- Mostafa, E.; Reinsberg, P.H.; Garcia-Segura, S.; Baltruschat, H. Chlorine species evolution during electrochlorination on boron-doped diamond anodes: In-situ electrogeneration of Cl2, Cl2O and ClO2. Electrochim. Acta 2018, 281, 831–840. [Google Scholar] [CrossRef]
- Souza, F.L.; Sáez, C.; Lanza, M.R.V.; Cañizares, P.; Rodrigo, M.A. The effect of the sp3/sp2 carbon ratio on the electrochemical oxidation of 2,4-D with p-Si BDD anodes. Electrochim. Acta 2016, 187, 119–124. [Google Scholar] [CrossRef]
- Umile, T.P.; Groves, J.T. Catalytic generation of chlorine dioxide from chlorite using a water-soluble manganese porphyrin. Angew. Chem. 2011, 50, 695–698. [Google Scholar] [CrossRef]
- Umile, T.P.; Wang, D.; Groves, J.T. Dissection of the mechanism of manganese porphyrin-catalyzed chlorine dioxide generation. Inorg. Chem. 2011, 50, 10353–10362. [Google Scholar] [CrossRef]
- Hicks, S.D.; Petersen, J.; Bougher, C.J.; Abu-Omar, M.M. Chlorite dismutation to chlorine dioxide catalyzed by a water-soluble manganese porphyrin. Angew. Chem. 2011, 50, 699–702. [Google Scholar] [CrossRef]
- Zdilla, M.J.; Lee, A.Q.; Abu-Omar, M.M. Bioinspired dismutation of chlorite to dioxygen and chloride catalyzed by a water-soluble iron porphyrin. Angew. Chem. 2008, 47, 7697–7700. [Google Scholar] [CrossRef]
- Qi, M.; Yi, T.; Mo, Q.; Huang, L.; Zhao, H.; Xu, H.; Huang, C.; Wang, S.; Liu, Y.; Hui, Z. Preparation of High-Purity Chlorine Dioxide by Combined Reduction. Chem. Eng. Technol. 2020, 43, 1850–1858. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; Monteiro, M.M.S.; de Melo Henrique, A.M.; Llanos, J.; Saez, C.; Dos Santos, E.V.; Rodrigo, M.A. A review on the electrochemical production of chlorine dioxide from chlorates and hydrogen peroxide. Curr. Opin. Electrochem. 2021, 27, 100685. [Google Scholar] [CrossRef]
- Romero-Fierro, D.; Bustamante-Torres, M.; Hidalgo-Bonilla, S.; Bucio, E. Microbial degradation of disinfectants. In Recent Advances in Microbial Degradation; Springer: Berlin/Heidelberg, Germany, 2021; pp. 91–130. [Google Scholar]
- Guggenberger, M.; Hettegger, H.; Zwirchmayr, N.S.; Hosoya, T.; Bacher, M.; Zaccaron, S.; Böhmdorfer, S.; Reiter, H.; Spitzbart, M.; Dietz, T. Degradation of the cellulosic key chromophore 2, 5-dihydroxy-[1,4]-benzoquinone (DHBQ) under conditions of chlorine dioxide pulp bleaching: Formation of rhodizonate as secondary chromophore—A combined experimental and theoretical study. Cellulose 2020, 27, 3623–3649. [Google Scholar] [CrossRef]
- Kucera, J. Biofouling of polyamide membranes: Fouling mechanisms, current mitigation and cleaning strategies, and future prospects. Membranes 2019, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Odeh, I.N.; Francisco, J.S.; Margerum, D.W. New pathways for chlorine dioxide decomposition in basic solution. Inorg. Chem. 2002, 41, 6500–6506. [Google Scholar] [CrossRef]
- Li, J.; Cassol, G.S.; Zhao, J.; Sato, Y.; Jing, B.; Zhang, Y.; Shang, C.; Yang, X.; Ao, Z.; Chen, G. Superfast degradation of micropollutants in water by reactive species generated from the reaction between chlorine dioxide and sulfite. Water Res. 2022, 222, 118886. [Google Scholar] [CrossRef]
- Kishimoto, N. State of the art of UV/chlorine advanced oxidation processes: Their mechanism, byproducts formation, process variation, and applications. J. Water Environ. Technol. 2019, 17, 302–335. [Google Scholar] [CrossRef]
- Saini, P.; Krishnan, A.; Yadav, D.; Hazra, S.; Elias, A.J. External Catalyst-Free Oxidation of Benzyl Halides to Benzoic Acids Using NaOH/TBHP in Water. Asian J. Org. Chem. 2021, 10, 2355–2359. [Google Scholar] [CrossRef]
- Rougé, V.; Allard, S.; Croué, J.-P.; von Gunten, U. In situ formation of free chlorine during ClO2 treatment: Implications on the formation of disinfection byproducts. Environ. Sci. Technol. 2018, 52, 13421–13429. [Google Scholar] [CrossRef]
- Wang, L.; Margerum, D.W. Hypohalite ion catalysis of the disproportionation of chlorine dioxide. Inorg. Chem. 2002, 41, 6099–6105. [Google Scholar] [CrossRef]
- Firak, D.S.; Farkas, L.; Náfrádi, M.; Alapi, T. Degradation of chlorinated and hydroxylated intermediates in UVA/ClO2 systems: A chlorine-based advanced oxidation process investigation. J. Environ. Chem. Eng. 2022, 10, 107554. [Google Scholar] [CrossRef]
- Philpott, M.J.; Hayes, S.C.; Reid, P.J. Femtosecond pump–probe studies of chlorine dioxide photochemistry in water and acetonitrile. Chem. Phys. 1998, 236, 207–224. [Google Scholar] [CrossRef]
- Chuang, Y.-H.; Wu, K.-L.; Lin, W.-C.; Shi, H.-J. Photolysis of Chlorine Dioxide under UVA Irradiation: Radical Formation, Application in Treating Micropollutants, Formation of Disinfection Byproducts, and Toxicity under Scenarios Relevant to Potable Reuse and Drinking Water. Environ. Sci. Technol. 2022, 56, 2593–2604. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Fan, M.; Yin, R.; Zhang, X.; Lei, Y.; Shang, C.; Yang, X. Micropollutant abatement and byproduct formation during the co-exposure of chlorine dioxide (ClO2) and UVC radiation. J. Hazard. Mater. 2021, 419, 126424. [Google Scholar] [CrossRef]
- Cosson, H.; Ernst, W.R. Photodecomposition of chlorine dioxide and sodium chlorite in aqueous solution by irradiation with ultraviolet light. Ind. Eng. Chem. Res. 1994, 33, 1468–1475. [Google Scholar] [CrossRef]
- Thøgersen, J.; Jepsen, P.U.; Thomsen, C.; Poulsen, J.A.; Byberg, J.; Keiding, S. Femtosecond photolysis of ClO2 in aqueous solution. J. Phys. Chem. A 1997, 101, 3317–3323. [Google Scholar] [CrossRef]
- Fang, J.; Fu, Y.; Shang, C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ. Sci. Technol. 2014, 48, 1859–1868. [Google Scholar] [CrossRef]
- Yin, R.; Blatchley, E.R., III; Shang, C. UV photolysis of mono-and dichloramine using UV-LEDs as radiation sources: Photodecay rates and radical concentrations. Environ. Sci. Technol. 2020, 54, 8420–8429. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅ OH/⋅ O− in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Buxton, G.; Subhani, M. Radiation chemistry and photochemistry of oxychlorine ions. Part 2.—Photodecomposition of aqueous solutions of hypochlorite ions. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1972, 68, 958–969. [Google Scholar] [CrossRef]
- Guo, K.; Wu, Z.; Shang, C.; Yao, B.; Hou, S.; Yang, X.; Song, W.; Fang, J. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water. Environ. Sci. Technol. 2017, 51, 10431–10439. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Katsumura, Y.; Ueda, K.; Ishigure, K. Reactions between some inorganic radicals and oxychlorides studied by pulse radiolysis and laser photolysis. J. Chem. Soc. Faraday Trans. 1997, 93, 1885–1891. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Ross, A.C.; Caballero, B.; Cousins, R.J.; Tucker, K.L. Modern Nutrition in Health and Disease; Jones & Bartlett Learning: Hoboken, NJ, USA, 2020. [Google Scholar]
- Nielsen, F.H. Manganese, molybdenum, boron, chromium, and other trace elements. Present Knowl. Nutr. 2012, 2012, 586–607. [Google Scholar]
- Li, L.; Yang, X. The essential element manganese, oxidative stress, and metabolic diseases: Links and interactions. Oxidative Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Russell, R.; Beard, J.L.; Cousins, R.J.; Dunn, J.T.; Ferland, G.; Hambidge, K.; Lynch, S.; Penland, J.; Ross, A.; Stoecker, B. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; A Report of the Panel on Micronutrients, Subcommittees on upper Reference Levels of Nutrients and of Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board Institute of Medicine; National Academies Press: Washington, DC, USA, 2001; p. 797. [Google Scholar]
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Asp. Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- Palacios, C. The role of nutrients in bone health, from A to Z. Crit. Rev. Food Sci. Nutr. 2006, 46, 621–628. [Google Scholar] [CrossRef]
- Chen, P.; Bornhorst, J.; Aschner, M.A. Manganese Metabolism in Humans. Front. Biosci. (Landmark Ed.) 2018, 23, 1655–1679. [Google Scholar] [CrossRef]
- Hoigné, J.; Bader, H. Kinetics of reactions of chlorine dioxide (OClO) in water—I. Rate constants for inorganic and organic compounds. Water Res. 1994, 28, 45–55. [Google Scholar] [CrossRef]
- Van Benschoten, J.E.; Lin, W.; Knocke, W.R. Kinetic modeling of manganese (II) oxidation by chlorine dioxide and potassium permanganate. Environ. Sci. Technol. 1992, 26, 1327–1333. [Google Scholar] [CrossRef]
- Wang, L.; Odeh, I.N.; Margerum, D.W. Chlorine dioxide reduction by aqueous iron (II) through outer-sphere and inner-sphere electron-transfer pathways. Inorg. Chem. 2004, 43, 7545–7551. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.; Carlson, K.; Gregory, D. The impact of ferrous ion reduction of chlorite ion on drinking water process performance. Water Res. 2001, 35, 4464–4473. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, J.; Zheng, X. Coupling technique for deep removal of manganese and iron from potable water. Environ. Eng. Sci. 2016, 33, 261–269. [Google Scholar] [CrossRef]
- Fábián, I.; Gordon, G. The kinetics and mechanism of the chlorine dioxide–iodide ion reaction. Inorg. Chem. 1997, 36, 2494–2497. [Google Scholar] [CrossRef]
- Stanbury, D.M.; Martinez, R.; Tseng, E.; Miller, C.E. Slow electron transfer between main-group species: Oxidation of nitrite by chlorine dioxide. Inorg. Chem. 1988, 27, 4277–4280. [Google Scholar] [CrossRef]
- Huie, R.E.; Neta, P. Kinetics of one-electron transfer reactions involving chlorine dioxide and nitrogen dioxide. J. Phys. Chem. 1986, 90, 1193–1198. [Google Scholar] [CrossRef]
- Fukutomi, H.; Gordon, G. Kinetic study of the reaction between chlorine dioxide and potassium iodide in aqueous solution. J. Am. Chem. Soc. 1967, 89, 1362–1366. [Google Scholar] [CrossRef]
- Kern, D.M.; Kim, C.-H. Iodine Catalysis in the Chlorite-Iodide Reaction1. J. Am. Chem. Soc. 1965, 87, 5309–5313. [Google Scholar] [CrossRef]
- Lengyel, I.; Li, J.; Kustin, K.; Epstein, I.R. Rate constants for reactions between iodine-and chlorine-containing species: A detailed mechanism of the chlorine dioxide/chlorite-iodide reaction. J. Am. Chem. Soc. 1996, 118, 3708–3719. [Google Scholar] [CrossRef]
- Lee, Y.; Von Gunten, U. Quantitative structure–activity relationships (QSARs) for the transformation of organic micropollutants during oxidative water treatment. Water Res. 2012, 46, 6177–6195. [Google Scholar] [CrossRef]
- Ganiev, I.; Timergazin, Q.; Kabalnova, N.; Shereshovets, V.; Tolstikov, G. Reactions of chlorine dioxide with organic compounds. Eur. Chem.-Technol. J. 2005, 7, 1–31. [Google Scholar] [CrossRef]
- Amor, H.B.; De Laat, J.; Dore, M. Mode d’action du bioxyde de chlore sur les composes organiques en milieu aqueux: Consommations de bioxyde de chlore et reactions sur les composes phenoliques. Water Res. 1984, 18, 1545–1560. [Google Scholar] [CrossRef]
- Wajon, J.E.; Rosenblatt, D.H.; Burrows, E.P. Oxidation of phenol and hydroquinone by chlorine dioxide. Environ. Sci. Technol. 1982, 16, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Hypochlorous Acid Chemistry in Mammalian Cells—Influence on Infection and Role in Various Pathologies. Int. J. Mol. Sci. 2022, 23, 10735. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, J.; Luo, X.; Zhang, C.; Zhou, Y. Removal of aniline using lignin grafted acrylic acid from aqueous solution. Chem. Eng. J. 2011, 172, 856–863. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, J.; Bai, X.; Lin, Y.; Zhou, W.; Li, J. Free chlorine formation in the process of the chlorine dioxide oxidation of aliphatic amines. Water Res. 2022, 217, 118399. [Google Scholar] [CrossRef]
- Hull, L.; Davis, G.; Rosenblatt, D.; Williams, H.; Weglein, R. Oxidations of amines. III. Duality of mechanism in the reaction of amines with chlorine dioxide. J. Am. Chem. Soc. 1967, 89, 1163–1170. [Google Scholar] [CrossRef]
- Rosenblatt, D.; Hull, L.; De Luca, D.; Davis, G.; Weglein, R.; Williams, H. Oxidations of amines. II. Substituent effects in chlorine dioxide oxidations. J. Am. Chem. Soc. 1967, 89, 1158–1163. [Google Scholar] [CrossRef]
- Andrzejewski, P.; Nawrocki, J. N-nitrosodimethylamine formation during treatment with strong oxidants of dimethylamine containing water. Water Sci. Technol. 2007, 56, 125–131. [Google Scholar] [CrossRef]
- Rosenblatt, D.H.; Hayes, A.J., Jr.; Harrison, B.L.; Streaty, R.A.; Moore, K.A. The reaction of chlorine dioxide with triethylamine in aqueous solution1. J. Org. Chem. 1963, 28, 2790–2794. [Google Scholar] [CrossRef]
- Selbes, M.; Kim, D.; Karanfil, T. The effect of pre-oxidation on NDMA formation and the influence of pH. Water Res. 2014, 66, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Schmidt, C.K.; Yoon, J.; von Gunten, U. Oxidation of N-nitrosodimethylamine (NDMA) precursors with ozone and chlorine dioxide: Kinetics and effect on NDMA formation potential. Environ. Sci. Technol. 2007, 41, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Yakupov, M.Z.; Shereshovets, V.V.; Imashev, U.B.; Ismagilov, F.R. Liquid-phase oxidation of thiols with chlorine dioxide. Russ. Chem. Bull. 2001, 50, 2352–2355. [Google Scholar] [CrossRef]
- Tan, H.; Wheeler, W.B.; Wei, C.I. Reaction of chlorine dioxide with amino acids and peptides: Kinetics and mutagenicity studies. Mutat. Res. 1987, 188, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Ison, A.; Odeh, I.N.; Margerum, D.W. Kinetics and mechanisms of chlorine dioxide and chlorite oxidations of cysteine and glutathione. Inorg. Chem. 2006, 45, 8768–8775. [Google Scholar] [CrossRef] [PubMed]
- Darkwa, J.; Olojo, R.O.; Chikwana, E.; Simoyi, R.H. Antioxidant Chemistry: Oxidation of l-Cysteine and Its Metabolites by Chlorite and Chlorine Dioxide. J. Phys. Chem. A 2004, 108, 5576–5587. [Google Scholar] [CrossRef]
- Napolitano, M.J.; Green, B.J.; Nicoson, J.S.; Margerum, D.W. Chlorine dioxide oxidations of tyrosine, N-acetyltyrosine, and dopa. Chem. Res. Toxicol. 2005, 18, 501–508. [Google Scholar] [CrossRef]
- Stewart, D.J.; Napolitano, M.J.; Bakhmutova-Albert, E.V.; Margerum, D.W. Kinetics and mechanisms of chlorine dioxide oxidation of tryptophan. Inorg. Chem. 2008, 47, 1639–1647. [Google Scholar] [CrossRef]
- Ogata, N. Denaturation of protein by chlorine dioxide: Oxidative modification of tryptophan and tyrosine residues. Biochemistry 2007, 46, 4898–4911. [Google Scholar] [CrossRef]
- Noss, C.I.; Hauchman, F.S.; Olivieri, V.P. Chlorine dioxide reactivity with proteins. Water Res. 1986, 20, 351–356. [Google Scholar] [CrossRef]
- Rougé, V. Chlorine Dioxide Oxidation in Water Treatment: Impact on Natural Organic Matter Characteristics, Disinfection Byproducts and Comparison with Other Oxidants; Curtin University: Bentley, WA, Australia, 2018. [Google Scholar]
- Bakhmutova-Albert, E.V.; Margerum, D.W.; Auer, J.G.; Applegate, B.M. Chlorine dioxide oxidation of dihydronicotinamide adenine dinucleotide (NADH). Inorg. Chem. 2008, 47, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Bryan, R.F. Biochemistry. Second Edition by D. Voet and J. G. Voet. Acta Crystallogr. Sect. D-Biol. Crystallogr. 1996, 52, 610. [Google Scholar] [CrossRef]
- Rubio-Casillas, A.; Cambra-Madrid, P. Farmacocinética y farmacodinamia del dióxido de cloro. E-CUCBA 2021, 16, 21–35. [Google Scholar] [CrossRef]
- Moore, G.S.; Calabrese, E.J.; DiNardi, S.; Tuthill, R.W. Potential health effects of chlorine dioxide as a disinfectant in potable water supplies. Med. Hypotheses 1978, 4, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, Y.; Inoue, N. First case of methemoglobinemia caused by a ClO2-based household product. Pediatr. Int. 2015, 57, 1182–1183. [Google Scholar] [CrossRef]
- Romanovsky, A.; Djogovic, D.; Chin, D. A Case of Sodium Chlorite Toxicity Managed with Concurrent Renal Replacement Therapy and Red Cell Exchange. J. Med. Toxicol. 2012, 9, 67–70. [Google Scholar] [CrossRef]
- Chejfec-Ciociano, J.M.; Martínez-Herrera, J.P.; Parra-Guerra, A.D.; Chejfec, R.; Barbosa-Camacho, F.J.; Ibarrola-Peña, J.C.; Cervantes-Guevara, G.; Cervantes-Cardona, G.A.; Fuentes-Orozco, C.; Cervantes-Pérez, E. Misinformation About and Interest in Chlorine Dioxide During the COVID-19 Pandemic in Mexico Identified Using Google Trends Data: Infodemiology Study. JMIR Infodemiol. 2022, 2, e29894. [Google Scholar] [CrossRef]
- Mostajo-Radji, M.A. Pseudoscience in the times of crisis: How and why chlorine dioxide consumption became popular in Latin America during the COVID-19 pandemic. Front. Political Sci. 2021, 25. [Google Scholar] [CrossRef]
- Burela, A.; Hernández-Vásquez, A.; Comandé, D.; Peralta, V.; Fiestas, F. Dióxido de cloro y derivados del cloro para prevenir o tratar la COVID-19: Revisión sistemática. Rev. Peru. De Med. Exp. Y Salud Pública 2021, 37, 605–610. [Google Scholar] [CrossRef]
- Soriano-Moreno, D.R.; Fernandez-Guzman, D.; Ccami-Bernal, F.; Rojas-Miliano, C.; Nieto-Gutierrez, W. Factors associated with the consumption of chlorine dioxide to prevent and treat COVID-19 in the Peruvian population: A cross-sectional study. BMC Public Health 2021, 21, 2109. [Google Scholar] [CrossRef]
- 111. La AEMPS Advierte de los Riesgos Graves Para la Salud por el Consumo de Dióxido de Cloro o MMS. Agencia Española De Medicam. Y Prod. Sanit. (AEMPS). 2020. Available online: https://www.aemps.gob.es/informa/la-aemps-advierte-de-los-riesgos-graves-para-la-salud-por-el-consumo-de-dioxido-de-cloro-o-mms/ (accessed on 25 October 2022).
- Vincenti, S.; de Waure, C.; Raponi, M.; Teleman, A.A.; Boninti, F.; Bruno, S.; Boccia, S.; Damiani, G.; Laurenti, P. Environmental surveillance of Legionella spp. colonization in the water system of a large academic hospital: Analysis of the four-year results on the effectiveness of the chlorine dioxide disinfection method. Sci. Total Environ. 2019, 657, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Southwell, K.L. Chlorine Dioxide Dry Fumigation in Special Collection Libraries. Libr. Arch. Secur. 2003, 18, 39–49. [Google Scholar] [CrossRef]
- Buttner, M.P.; Cruz, P.; Stetzenbach, L.D.; Klima-Comba, A.K.; Stevens, V.L.; Cronin, T. Determination of the Efficacy of Two Building Decontamination Strategies by Surface Sampling with Culture and Quantitative PCR Analysis. Appl. Environ. Microbiol. 2004, 70, 4740–4747. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, H.; Poppendieck, D.; Corsi, R.L. Chlorine dioxide reactions with indoor materials during building disinfection: Surface uptake. Environ. Sci. Technol. 2009, 43, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.C.; Wu, C.; Andriychuk, L.A.; Martín, J.M.; Brasel, T.L.; Jumper, C.A.; Straus, D.C. Effect of Chlorine Dioxide Gas on Fungi and Mycotoxins Associated with Sick Building Syndrome. Appl. Environ. Microbiol. 2005, 71, 5399–5403. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Lowe, J.J.; Gibbs, S.G.; Iwen, P.C.; Smith, P.W.; Hewlett, A.L. Impact of chlorine dioxide gas sterilization on nosocomial organism viability in a hospital room. Int. J. Environ. Res. Public Health 2013, 10, 2596–2605. [Google Scholar] [CrossRef]
- Burela, A.; Hernández-Vásquez, A.; Comandé, D.; Peralta, V.; Fiestas, F. Chlorine dioxide and chlorine derivatives for the prevention or treatment of COVID-19: A systematic review. Rev. Peru. De Med. Exp. Y Salud Publica 2021, 37, 605–610. [Google Scholar] [CrossRef]
- Silva, A.A.; Orantes, L.D.C.; Enriquez, K.O.G.; Perez, F.S.P.; Basilio, A.; Jimenez, Y.L.C.; Díaz-Gutiérrez, S.P. Chemical pneumonitis secondary to chlorine dioxide consumption in a patient with severe Covid-19. Platelets 2020, 280, 261000. [Google Scholar]
- Lardieri, A.; Cheng, C.; Jones, S.C.; McCulley, L. Harmful effects of chlorine dioxide exposure. Clin. Toxicol. 2021, 59, 448. [Google Scholar] [CrossRef]



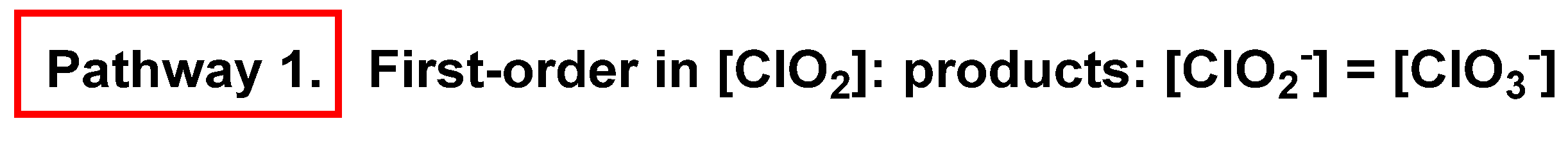

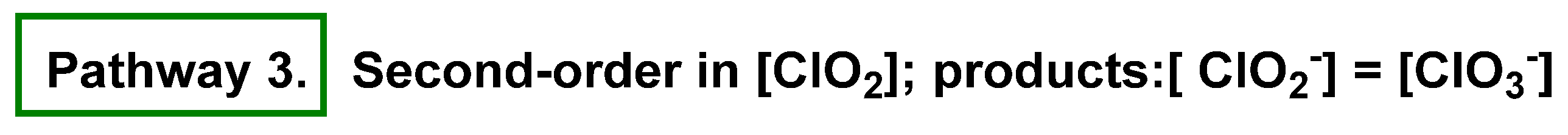
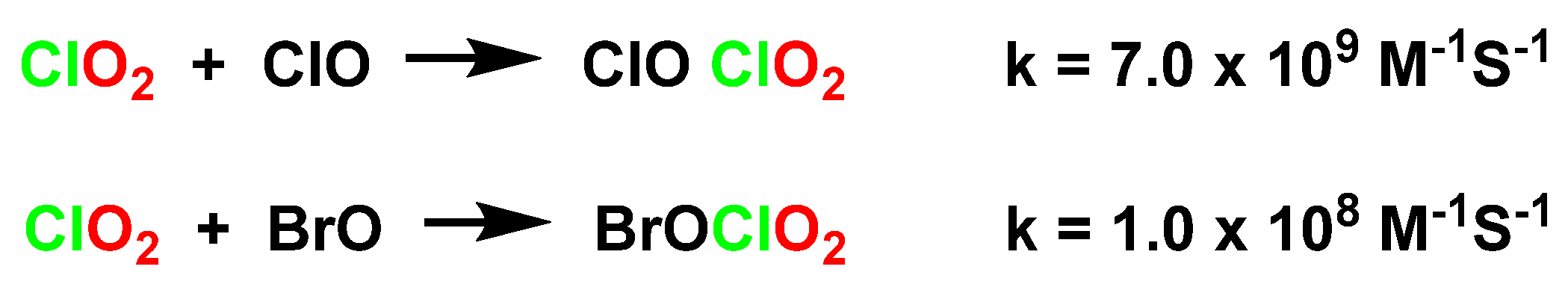



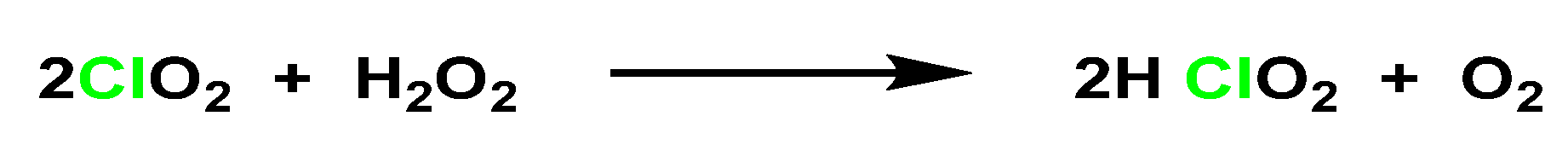
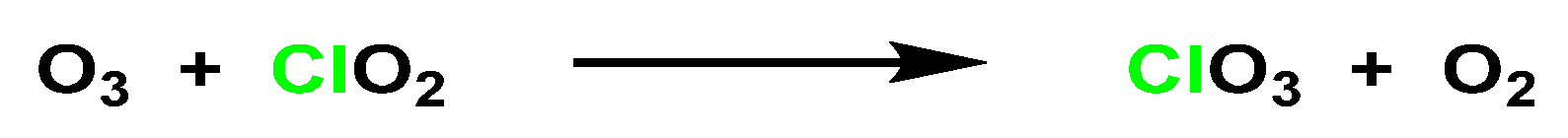





| Compound | Solvent | pH | T °C | K (M−1 s−1 ) |
|---|---|---|---|---|
| Phenoxide ion | H2O | 23 | 4.9 × 107 | |
| Phenol | H2O | 0.24 | ||
| 2-Chlorophenoxide ion | H2O | 2–5 | 23 | 3.5 x 107 |
| 2-Chlorophenol | H2O | 2–5 | 23 | 1.5 |
| Compound | pH | T °C | K (M−1 s−1 ) | Refs |
|---|---|---|---|---|
| Benzylamine | 8.96 | 25.0 | 4.1 × 10−2 | [86] |
| Benzyl-tert-butylamine | 8.4 | 25.0 | 2.9 × 102 | [86] |
| N,N- dimethy-3-methoxybenzylamine | 27.0 | 2.9 × 104 | [87] | |
| Methylamine | 7–10 | 25.0 | <1 | [85] |
| Dimethylamine | 6.8–9.3 | 24.0 | 5 × 102 | [88] |
| Trimethylamine | 23.0 | 6 × 104 | [89] |
| Compound | pH | T °C | K (M−1 s−1 ) |
|---|---|---|---|
| Peptides | |||
| Glutathione | 5.9 | 25.0 | 1.4 × 108 |
| Proteins | |||
| Bovine serum albumin | 7.0 | 25.0 | 6.4 |
| Glucosa-6-fosfato deshidrogenasa | 7.0 | 25.0 | 9.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrés, C.M.C.; Lastra, J.M.P.d.l.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Chlorine Dioxide: Friend or Foe for Cell Biomolecules? A Chemical Approach. Int. J. Mol. Sci. 2022, 23, 15660. https://doi.org/10.3390/ijms232415660
Andrés CMC, Lastra JMPdl, Andrés Juan C, Plou FJ, Pérez-Lebeña E. Chlorine Dioxide: Friend or Foe for Cell Biomolecules? A Chemical Approach. International Journal of Molecular Sciences. 2022; 23(24):15660. https://doi.org/10.3390/ijms232415660
Chicago/Turabian StyleAndrés, Celia María Curieses, José Manuel Pérez de la Lastra, Celia Andrés Juan, Francisco J. Plou, and Eduardo Pérez-Lebeña. 2022. "Chlorine Dioxide: Friend or Foe for Cell Biomolecules? A Chemical Approach" International Journal of Molecular Sciences 23, no. 24: 15660. https://doi.org/10.3390/ijms232415660
APA StyleAndrés, C. M. C., Lastra, J. M. P. d. l., Andrés Juan, C., Plou, F. J., & Pérez-Lebeña, E. (2022). Chlorine Dioxide: Friend or Foe for Cell Biomolecules? A Chemical Approach. International Journal of Molecular Sciences, 23(24), 15660. https://doi.org/10.3390/ijms232415660







